Abstract
The multifaceted extracellular milieu presents biochemical and biophysical stimuli that influence stem cell differentiation. Two-dimensional (2D) micropatterned substrates allow the presentation of these cues in spatially defined geometries that have been demonstrated to guide stem cell fate decisions. Leveraging stem cells to reconstruct microvasculature, made up of an inner lining of endothelial cells (ECs) supported by pericytes, is critical to tissue-engineering advances; thus, methods to improve endothelial differentiation efficiency are vital to these efforts. In this study, we examine the hypothesis that the diameter of micropatterned islands influences endothelial differentiation from human induced pluripotent stem cells (hiPSCs). Comparing island diameters of 80, 140, 225 and 500 μm, we found that co-cultures of control ECs and pericytes did not yield variable ratios of cell types; however, when hiPSCs were differentiated toward a bicellular population of ECs and pericytes on these varying micropattern feature sizes, we found that smaller islands promoted EC differentiation efficiency, yielding a derived population composed of 70% ECs, which exhibited a greater sprouting propensity. Differentiation on the largest feature size exhibited a smaller EC yield, similar to that on non-patterned substrates. Taken together, these data demonstrate that micropatterned islands of varying diameters can be used to modulate EC differentiation efficiency.
Keywords: cell co-culture, endothelial cells, micropatterning, pericytes, pluripotent stem cells, tissue engineering
1. Introduction
Vascular reconstruction depends on the interplay of multiple cell types, biochemical cues and biophysical stimuli (Jain, 2003). The extracellular matrix (ECM), which has numerous functions, including presentation of instructive cues and maintenance of tissue integrity, is a key regulator in vascular development and regeneration. Advances in tissue engineering allow researchers to harness these instructive signals in precise microenvironments, offering novel therapies for vascular repair (Zhang et al., 2011).
Of the multiple cell types that make up blood vessel architecture, endothelial cells (ECs) form the inner lining of the vascular system and are able to form nascent structures intrinsically. Pericytes are recruited to these structures to confer stabilization and support (Stratman et al., 2009). Because all tissues, both native and engineered, require a functional blood supply for survival, rebuilding blood vessels in the laboratory continues to be a burgeoning field of study (Kusuma and Gerecht, 2010; Blinder et al., 2014).
Human pluripotent stem cells (hPSCs) are an attractive starting source for this endeavour because of their ability to differentiate into all cell types of the body and to self-renew indefinitely in culture, allowing the generation of clinically relevant quantities of cell types of interest. Human embryonic stem cells (hESCs), one subclass of hPSCs, are derived from the developing blastocyst and currently make up much of hPSC research. A newer subclass of hPSCs, human induced PSCs (hiPSCs), are derived by reprogramming somatic cells, conferring patient specificity (Mali and Cheng, 2012). Our previous studies have demonstrated the derivation of a bicellular population of ECs and pericytes from hPSCs after 12 days in a two-dimensional (2D), feeder-free manner (Kusuma et al., 2013). More recently, we have demonstrated that using low-oxygen (~5% O2) culture conditions to this differentiation scheme (either over the entire 12 day differentiation or just during the first 6 days) enhances endothelial differentiation potential, yielding ECs with appropriate membrane localization of vascular endothelial cadherin (VEcad), a phenotype of mature derivatives, after just 12 days (Kusuma et al., 2014; Kusuma et al., 2014). In these studies, we have extensively examined the phenotype and functionality of EC and pericyte derivatives both in vitro and in vivo (Wanjare et al., 2014).
Stem cell fate is predicated by stimuli in the surrounding milieu (Dickinson et al., 2011; Hazeltine et al., 2013). Previous studies have demonstrated a correlation between microenvironment feature size and stem cell fate decisions. Human PSCs differentiated in suspended cell aggregates, or embryoid bodies (EBs), exhibited size-dependent differentiation decisions (Hwang et al., 2009); EBs with a controlled diameter of 450 μm demonstrated a preferential differentiation towards the cardiac lineage, whereas smaller EBs (~150 μm diameter) favoured endothelial lineages. Micropatterning offers a 2D approach to control cellular behaviour, using instructive cues in the form of distinct adherent geometrically defined patterns that typically consist of extracellular matrix (ECM) proteins. Mouse ESCs cultured on fibronectin circular micropatterns exhibited the greatest potential to differentiate toward cardiomyocytes on 200 μm diameters, compared to 100, 300 and 400 μm diameters (Sasaki et al., 2009). On circular Matrigel patterns, cultured hESCs differentiated toward mesoderm on larger islands (800 and 1200 μm), but toward endoderm fates on smaller geometries (200 and 400 μm) (Peerani et al., 2007; Lee et al., 2009). An adult stem cell population, mesenchymal stem cells (MSCs), demonstrated preferential differentiation toward adipocytes on smaller fibronectin islands, whereas larger islands promoted osteoblast differentiation (McBeath et al., 2004). Taken together, these studies reveal that ECM proteins are not the lone determinant of fate specification; rather, their spatially defined presentation is also a key influence. Moreover, the influence of constrained 2D environments on endothelial differentiation from hiPSCs has not been previously examined.
In the current study, we harnessed our previous co-differentiation strategy, which guides hPSCs toward a bicellular population of ECs and pericytes under low oxygen conditions, to test the hypothesis that micropattern diameter affects endothelial differentiation potential from the hiPSC line, BC1.
2. Materials and methods
2.1. Cell culture
2.1.1. HUVECs culture
Human umbilical vein ECs (HUVECs) were cultured as previously described (Kusuma et al., 2012). Briefly, HUVECs (Promocell) were cultured in endothelial cell growth medium (ECGM; PromoCell) supplemented with 2% fetal bovine serum (FBS). The medium was changed every other day. Cells were passaged every 3–4 days with 0.05 trypsin/0.1% ethylenediamine tetra-acetic acid (EDTA; Invitrogen) and maintained in a humidified incubator at 37°C in 5% CO2 atmosphere.
2.1.2. Pericytes culture
Human placental pericytes (passages 3–5, Promocell) were cultured in the specified pericyte growth medium (Promocell) and were passaged every 3–4 days, using a detachment kit (Promocell), as previously described (Wanjare et al., 2014).
2.1.3. HUVECs/pericytes co-culture
HUVECs and pericytes were seeded onto micropatterned coverslips (CYTOO) at a 1:1 ratio with a total cell number of 50 000 in endothelial growth medium 2 (EGM2; Lonza) containing 10% FBS.
2.1.4. hiPSCs culture and differentiation
A human iPSC line, BC1 (Chou et al., 2011; Cheng et al., 2012), was dissociated into a single-cell suspension and seeded onto collagen IV-coated dishes (Trevigen, CellStar) under 5% O2 conditions, as described previously (Kusuma et al., 2013, 2014). Briefly, differentiating cells were exposed to 5% O2 conditions for 6 days, collected through digestion with TrypLE (Invitrogen), separated with a 40 μm strainer and reseeded on fibronectin-micropatterned coverslips (CYTOO) at 50 000 cells/coverslip in EC differentiation medium, which was made up of ECGM (Promocell) supplemented with 2% FBS (Promocell), 50 ng/ml vascular endothelial growth factor (VEGF; Roche), 10 μM SB431542 (Tocris) and 0.1% penicillin–streptomyocin (Life Technologies). The medium was changed on day 3 and the samples were fixed on day 6. Cells were imaged daily using an inverted light microscope (Olympus).
2.2. Immunofluorescence
Cells were prepared for immunofluorescence as previously described (Kusuma et al., 2012). Briefly, fixed cells were blocked in 1% bovine serum albumin (BSA; Sigma-Aldrich), treated with 0.1% Triton-X (Sigma-Aldrich) and incubated with mouse anti-human VEcad (1:200; Santa Cruz Biotechnology), rabbit anti-human SM22 (1:200; Abcam) or rabbit anti-human PDGFRβ (1:100; Santa Cruz Biotechnology), followed by anti-mouse FITC (1:40; Sigma) or anti-rabbit IgG AlexaFluor 488 conjugate (1:1000; Molecular Probes) and DAPI (1:1000; Roche Diagnostics), all at room temperature in the dark. The immunolabelled cells were examined using a fluorescence microscope (Olympus BX60).
2.3. Quantification
Image processing and analysis were conducted using a custom-written MATLAB algorithm (see supporting information). Subsequent to preprocessing the images via a global background subtraction scheme, total nuclei were enumerated by finding regional maximum pixel intensities. Additionally, co-localized nuclei with corresponding image channels representing VEcad expression were quantified.
2.4. Graphs and statistics
All analyses were performed in triplicate samples from at least three independent experiments. A minimum of 30 patterns of each size were analysed per experiment. One-way ANOVA with Bonferroni post hoc test were performed to determine significance (GraphPad Prism 4.02).
3. Results
3.1. HUVECs/pericytes co-culture on circular micropatterns
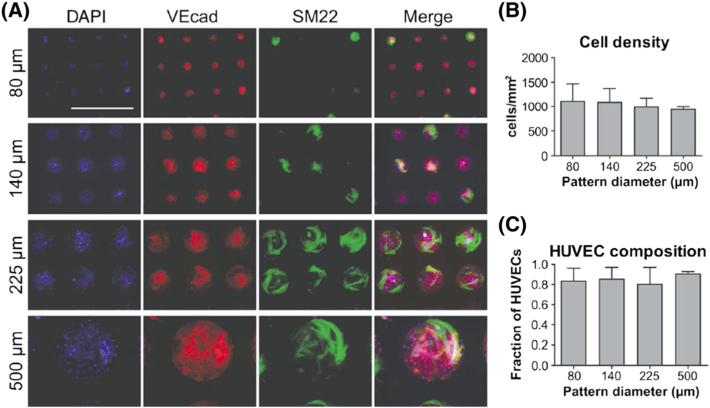
We studied circular fibronectin micropatterns with diameters of 80, 140, 225 and 500 μm. To confirm preferential attachment of control cells on fibronectin micropatterns and to determine whether these cell types preferentially attach to a particular feature size, control ECs (i.e. HUVECs) and pericytes were seeded at a 1:1 ratio on patterned coverslips and cultured for 2 days, allowing for cell adhesion, spreading and the re-establishment of junctional proteins between cells. A 1:1 ratio of tissue-derived vasculogenic cells was chosen to ensure that an uneven ratio did not skew adhesion propensity, and to eliminate the chance of cellular plasticity that hPSC derivatives may exhibit. Both cell types exhibited preferential attachment to the patterned regions (Figure 1A). HUVECs, indicated by VEcad, and pericytes, indicated by SM22, co-cultured on patterns, demonstrate spread pericytes growing above as well as among the HUVECs monolayer (Figure 1A). Quantification of cell number/pattern size revealed that pattern size did not influence cell growth (Figure 1B). Co-culturing these two cell types revealed a preferential attachment of HUVECs to the patterns. Quantification of the fraction of HUVECs/pattern size showed that patterns were predominantly covered by HUVECs (Figure 1C). This finding could be due to preferential attachment of HUVECs to fibronectin surfaces or the smaller size of HUVECs compared to pericytes, which typically take on a more spread morphology. Overall, however, the fraction of HUVECs remained similar between the different pattern sizes, demonstrating that differentiated ECs and pericytes do not demonstrate preferential attachment to any particular feature size.
Figure 1.
Co-culture of HUVECs and pericytes on circular micropatterns of varying diameter and assessed for: (A) VEcad (red) and SM22 (green) expression (nuclei in blue; scale bar = 500 μm); (B) total cell density; and (C) fraction of HUVECs on patterns
3.2. Differentiation of hiPSCs toward EVCs on circular micropatterns
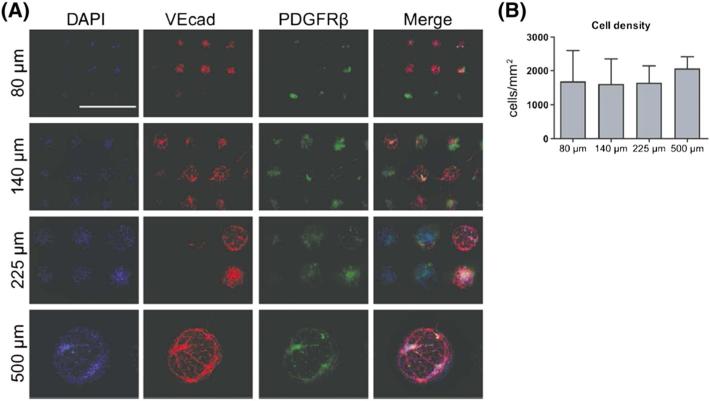
Next, to discern whether micropattern size influences endothelial differentiation potential from hiPSCs, we cultured differentiating cells on micropatterned coverslips. Human iPSCs were dissociated into a single-cell suspension and differentiated for 6 days on collagen IV-coated dishes under 5% O2 conditions. Using this low-oxygen priming method, we obtained approximately 50% positive VEcad cells, on average, from three independent experiments, as previously reported (Kusuma et al., 2014). In the current study, cells were differentiated for the first 6 days under 5% O2 conditions, but on day 6 these progenitors were transferred to coverslips containing circular micropatterns with diameters of 80, 140, 225 or 500 μm. We confirmed that the second 6 days of differentiation on a fibronectin substrate does not alter VEcad composition of EVCs compared to differentiation on collagen IV, as we have utilized in our previous study (Kusuma et al., 2013) (see supporting information, Figure S1). After an additional 6 days, we found that the cells demonstrated preferential attachment to the patterned regions and spread to confluence on the patterns (Figure 2A). We stained for VEcad and platelet-derived growth factor receptor-β (PDGFRβ) to determine our EC and pericyte populations, respectively. As in our bulk differentiation conditions (Kusuma et al., 2014), we could observe derived ECs that took on a cobblestone-like morphology and exhibited VEcad appropriately localized to the cellular membrane. Derived PDGFRβ-positive (PDGFRβ+) pericytes were observed in close contact with VEcad-positive (VEcad+) cells. Quantification of total cell number revealed that cell growth did not depend on pattern size (Figure 2B).
Figure 2.
Co-differentiation of hiPSCs on circular micropatterns. Differentiated hiPSC-BC1 cells on micropatterns were assessed for: (A) endothelial and pericyte differentiation, indicated by VEcad (red) and PDGFR β (green), respectively (nuclei in blue; scale bar = 500 μm); (B) cell density/pattern size
3.3. Quantitative comparison of endothelial differentiation potential on varying micropattern sizes
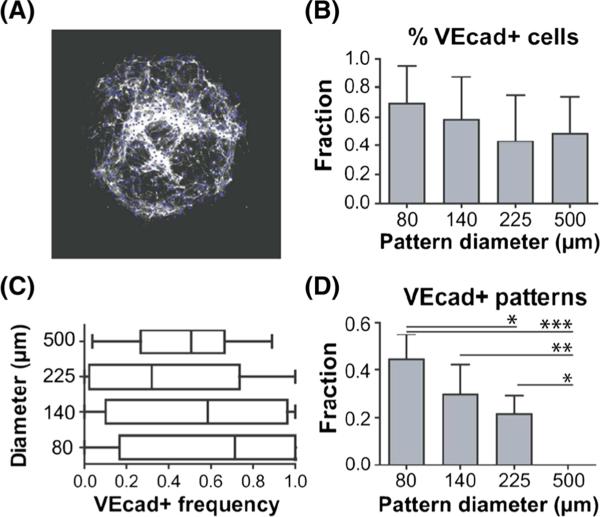
We developed a MATLAB code to quantify the total number of VEcad+ cells per individual pattern. The programme produced black and white images of the VEcad stain with corresponding nuclei positive for VEcad (Figure 3A). Quantification of endothelial derivatives revealed that, unlike control HUVECs and pericytes co-culture, we could obtain different fractions of ECs on the varying pattern sizes. Differentiation on the smallest diameter, 80 μm, yielded approximately 67% VEcad+ cells across all patterns of this feature size (Figure 3B). Quantification over each individual pattern revealed a median value of 71% VEcad+ cells (Figure 3C). Differentiation on 140 μm patterns yielded both mean and median values of 58% VEcad+ cells. Patterns with 225 μm diameters yielded 42% VEcad+ cells, with a median value of 32% per individual pattern. Comparison over these three feature sizes reveals a great endothelial differentiation potential on smaller feature sizes. When we differentiated cells on 500 μm patterns; however, we obtained approximately 50% VEcad+ cells, closely matching our previously published results on non-patterned dishes (Kusuma et al., 2014). Next, we quantified the fraction of patterns that only contained VEcad+ cells without any PDGFRβ+ cells. We could also observe that homogeneous EC patterns were more prevalent at the smallest pattern size (Figure 3D). The fractions decreased at 140 and 225 μm. Pure EC populations were not observed on 500 μm patterns.
Figure 3.
Quantification of endothelial differentiation potential. (A) Output of Matlab analysis and quantification of the (B) total percentage of VEcad-positive cells over all patterns of each feature size; (C) distribution of VEcad-positive cells for individual patterns; and (D) fraction of pure VEcad-positive patterns for each feature size; *p < 0.05; **p < 0.01; ***p < 0.001
3.4. Endothelial sprouting on varying micropattern sizes
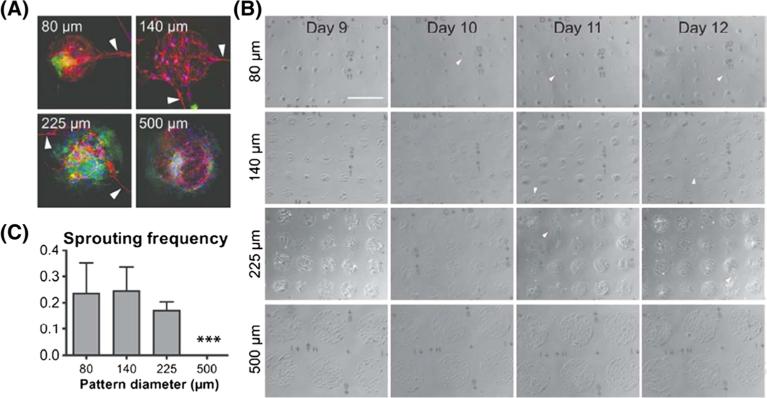
We could also observe sprouting beyond the confined patch area from EVCs in all pattern sizes < 500 μm (Figure 4A). Sprouting is an important stage in the angiogenic cascade, initiating the growth of new blood vessels. To characterize this phenotype, we first assessed whether this behaviour was cell type-specific. Immunofluorescence analysis revealed that our VEcad+ ECs were the lone cell type to sprout from patterns, a phenotype that has been described in other derived EC cultures (Hwang et al., 2009). Imaging patterns over time allowed us to examine the kinetics of sprouting formation (Figure 4B). We found that sprouting primarily occurred on the small micro-pattern feature sizes around day 10, but were largely absent from large micropattern feature sizes until 11–12 days. Quantification of sprouting frequency within each feature size after 12 days corroborated our EC differentiation results; sprouting frequency was much greater in smaller micropatterned islands (i.e. 80 and 140 μm diameters) compared to larger islands (i.e. 225 μm) (Figure 4C). No sprouting was observed outside of 500 μm diameter islands.
Figure 4.
Characterization of sprouting propensity. (A) Immunofluorescent images of instances of sprouting on all pattern sizes; VEcad in red, PDGFRβ in green and DAPI in blue. (B) Light microscope images depicting emergence of sprouting over time on pattern sizes; scale bar = 500 μm. (C) Quantification of sprouting frequency; arrowheads, instances of sprouting
4. Discussion
The regeneration of functional blood vessels in the laboratory benefits a myriad of downstream applications, such as tissue engineering and vascular regenerative medicine; the differentiation of vascular cell types from hPSCs is vital to these efforts in order to recreate patient-specific vasculature. Toward this end, spatial presentation of ECM has been realized as an important cue in the vascular system (Raghavan et al., 2010).
Previous studies on the effect of circular micropatterns on somatic cell differentiation have been primarily centred on the cardiac lineage (Sasaki et al., 2009). Here we present the observation that geometric patterns can also influence the differentiation of ECs from the hiPSC line BC1. Our previous studies on non-patterned surfaces revealed that exposure to low oxygen tension augments endothelial differentiation capacity of hPSCs, yielding approximately 50% VEcad+ cells from the hiPSC line BC1 (Kusuma et al., 2014). In the current study, we added another layer of complexity to the cellular milieu by confining the growth area to micropatterned substrates. We found that micropatterned islands with 80 μm diameters further increased differentiation to yield 70% VEcad+ cells. Moreover, almost 50% of these patterns were covered with ECs only, without pericyte infiltration. We suspect that this preference for small diameter size is due to the well-accepted notion of pericyte-mediated endothelial growth inhibition.
With increasing diameter size, EC differentiation yield decreased; however, differentiation on 500 μm islands, our largest tested pattern size, more closely resembled differentiation on non-patterned substrates, yielding approximately 50% VEcad+ cells (Kusuma et al., 2014). When we quantified cell growth on the tested pattern sizes, we observed that it was independent of pattern size, suggesting that the distinct differentiation capabilities were not dependent on cell proliferation.
We anticipate that our finding that smaller diameter size promotes EC differentiation has a lower feature size limit, as previous studies have demonstrated that islands with approximately 10 μm diameter promote apoptosis (Chen et al., 1997). Furthermore, we anticipate that islands with diameters > 500 μm will not exhibit distinct VEcad+ yields and rather continue to follow a 50% VEcad+ yield found under non-patterned differentiation conditions.
While the focus of the current study is endothelial differentiation, the system presented here could be used as a platform to further study the dynamic interactions between hiPSC-derived ECs and pericytes. First, employing a 2D substrate facilitates high-resolution imaging of homogeneous populations of ECs or pericytes, or co-cultures of both cell types. Second, because our differentiation scheme relies on the co-derivation of both vascular cell types, we anticipate that differentiation on micropatterned islands will help to facilitate the derivation of populations with specific EC:pericyte ratios, which vary based on tissue type.
Taken together, our results demonstrate an important role for ECM feature size in augmenting endothelial differentiation yield from hiPSCs in a controlled manner amenable for clinical translation.
Supplementary Material
Acknowledgements
We thank Dr Linzhao Cheng for providing the hiPSC line BC1. We gratefully acknowledge support for this study by predoctoral awards from the American Heart Association (to S.K.), the National Institutes of Health (NIH; Grant Nos F31HL112644 and R01HL107938, to S.K.) and the National Science Foundation (NSF; Grant No. 1054415, to S.G.).
Footnotes
Supporting information may be found in the online version of this article.
Conflict of interest
The authors have declared that there is no conflict of interest.
Supporting information on the internet
The following supporting information may be found in the online version of this article:
Supplementary 1. No effect of ECM on differentiation capability; flow-cytometry plots for VEcad of EVCs differentiated on either collagen IV or fibronectin substrates.
References
- Blinder YJ, Mooney DJ, Levenberg S. Engineering approaches for inducing blood vessel formation. Curr Opin Chem Eng. 2014;3:56–61. [Google Scholar]
- Chen CS, Mrksich M, Huang S, et al. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Cheng L, Hansen NF, Zhao L, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson LE, Kusuma S, Gerecht S. Reconstructing the differentiation niche of embryonic stem cells using biomaterials. Macromol Biosci. 2011;11:36–49. doi: 10.1002/mabi.201000245. [DOI] [PubMed] [Google Scholar]
- Hazeltine LB, Selekman JA, Palecek SP. Engineering the human pluripotent stem cell microenvironment to direct cell fate. Biotechnol Adv. 2013;31:1002–1019. doi: 10.1016/j.biotechadv.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YS, Chung BG, Ortmann D, et al. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Kusuma S, Facklam A, Gerecht S. Characterizing human pluripotent stem cell-derived vascular cells for tissue engineering applications. Stem Cells Dev. 2014 doi: 10.1089/scd.2014.0377. DOI: 10.1089/scd.2014.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma S, Gerecht S. Engineering blood vessels using stem cells: innovative approaches to treat vascular disorders. Expert Rev Cardiovasc Ther. 2010;8:1433–1445. doi: 10.1586/erc.10.121. [DOI] [PubMed] [Google Scholar]
- Kusuma S, Peijnenburg E, Patel P, et al. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler Thromb Vasc Biol. 2014;34:913–920. doi: 10.1161/ATVBAHA.114.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma S, Shen YI, Hanjaya-Putra D, et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:12601–12606. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma S, Zhao S, Gerecht S. The extra-cellular matrix is a novel attribute of endothelial progenitors and of hypoxic mature endothelial cells. FASEB J. 2012;26:4925–4936. doi: 10.1096/fj.12-209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LH, Peerani R, Ungrin M, et al. Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Res. 2009;2:155–162. doi: 10.1016/j.scr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Mali P, Cheng L. Concise review: human cell engineering: cellular reprogramming and genome editing. Stem Cells. 2012;30:75–81. doi: 10.1002/stem.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Peerani R, Rao BM, Bauwens C, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Nelson CM, Baranski JD, et al. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng A. 2010;16:2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki D, Shimizu T, Masuda S, et al. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30:4384–4389. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, et al. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M, Kusuma S, Gerecht S. Defining differences among perivascular cells derived from human pluripotent stem cells. Stem Cell Rep. 2014;2:561–575. doi: 10.1016/j.stemcr.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xiao Y, Hsieh A, et al. Micro-and nanotechnology in cardiovascular tissue engineering. Nanotechnology. 2011;22:494003. doi: 10.1088/0957-4484/22/49/494003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.