Abstract
Objective
To determine if the use of a sex specific standard to define small for gestational age (SGA) will improve prediction of stillbirth.
Study design
We performed a retrospective cohort study of singleton pregnancies excluding anomalies, aneuploidy, undocumented fetal sex or birthweight. SGA was defined as birthweight < 10th percentile by the non-sex specific and sex specific Alexander standards. The association between SGA and stillbirth using these standards was assessed using logistic regression.
Results
Among 57,170 pregnancies meeting inclusion criteria, 319 (0.6%) pregnancies were complicated by stillbirth. The area under the receiver operating characteristics curve for the prediction of stillbirth was greater for the sex-specific compared to the non-sex specific standard (0.83 vs. 0.72 P< 0.001).
Conclusion
Our findings suggest adoption of a sex specific standard for diagnosis of SGA as it is more discriminative in identifying the SGA fetus at risk for stillbirth.
Keywords: Sex specific growth, small for gestational age, SGA, stillbirth
Introduction
Sex specific differences in birthweight have been described since the 1950s1; females tend to be smaller than males.2 Studies on fetal growth have not only confirmed that females are notably smaller than males, but that this difference in growth exists throughout gestation, with an increase in overall growth-disparity between sexes with increasing gestational age.3, 4 Growth patterns of individual fetal biometric parameters have also been shown to differ between males and females.4, 5, 6 These differences result in a unique sex specific growth trajectory which was described by Alexander et al. in the development of a national fetal growth standard.7 Despite the evidence of sex differences in fetal growth and improved sonographic accuracy of sex specific fetal weight estimation,6, 8 sex specific growth standards are not routinely utilized in the United States.
Over the last 20 years, little improvement has been made in stillbirth rates in high-income countries9. In the United States, one out of every two-hundred pregnancies reaching 22 weeks ends in stillbirth,10 and birthweight has been demonstrated, through time, and over varying populations, to be one of the strongest epidemiological associations with perinatal mortality.11 Given the evidence of sex differences in fetal growth, our objective was to determine if a sex specific fetal growth standard, compared to a non-sex specific growth standard, will improve the prediction of stillbirth among fetuses that are small for gestational age (SGA).
Materials and Methods
We conducted a retrospective cohort study of singleton pregnancies using our prospectively collected perinatal database from January 1990 to December 2009 at Washington University in Saint Louis. Approval for the study was obtained from our institutional review board. Our perinatal database is a large validated system updated and maintained daily by a dedicated perinatal research nurse; details of the methods for data collection and follow-up have been described previously.12 Briefly, our academic tertiary care center is a major referral center for Missouri, Southern and Central Illinois and Northeast Arkansas. Maternal demographics, medical, and obstetric history are obtained using a self-report questionnaire at the initial ultrasound visit. Follow-up information is obtained from the medical record or through contact with the patient and referring physician if the patient delivers outside our hospital system.
We excluded pregnancies complicated by fetal anomalies and aneuploidy. Pregnancies with missing birthweight and sex status were also excluded. Gestational age was confirmed by ultrasound criteria. Gestational age was reassigned if there was a discrepancy of ± 5 days in the first trimester or ± 10 days in the second trimester. Ultrasound examinations were performed by certified obstetric sonographers and final diagnoses were made by board certified Maternal Fetal Medicine specialists.
SGA was defined as birthweight < 10th percentile by the non-sex specific growth standard and the sex specific growth standard as published by Alexander et al.7 The primary outcome examined was stillbirth, defined as intrauterine fetal death ≥ 20 weeks gestation. Univariable analysis was used to compare maternal demographic, medical, and pregnancy characteristics between stillbirths and live births. Descriptive statistics were performed using χ2 for categorical variables and Student’s t-test for continuous variables.
Odds ratios (OR) for SGA stillbirth were calculated for both the sex specific and non-sex specific definitions of SGA. Multivariable logistic regression was used to obtain adjusted odds ratios (aOR). Covariates were selected based on biologic plausibility, and factors identified in the literature. Maternal age, nulliparity, race, body mass index (BMI), smoking, chronic hypertension (CHTN) and pre-gestational diabetes were included in the final model. Fit for each final model was assessed using the Hosmer-Lemeshow goodness-of-fit test.13 To compare the predictive abilities of the two growth standards, receiver operating characteristics (ROC) curves were developed for the prediction of stillbirth among SGA pregnancies as defined by the non-sex specific standard and the sex specific standard. The areas under the ROC curves (AUC) were compared using the nonparametric test by DeLong et al.14 The screening efficiencies for the prediction of stillbirth among SGA pregnancies were calculated for the non-sex specific and the sex specific standards. Screening efficiencies included sensitivity, specificity, negative predictive value, positive predictive value, negative likelihood ratios and positive likelihood ratios. Statistical analysis was performed using STATA 12.0. Tests with p< 0.05 were considered statistically significant.
Results
Of 57, 170 pregnancies meeting inclusion criteria, 28,954 (50.6%) fetuses were female and 28,216 (49.4%) male. In all, 319 (0.6%) pregnancies were complicated by stillbirth. Table 1 demonstrates relevant demographic characteristics of the study cohort comparing maternal and obstetric characteristics of pregnancies with female fetuses to those with male fetuses. There were significantly more male SGA pregnancies than female SGA pregnancies when SGA was defined by either of the two growth standards: non-sex specific (9.0% vs. 5.8% p<0.01) and sex specific (11.7% vs. 5.6% p<0.01). Pregnancies with female fetuses were similar to pregnancies with male fetuses for the remaining variables tested (Table 1). Table 2 demonstrates relevant demographic characteristics of the study cohort comparing pregnancies complicated by stillbirth to pregnancies resulting in a live birth. Mothers experiencing stillbirth had a median age of 2 years younger than mothers with live births (29 (23,35) vs. 31 (26, 35), and the frequency of AMA mothers did not differ between groups (p=0.26). Additionally, 5.9% more nulliparous mothers experienced stillbirth over livebirth (44.5% vs. 38.6%, p=0.02). Race was also significantly different among women experiencing stillbirth and livebirth. African American women accounted for 42.3% of the stillbirths but only 22.7% of the population whereas white women accounted for 45.1% of stillbirths but made up 61.8% of the population. Finally, the potentially modifiable risk factors BMI, smoking, and pre-gestational diabetes were significantly greater among stillbirths compared to livebirths (Table 2), whereas chronic hypertension was statistically greater in the livebirth group compared to the stillbirth group but only by 0.1% making the clinical significance questionable (5.7% vs. 5.6% p<0.01) (Table 2).
Table 1.
Maternal and Pregnancy characteristics of female and male fetuses
| Characteristic | Female (28,954) | Male (28,216) |
|---|---|---|
| Age (y)* | 31 (26, 35) | 31 (26, 35) |
| AMA (%) | 8,466 (29.2) | 8,408 (29.8) |
| Nulliparous (%) | 11,267 (38.9) | 10,796 (38.3) |
| Race | ||
| African American (%) | 6,567 (22.7) | 6,426 (22.8) |
| White (%) | 17,833 (61.6) | 17,471 (62.0) |
| Other (%) | 4,554 (15.7) | 4,319 (15.3) |
| BMI (kg/m2)* | 24.8 (21.7, 29.7) | 24.7 (21.7, 29.5) |
| Smoking (%) | 3,139 (10.9) | 3,099 (11.0) |
| Chronic hypertension (%) | 695 (2.4) | 729 (2.6) |
| Preeclampsia (%) | 2,313 (8.1) | 2,269 (8.1) |
| Pre-gestational diabetes (%) | 582 (2.0) | 525 (1.9) |
| Gestational Diabetes (%) | 1,555 (5.4) | 1,463 (5.2) |
| SGA non-sex specific (%)† | 1,683 (5.8) | 2,526 (9.0) |
| SGA sex specific (%)† | 1,465 (5.6) | 3,307 (11.7) |
| Stillbirth (%) | 177 (0.6) | 142 (0.5) |
Median (interquartile range)
p<0.05
Table 2.
Maternal Characteristics of Stillbirths and Live Births
| Characteristic | Stillbirth (319) | Live birth (56,851) |
|---|---|---|
| Age (y)*† | 29 (23,35) | 31 (26,35) |
| AMA (%) | 85 (26.7) | 16,789 (29.5) |
| Nulliparous (%)† | 142 (44.5) | 21,921 (38.6) |
| Race | ||
| African American (%)† | 135 (42.3) | 12,858 (22.6) |
| White (%)† | 144 (45.1) | 35,160 (61.8) |
| Other (%) | 40 (12.5) | 8,833 (15.5) |
| BMI (kg/m2)*† | 25.9 (22.7,33.0) | 24.8 (21.7,29.5) |
| Smoking (%)† | 48 (15.1) | 6,190 (10.9) |
| Chronic hypertension (%)† | 18 (5.6) | 1,406 (5.7) |
| Preeclampsia (%) | 21 (6.7) | 4,561 (1.9) |
| Pre-gestational diabetes (%)† | 13 (4.1) | 1,094 (1.9) |
| Gestational Diabetes (%) | 15 (4.8) | 3,003 (5.3) |
Median (interquartile range)
p<0.05
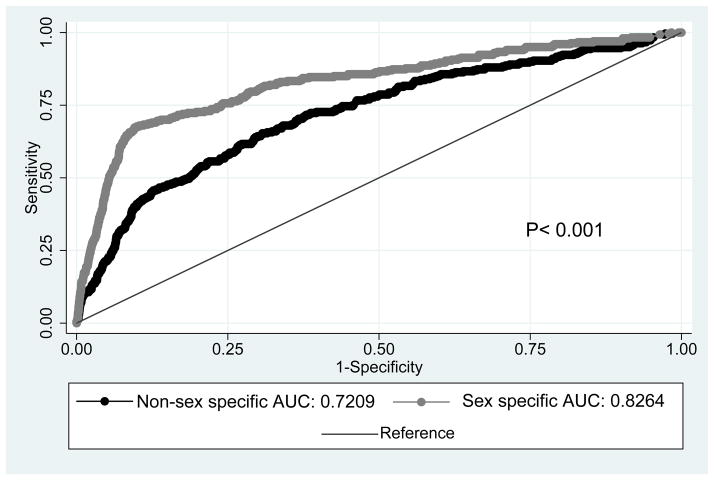
Using the non-sex specific growth standard, 102 of 319 stillbirths were defined as SGA, giving a 32% screen positive rate of the non-sex specific standard (Table 3). Using the sex specific growth standard to define SGA, 204 of 319 stillbirths were SGA, giving a 64% screen positive rate for the sex specific standard. The odds of stillbirth when SGA is defined by the sex specific standard was nearly 4-fold higher than the odds of stillbirth when SGA was defined by the non-sex specific standard (aOR 19.6 (95% CI 15.3–25.1) vs. aOR 5.26 (95% CI 4.1–6.7)). ROCs showed that defining SGA by the sex specific standard demonstrates better discrimination for the prediction of stillbirth compared to the non-sex specific standard (Figure 1) (AUC 0.826 vs. AUC 0.721 p<0.001).
Table 3.
Association between SGA and stillbirth for the non-sex specific and sex specific growth standards
| Growth Standard | Screen Positive (n stillbirths=319) | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|---|
| Non-sex specific n(%) | 102 (32%) | 6.04 (4.7–7.0) | 5.26 (4.1–6.8) |
| Sex specific n(%) | 204 (64%) | 20.30 (16.0–25.8) | 19.6 (15.3–25.0) |
Adjusted for maternal age, nulliparity, African American race, BMI, smoking, CHTN, and pre-gestational diabetes
Figure 1.
ROC curves for the prediction of stillbirth for SGA as defined by non-sex specific and sex specific growth standards. Adjusted for maternal age, nulliparity, African American race, BMI, smoking, CHTN and pre-gestational diabetes.
Further characterization of the diagnostic utility of the two growth standards is presented in Table 4. While sensitivities and positive predictive values for stillbirth were poor for both standards, each diagnostic test characteristic was superior for the sex specific standard. For a fixed false-positive of 10%, the detection rate of the sex specific standard was 70%, compared with 44% for the non-sex specific standard.
Table 4.
Test characteristics of non-sex specific, sex specific and combination of the two standards to predict stillbirth for SGA pregnancies
| Growth Standard | Sensitivity | Specificity | PPV* | NPV* | +LR* (95% CI) | −LR* (95% CI) |
|---|---|---|---|---|---|---|
| Non-sex specific | 32.0% | 92.8% | 2.4% | 99.6% | 4.43 (3.8–5.2) | 0.73 (0.7–0.8) |
| Sex specific | 63.9% | 92% | 4.3% | 99.8% | 7.96 (7.3–8.7) | 0.39 (0.3–0.5) |
NPV (negative predictive value), PPV (positive predictive value), +LR (positive likelihood ratio), −LR (negative likelihood ratio).
Discussion
In this study, we found SGA defined by a sex-specific growth standard is associated with greater odds of stillbirth than SGA defined by a non-sex specific growth standard. In addition, the sex specific standard was better at discriminating the SGA fetuses at risk for stillbirth. Further, use of the sex specific standard was associated with a higher detection of stillbirth for a fixed false-positive rate. Our findings demonstrate the superiority of a sex specific growth standard for the prediction of stillbirth among SGA fetuses and support the clinical utility of sex specific standards of fetal growth.
Historically, the difference in growth between males and females was not thought to become significant until late in the third trimester, making the clinical significance of detecting such growth differences in the antenatal period questionable.2 However, through a prospective longitudinal study of 96 women, Parker et al. demonstrated the divergence of sex specific intrauterine fetal growth starting at approximately 20 weeks gestation and this sex disparity in growth increases as gestational age advances.4
The role of fetal sex in the accuracy of ultrasound to predict birthweight was investigated by Melamed et al in a retrospective cohort study of over three-thousand women.5 The authors tested the accuracy of 8 published regression models among male and female fetuses and found the likelihood of estimated fetal weight prediction within 10% of birthweight was 30% higher for male fetuses. The systematic error was found to be greater for female fetuses regardless of the model used. However, the authors questioned the clinical significance of the relatively small magnitude of the differences in accuracy between male and female fetuses.
In follow-up to their 2011 study, Melamed and colleagues compared 9 existing models for the estimation of fetal weight to their own sex-specific model.15 The Melamed sex specific models performed with greater accuracy. The authors found the improved accuracy of the sex specific models was not observed at extremes of weight, below the first birthweight quartile and above the third birthweight quartile.
Most recently, in 2013, Melamed and colleagues published a retrospective cross-sectional investigation of over 12,000 fetal weight estimations to evaluate the effect of fetal sex on fetal growth patterns in the second and third trimester.6 With a large cohort the authors were able to use contemporary techniques to confirm findings of previous studies demonstrating the independent effect of fetal sex on the relationship between biometric parameters. 4, 8, 16, 17 In addition, using regression analysis, the authors were able to show sex specific differences in growth unique to each biometric parameter. Although the current literature supports the use of a sex specific model to estimate fetal weight, stratified analyses by both Seimer and Melamed suggest the SGA fetus may not benefit from the improved accuracy of a sex specific model for the estimation of fetal weight. The accurate prediction of fetal weight is important, but most fetuses will be normally grown, and it is the fetus at the extremes of growth that are at risk for poor outcomes. Therefore, if we are not able to improve the accuracy of ultrasound for the extremes of weight, then the clinical utility of such models remain unclear.
Our study differs from the studies of Seimer and Melamed in a fundamental way. Unlike the previous authors, we did not set out to evaluate the accuracy of ultrasound; rather, our interest was in the ability of SGA diagnosed based on a population based growth standard to accurately predict stillbirth. We designed our study to evaluate the relationship between SGA and stillbirth for two reasons. First, stillbirth is a potentially modifiable important outcome of interest. Second, as demonstrated by the overall low positive predictive value of both growth standards, many small fetuses do not result in stillbirth. Refining our ability to predict SGA fetuses at risk for stillbirth has the potential to lead to improved surveillance of the small fetus truly at risk for stillbirth, and reduce unnecessary intervention and improved resource utilization for the fetus not at risk for stillbirth.
The major strength of our study was in our well maintained, database with rich patient-level data which has been validated in previous studies. This cohort is sufficiently large to to examine the relatively rare, but important outcome of stillbirth. Second, because our large cohort included over 300 stillbirths we were able to perform logistic regression to adjust for multiple confounders. Finally, because we applied both growth standards to the same cohort of patients we minimized selection bias and confounding.
Our study was performed retrospectively, as such, it is subject to the inherent limitations of all retrospective studies. A factor that limits all studies involving measures of fetal weight is the issue of whether to use ultrasound estimates of fetal weight or birthweight. We chose to use birthweight to define SGA, which is potentially problematic as the timing of demise in relation to timing of birth is often unknown. However, use of Ultrasound estimated fetal weight is complicated by the timing of ultrasound in relation to delivery, and the known inaccuracies of ultrasound.18, 19 Further, we were interested in examining the accuracy of the growth standard to predict stillbirth as opposed to the accuracy of ultrasound to predict birthweight. Therefore, we chose to use birthweight as it seemed to be the most reasonable measure of weight to answer our study question.
In conclusion, while Alexander et al. included both a sex-specific population based growth reference and a non-sex specific reference only the non-sex specific reference has become the widely used growth standard in the United States.7 Our findings demonstrate that the sex specific growth standard performs better than the non-sex specific standard as a tool to best identify SGA fetuses at risk for stillbirth. Adoption of the sex specific growth standard into clinical practice may be a simple way to improve the identification of the SGA fetus at risk for stillbirth.
Acknowledgments
Dr. Trudell is supported by NIH T32 Grant 5T32HD055172-05 George Macones (PI) and the Washington University Institute of Clinical and Translational Sciences Grant UL1TR000448 Bradley Evanoff (PI)
Abreviations
- SGA
small for gestational age
- OR
odds ratio
- aOR
adjusted odds ratio
- BMI
body mass index, kg/m2
- CHTN
chronic hypertension
- ROC
receiver operating characteristics curve
- AUC
area under the receiver operating characteristics curve
Footnotes
Conflicts of interest statement: The authors have no conflict of interest to disclose.
Presented as a poster presentation at the 33rd annual meeting of the Society of Maternal Fetal Medicine, February 11–16, 2013, San Francisco, CA. Abstract #299
References
- 1.Fraccaro M. A contribution to the study of birth weight based on an Italian sample. Annals of human genetics. 1956;20(4):282–297. doi: 10.1111/j.1469-1809.1955.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 2.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 3.Thomson AM, Billewicz WZ, Hytten FE. The assessment of fetal growth. The Journal of obstetrics and gynaecology of the British Commonwealth. 1968;75(9):903–916. doi: 10.1111/j.1471-0528.1968.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 4.Parker AJ, Davies P, Mayho AM, Newton JR. The ultrasound estimation of sex-related variations of intrauterine growth. Am J Obstet Gynecol. 1984;149(6):665–669. doi: 10.1016/0002-9378(84)90255-2. [DOI] [PubMed] [Google Scholar]
- 5.Melamed N, Ben-Haroush A, Meizner I, Mashiach R, Glezerman M, Yogev Y. Accuracy of sonographic weight estimation as a function of fetal sex. Ultrasound Obstet Gynecol. 2011;38(1):67–73. doi: 10.1002/uog.8914. [DOI] [PubMed] [Google Scholar]
- 6.Melamed N, Meizner I, Mashiach R, Wiznitzer A, Glezerman M, Yogev Y. Fetal sex and intrauterine growth patterns. J Ultrasound Med. 2013;32(1):35–43. doi: 10.7863/jum.2013.32.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 8.Schild RL, Sachs C, Fimmers R, Gembruch U, Hansmann M. Sex-specific fetal weight prediction by ultrasound. Ultrasound Obstet Gynecol. 2004;23(1):30–35. doi: 10.1002/uog.942. [DOI] [PubMed] [Google Scholar]
- 9.Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. 2011;377(9774):1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 10.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, et al. Stillbirths: the way forward in high-income countries. Lancet. 2011;377(9778):1703–1717. doi: 10.1016/S0140-6736(11)60064-0. [DOI] [PubMed] [Google Scholar]
- 11.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? American journal of epidemiology. 2006;164(4):303–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 12.Odibo AO, Francis A, Cahill AG, Macones GA, Crane JP, Gardosi J. Association between pregnancy complications and small-for-gestational-age birth weight defined by customized fetal growth standard versus a population-based standard. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(3):411–417. doi: 10.3109/14767058.2010.506566. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DWLS. Applied logistic regression. 2. Wiley; New York (NY): 2000. [Google Scholar]
- 14.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 15.Melamed N, Yogev Y, Ben-Haroush A, Meizner I, Mashiach R, Glezerman M. Does use of a sex-specific model improve the accuracy of sonographic weight estimation? Ultrasound Obstet Gynecol. 2012;39(5):549–557. doi: 10.1002/uog.10064. [DOI] [PubMed] [Google Scholar]
- 16.Pang MW, Leung TN, Sahota DS, Lau TK, Chang AM. Customizing fetal biometric charts. Ultrasound Obstet Gynecol. 2003;22(3):271–276. doi: 10.1002/uog.196. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzler P, Bland JM, Holden D, Campbell S, Ville Y. Sex-specific antenatal reference growth charts for uncomplicated singleton pregnancies at 15–40 weeks of gestation. Ultrasound Obstet Gynecol. 2004;23(1):23–29. doi: 10.1002/uog.966. [DOI] [PubMed] [Google Scholar]
- 18.Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound Obstet Gynecol. 2005;25(1):80–89. doi: 10.1002/uog.1751. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan SP, Beydoun H, Chang E, Sandlin AT, Dahlke JD, Igwe E, et al. Prenatal Detection of Fetal Growth Restriction in Newborns Classified as Small for Gestational Age: Correlates and Risk of Neonatal Morbidity. Am J Perinatol. 2013 doi: 10.1055/s-0033-1343771. [DOI] [PubMed] [Google Scholar]