Abstract
Inflammatory responses to biomaterials heavily influence the environment surrounding implanted devices, often producing foreign body reactions. The macrophage is a key immunomodulatory cell type consistently associated with implanted biomaterials and routinely employed in short term in vitro cell studies of biomaterials aiming to reproduce host responses. Inconsistencies within these studies, including differently sourced cells, different durations of culture, and assessment of different activation markers, lead to many conflicting results in vitro that confound consistency and conclusions. We hypothesize that different experimentally popular monocyte-macrophage cell types have intrinsic in vitro culture-specific differences that yield conflicting results. Recent studies demonstrate changes in cultured macrophage cytokine expression over time, leading to the hypothesis that changes in macrophage phenotype also occur in response to extended culture. Here, macrophage cells of different transformed and primary-derived origins were cultured for 21 days on model polymer biomaterials. Cell type-based differences in morphology and cytokine/chemokine expression as well as changes in cell surface biomarkers associated with differentiation stage, activation state, and adhesion were compared. Results reflect consistent macrophage development towards an M2 phenotype via up-regulation of the macrophage mannose receptor for all cell types following 21-day extended culture. Significantly, implanted biomaterials experiencing the foreign body response and encapsulation in vivo often elicit a shift towards an analogous M2 macrophage phenotype. In vitro “default” of macrophage cultures, regardless of lineage, to this M2 state in the presence of biomaterials at long culture periods is not recognized but has important implications to in vitro modeling of in vivo host response.
Keywords: In vitro, Foreign Body Response, Cytokine, Biocompatibility, Cell Activation, Cell Culture, Inflammatory phenotype
Introduction
In vitro cell-based assays are ubiquitously used to assess cell responses to biomaterials [1-12]. However, the use of different cell lines, different time points, passages, media, and different markers of inflammation provide diverse sets of data and associated conflicting interpretations and conclusions [13, 14]. A recent cell culture report for cytokine production by human monocyte-derived macrophages for as long as ten days in culture showed peak inflammatory cytokine expression at early time points, followed by reductions and a return to basal levels [13]. This shift in cytokine expression over time led to the hypothesis that macrophages undergo distinct phenotypic changes on biomaterial surfaces during culture [13]. Significantly, understanding macrophage phenotypic changes in contact with implanted biomaterials is essential to controlling the host foreign body reaction. Ultimately, in vivo correlation or validation of this or any in vitro-based hypothesis is required. Initial understanding of the limitations of the in vitro test bed seems prudent for such a comparison.
Macrophages are phagocytic cells involved in inflammation, wound healing, infection, and the host response to implanted materials. They are proposed to represent a continuum of different phenotypes depending on their tissue location, environment, differentiation stage, and activation state. Recently efforts have been made to clarify this diverse range of macrophage activation states based either on the activator used [15], categorization across an arbitrary color wheel of phenotypes [16] or placing them into M1, M2a, M2b, and M2c subsets [17]. Cellular heterogeneity is based on select cell surface markers and cytokine expression upon both activation and during different stages of cell differentiation [17]. Though assessing macrophage status is complex and may be constantly variable [18], two primary distinctions, the M1 and M2 phenotypes,[19] are currently popularly applied as a simplified framework to distinguish two different macrophage states [17, 20]. Cell markers distinguish macrophages polarized towards these opposite M1 and M2 ends of this dichotomy. M1, also known as classically activated, macrophages can be induced, among others, by soluble stimulants such as IFN-γ and LPS/TNF-α, have antimicrobial and cytotoxic properties, and express specific Toll-like receptors (e.g., TLR-4) [21]. M2, or alternatively activated, macrophages are associated with anti-inflammation [15], immune-regulation, tissue remodeling [17], and importantly, the foreign body response [19, 22, 23], and are distinguished by increased macrophage mannose receptor (MMR, CD206) expression [24]. The M1/M2 macrophage dichotomy has been used increasingly in biomaterials inflammatory assessments to characterize materials both in vitro [25, 26] and in vivo [26, 27].
Here we compare 21-day cultured responses by both primary and common immortalized, transformed secondary macrophage cell lines at different stages of activation and differentiation to probe and distinguish effects of extended culture on phenotypic markers. Variations in cell morphology, cytokine secretion, and external receptor expression were noted in 21-day (long-term) cultures, a time-point relevant to in vivo maturation of certain aspects of the host foreign body response [28, 29].
Materials and Methods
Model biomaterial culture surfaces and surface preparation
Model and control solid two-dimensional cell culture materials used in this study have been characterized previously for in vitro cultures: standard tissue culture polystyrene (TCPS, 15×100mm petri dishes, Falcon®, BD Biosciences, San Diego, USA); poly-L-lactide (PLLA, Polysciences Inc., Warrington, USA) and Teflon-AF® (DuPont Fluoroproducts, USA)[30]. Teflon-AF® surfaces were prepared as previously reported[31, 32]: PS petri dishes (ø=100mm) were coated with Teflon-AFTM (3 mL of 0.1% solution diluted from stock in 3M™ Fluorinert™ Electronic Liquid FC-40 solvent, 3M Corp. St. Paul, USA) prior to overnight vacuum exposure at 65°C. PLLA surfaces (50,000 MW) were prepared as described previously by solvent casting a 0.2% w/v solution of PLLA in methylene chloride[31, 32]: glass petri dishes (ø=100mm) were coated with 10mL of PLLA solution, loosely covered, and allowed to dry in a fume hood for approximately one hour. Teflon-AF® and PLLA-coated plates were then sterilized inside a laminar flow hood after misting with 70% ethanol in cell-grade water (Hyclone®, Logan, UT) by treatment with culture-hood UV light for 15 minutes (a process shown to have no detectable effect on surface chemistry by XPS analysis)[33]. All surfaces and cell culture materials were tested for the presence of contaminating endotoxin using a PyrogeneTM Assay kit (Cambrex, East Rutherford, USA), and determined to be below the kit detection limit (0.02 EU/mL).
Primary murine macrophage cell harvest
Specific-pathogen-free female C57BL/6 mice (6-8 weeks old, Jackson Laboratory, Bar Harbor, USA) were acclimated and maintained in University of Utah comparative medicine facilities and given sterile water and mouse chow for the duration of the experiments. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) have been observed; all experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee of the University of Utah.
Bone marrow cells were harvested from murine tibias and femurs and differentiated into macrophage cells using previously described methods [32, 34]. Briefly, bone marrow cells were flushed from bones, and then differentiated into bone-marrow derived macrophages (BMMΦ) by incubating bone marrow cells in complete DMEM (cDMEM, DMEM supplemented with 10% heat-inactivated FBS, 10% conditioned media supernatant from L-929 fibroblast cell cultures (ATCC, Manassas, VA), 1% penicillin-streptomycin (Hyclone®, Logan, UT), 0.01M Hepes buffer (Hyclone®, Logan, UT), 1mM sodium pyruvate (Hyclone®, Logan, UT), and 1% MEM non-essential amino acids solution (Hyclone®, Logan, UT)). BMMΦs were maintained in this culture medium in all experiments for the duration of the study. According to previously established methods, primary cells were cultured for 7 days on TCPS with media changes every 2 days before they were utilized in experiments [32, 34]. Adherent (i.e., stromal) cells were selected as mature macrophages (BMMΦs) for further studies and were used at passage 1 [34]. Replicates are defined as cells from different mice and a minimum of three replicates were completed for all experiments.
Immortalized murine cell (secondary cell line) culture
Adherent murine (monocyte-)macrophage cell lines IC-21, J774A.1 and RAW 264.7 were purchased as virally transformed, immortalized oncogenic phenotypes (ATCC, Manassas, USA). RAW 264.7 and IC-21 cells were cultured in RPMI-1640 (Mediatech, Inc, Herndon, USA) and J774A.1 cells were cultured in DMEM (Mediatech, Inc, Herndon, USA) per vendor standard culture recommendations. All growth media used to culture cell lines were supplemented with 10% FBS (Hyclone®, Logan, USA), 1% penicillin-streptomycin (Gibco, Carlsbad, USA), and 0.01 M Hepes buffer (Sigma, St. Louis, USA). Endotoxin addition (e.g., LPS) was not used to activate cultured macrophages. Cell cultures were maintained below 80% confluence in TCPS flasks and passaged by incubation with divalent cation-free Dulbecco’s Phosphate Buffered Saline (dPBS Hyclone®, Logan, USA) prior to scraping with a rubber scraper. All cells were used at or below passage number 20 as received from ATCC and incubated under standard conditions. Replicates are defined as cells harvested from different passages and/or flasks. A minimum of three replicates weas performed for each experiment.
Extended Cell Culture
Cell lines and mature primary BMMΦs were passaged and seeded at 10,000 cells/cm2, which yielded approximately 80% confluence onto test surfaces on Day 0 for continuous 21-day culture. Media was changed every 24 hours for 21 days and cells were imaged prior to all media changes. To prevent protein degradation, supernatants from each culture were collected at days 1, 2, 3, 7, 14, and 21 and stored at −80°C until needed for cytokine analysis. Cells were removed from surfaces on Day 21 by incubation with non-cationic PBS (Gibco, Carlsbad, USA) followed by gentle scraping and analysis by flow cytometry for phenotypic surface markers. Three replicates were performed for each cell type on each surface.
Cell imaging
Live cells were photographed using phase contrast microscopy using a Nikon Eclipse TE 2000-U microscope (Nikon Inc., Torrance, USA), a Photometrics CoolSnap ES camera (Roper Scientific, Tucson, USA), and Metamorph™ software (Molecular Devices, Downingtown, USA). The mean counts of at least 5 randomly selected frames were used to estimate the number of cells on each plate. Cell images were analyzed for the number of cells per 40X field.
Flow cytometric analysis of cell surface markers
Control cells (freshly differentiated BMMΦ or secondary cell lines from stock flasks), and cells from 21-day cultures were removed from culture surfaces. Cell suspensions were incubated with purified monoclonal antibodies (MAbs) CD16/32 (clone 93, rat IgG2a anti-mouse, eBiosciences, San Diego, USA) using at least 1μg of MAbs per million cells in 100μL of staining solution (PBS with 1% FBS and 0.01% w/w NaN3) at 4°C for 15 minutes to block Fc receptors [35]. After rinsing Fc-blocked cells twice with staining solution, cell suspensions were transferred to a 96-well plate for staining with 1μg of fluorescently conjugated MAbs diluted to 100μL with staining solution at 4°C for 30 minutes in the dark. MAbs against CD11b (clone M1/70, rat anti-mouse IgG2b), CD18 (clone m18/2, rat anti-mouse IgG2a), CD11c (clone N418, armenian hamster anti-mouse IgG), CD54 (clone YN1/1.7.4, rat anti-mouse IgG2b), F4/80 (clone BM8, rat anti-mouse IgG2a), Fc (clone 93, rat anti-mouse IgG2a), CD14 (clone Sa2-8, rat anti-mouse IgG2a), CD40 (clone MR5D3, rat anti-mouse IgG2a), TLR-4 (clone UT41, mouse IgG1, shown to cross-react with mouse, rat, and human), and CD206 (macrophage mannose receptor, MMR, clone MR5D3, rat anti-mouse IgG2a) were used in this study. All MAbs were purchased from AbD Serotec Inc (Raleigh, USA) or eBioscience (San Diego, USA) as direct conjugates to Alexa Fluor 488 (TLR-4) or FITC (all others). Cells were rinsed twice with additional staining solution to reduce background fluorescence from unbound antibody prior to analysis. Data acquisition and analysis for this study used a FACScan (BD Biosciences, Mountain View, USA), CellQuest software (BD Biosciences, San Jose, USA), and WinMDI 2.9 software (J. Trotter, The Scripps Research Institute, La Jolla, USA).
Quantitation of cytokine expression over time
Cytometric bead array (CBA) kits were purchased from BD Biosciences (USA) and used per manufacturer’s instructions. Supernatants (obtained from respective culture media) from each culture were collected at days 1, 2, 3, 7, 14, and 21. CBA assays were performed on a minimum of 3 replicates for all time points, for all surfaces, and for all cytokines/chemokines available. MIP-1β, TNF, RANTES, and MCP-1 were all secreted above the limit of detection for the CBA assay. GM-CSF, IFN-γ, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p70, and IL-13 were all secreted below the assay’s limit of detection (data not shown). A 10-point, non-linear, standard curve with known spiked amounts of cytokines was used to generate quantitative data, according to CBA manufacturer’s instructions. Data acquisition and analysis for this study used a FACScan (BD Biosciences, Mountain View, USA), CellQuest software (BD Biosciences, San Jose, USA), and WinMDI 2.9 software (J. Trotter, The Scripps Research Institute, La Jolla, USA). The estimated number of cells per plate (from cell density numbers described above) was used to normalize the data to control for cell population fluctuations.
Statistical Analysis
Bar graphs reflect the average of at least n=3 ± SEM. Statistical significance was determined using a Single-Factor ANOVA followed by post-hoc student’s t-tests. P-values less than 0.05 were considered significant. Samples were compared against the control, which represented the Day 1 time point for each condition. Comparisons were selected in advance and reported individually rather than as a group and therefore were not appropriate for multiple comparison’s correction [36]. Detectable limits for each assay were determined using the value of the control assay signal at 0 (background) plus 3 times the standard deviation of the mean.
Results
Cell morphology and density depend on cell type and culture surface
Cell morphologies for all cell types on all surfaces at Days 1 and 21 are shown in Figure 1. In contrast to cultures of IC-21, J774A.1, and RAW 264.7 immortalized cell lines, BMMΦ cells exhibited surface-dependent morphology at both time points. At Day 1, BMMΦ cells cultured on TCPS surfaces displayed a spiny, oblong morphology. This morphology was not observed when these cells were cultured on the more hydrophobic Teflon-AF® or PLLA surfaces. Over time, BMMΦ morphology continued to be surface-dependent: BMMΦ cultured on Teflon-AF® surfaces developed large lamellipodia, but on TCPS and PLLA surfaces, maintained a spiny, oblong morphology. This phenomenon was more pronounced for BMMΦs cultured on PLLA surfaces.
Figure 1.
Phase contrast images showing cell morphologies compared for various monocyte(macrophage) cell types before and after extended in vitro culture.
IC-21, J774A.1, and RAW 264.7 cells displayed similar morphologies regardless of polymer surface (Figure 1). They proliferated to confluence before Day 21. By Day 21, each possessed rounded cell morphologies distinct from their original Day 1 morphology (Figure 1).
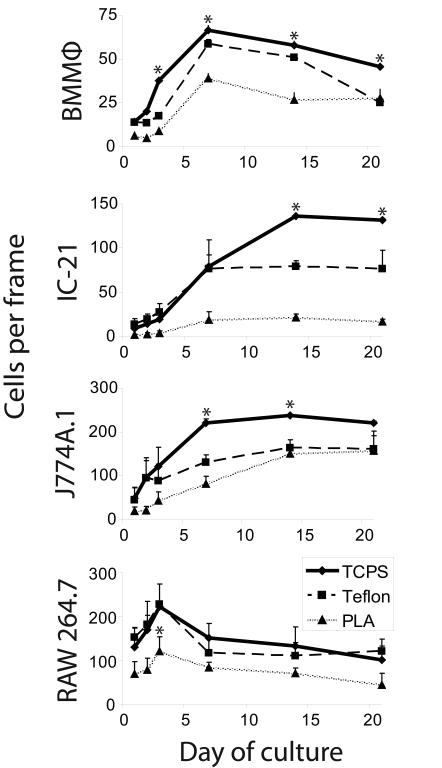
Cellular proliferation rates in these same cultures are represented in Figure 2 as the number of cells per 40X frame over a 21-day period for each cell type across all surfaces. Overall, proliferation of BMMΦ cells was highest on TCPS surfaces compared to Teflon-AF® or PLLA surfaces. Similarly, IC-21 cells had higher proliferation rates on TCPS than on Teflon-AF® or PLLA surfaces; however, these differences were not observed until after Day 7 of culture. J774A.1 cells also displayed comparable proliferation trends to BMMΦ cells on each surface, but had much higher total cell numbers. RAW 264.7 cells only proliferated during Days 1 through 3 on all surfaces and thereafter the number of cells decreased (Figure 2). A previous short term study on identical surfaces observed similar cell proliferation trends for these murine immortalized cell lines to 4 days [31]. Additionally, similar numbers of RAW 264.7 cells per 40X field were observed between TCPS and Teflon-AF® surfaces at all time points. TCPS, the gold standard substrate for cell culture, resulted in the highest population densities for all cell types (Figure 2). PLLA surfaces induced the lowest cell numbers for all cells assays (Figure 2), potentially due to surface chemistry and hydrolytic chemistry changes as they degrade [37].
Figure 2.
Viable macrophages on various biomaterials over 21 days indicate that cell proliferation over time is cell type-, source- and surface-dependent. Asterisks distinguish significant changes (p<0.05) from TCPS at each time point.
Macrophage cytokine expression depends on cell type and culture surface
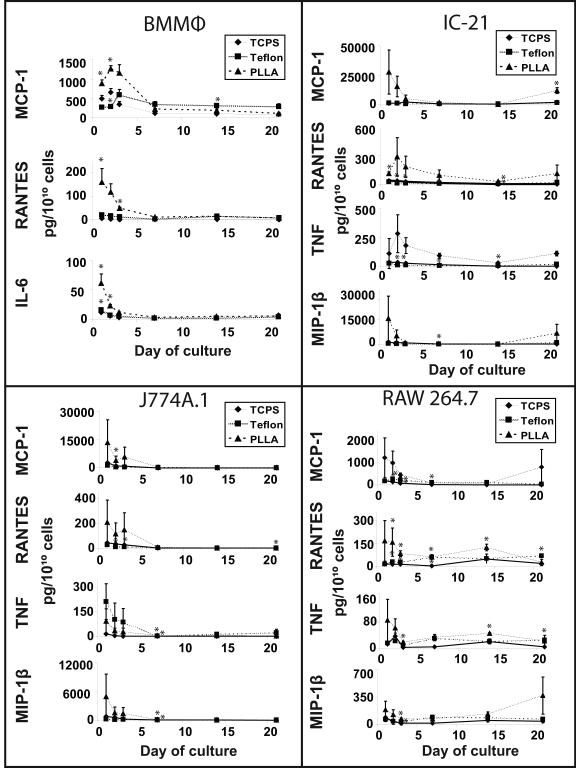
Cytometric bead array (CBA) assays assessed cytokine and chemokine expression in all cell culture supernatants collected over 21 days. Supplementary Figure 1 represents chemokine or cytokine concentrations expressed as pg/mL from supernatants of BMMΦ, IC-21, J774A.1, and RAW 264.7 cultures, respectively. Figure 3 shows the same data represented as pg of analyte per 1010 cells to normalize against changes in cell growth/density on these surfaces over time. Expression of only five analytes from the 14-analyte CBA assay panel was detectable (Figure 3 and Table 1). These include two chemokines (RANTES and MCP-1) and three inflammatory cytokines (TNF-α, IL-6, and MIP-1β). While BMMΦ supernatants contained only the inflammatory cytokine IL-6, supernatants collected from IC-21, J774A.1, and RAW 264.7 contained both TNF-α and MIP-1β (Figure 3 and Table 1). Supernatants from BMMΦ cell cultures plated on different surfaces showed several significant differences in their cytokine and chemokine contents. Those cultured on TCPS surfaces contained higher concentrations of MCP-1 at Day 2 than those cultured on Teflon-AF® or PLLA surfaces, while at Day 7, MCP-1 supernatant concentrations obtained from cells on Teflon-AF® surfaces were much higher than those cultured on TCPS or PLLA surfaces. By contrast, RANTES and IL-6 expression were significantly higher in supernatants obtained at early time points of BMMΦ cells cultured on PLLA surfaces. However, supernatants from BMMΦ cells cultured on PLLA surfaces had higher levels of MCP-1, RANTES and IL-6 than those cultured on TCPS or Teflon-AF® surfaces, on a per cell basis (3). Cytokine/chemokine responses normalized to cell number from BMMΦs indicate that secretion is up-regulated upon initial surface adhesion and drops to basal levels in culture by Day 7 (Figure 3).
Figure 3.
Expression of cytokines/chemokines detected from culture media by the cytometric bead array assay over 21 days depends on culture surface and cell culture time. Cytokine/chemokine expression was normalized to cell number per plate at each timepoint. (line shown to guide the eye only). Asterisks distinguish significant changes (p<0.05) from TCPS at each time point.
Table 1.
Summary of cytokines/chemokines detected in macrophage media supernatants from cell cultures at many time points along the 21-day culture period.
| BMMϕ | IC-21 | J774A.1 | RAW 264.7 | |
|---|---|---|---|---|
| RANTES | + | + | + | + |
| MCP-1 | + | + | + | + |
| IL-6 | + | |||
| TNF | + | + | + | |
| MIP-1β | + | + | + |
MCP-1, RANTES, TNF and MIP-1β were detected in supernatants obtained from immortalized IC-21, J774A.1 and RAW 264.7 cell cultures over a 21-day period (Figure 3 and Table 1). In contrast to supernatants obtained from similar cultures of BMMΦ cells, no significant differences in cytokine and chemokine expression was noted in supernatants obtained from IC-21, J774A.1, or RAW 264.7 cells cultured on different surfaces. Interestingly, when data were represented as pg of analyte per 1010 cells, immortalized cell lines displayed a similar cytokine/chemokine concentration trend to BMMΦ cell cultures, showing an initial burst of expression that dropped to basal levels by Day 7 (Figure 3).
Changes in expression of cell surface markers following extended macrophage culture on model biomaterials depend on cell type
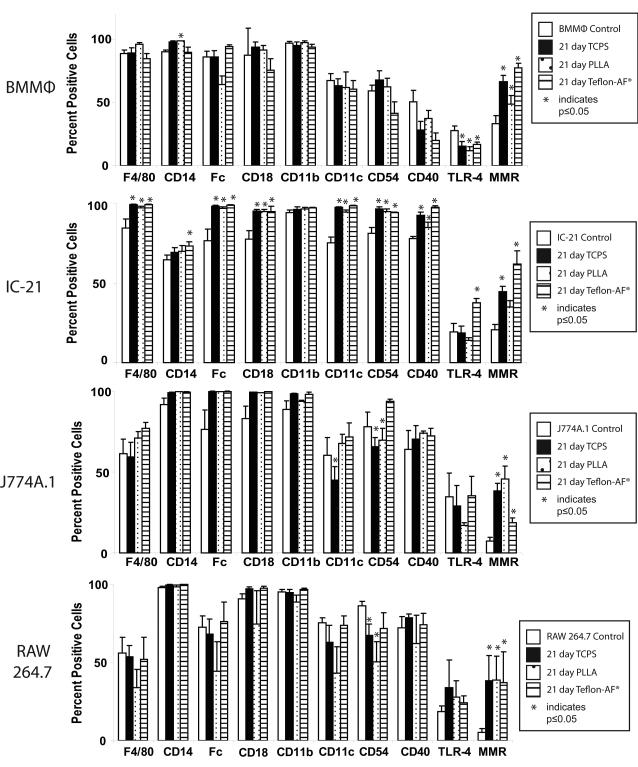
Expression changes for cell surface markers associated with macrophage maturation differentiation, adhesion, and activation in both the primary BMMФs and secondary cell lines were compared. The markers chosen are associated with differentiation maturation (F4/80, CD14, Fc receptors), integrin expression (CD18, CD11b, and CD11c), intracellular adhesion (CD54) and cell activation (CD40, TLR-4 and MMR). Changes in relative expression of these markers over the time-course of the study were determined using flow cytometry. The mean fluorescence channel (MFC) for each marker within each cell population is listed in Supplementary Figure 2. Comparative analysis of the mean fluorescence channel (MFC) was used to determine the percentage of positive cells for each marker within each cell population, which is expressed graphically in Figure 4 and in a table in Supplementary Figure 3. Statistically significant changes in expression of each surface marker for all cell types and culture surface on a percent positive basis are summarized in Table 2.
Figure 4.
Flow cytometry data indicating changes in macrophage surface marker expression based on percent positive cells for maturation differentiation (F4/80, CD14, Fc), intracellular adhesion (CD54), and activation (CD40, TLR-4, and MMR) before and after 21 days of culture. Asterisks indicate p<0.05 compared to control (Day 1 time point).
Table 2.
Summary of statistically significant changes in cell surface marker expression on a percent positive cell basis following 21 days of in vitro culture. Only statistically significant (p<0.05 compared to Day 1 time point) changes are reported. Significant increases are shown in black arrows, and significant decreases are shown in gray arrows.
| BMMϕ | IC-21 | J774A.1 | RAW 264.7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCPS | PLLA | Teflon-AF® | TCPS | PLLA | Teflon-AF® | TCPS | PLLA | Teflon-AF® | TCPS | PLLA | Teflon-AF® | ||
| Markers of macrophage maturity |
F4/80 | ↑ | ↑ | ↑ | |||||||||
| CD14 | ↑ | ↑ | |||||||||||
| Fc | ↑ | ↑ | ↑ | ||||||||||
| Macrophage adhesion molecules |
CD18 | ↑ | ↑ | ↑ | |||||||||
| CD11b | |||||||||||||
| CD11C | ↑ | ↑ | ↑ | ↓ | |||||||||
| CD54 | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ | ||||||
| Macrophage activation markers |
CD40 | ↑ | ↑ | ↑ | |||||||||
| TLR-4 | ↓ | ↓ | ↓ | ↑ | |||||||||
| MMR | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |
BMMΦ cells maintained generally stable cell surface marker expression following 21-day cultures on different model surfaces. The only significant changes noted were a decrease in the percent positive cells for TLR4 and an increase in MMR on all surfaces (TCPS, Teflon-AF®, PLLA) and an increase in CD14 on PLLA (Figure 4 and Table 2).
IC-21 cells displayed the greatest statistically significant changes in surface protein expression following extended culture compared to all cell types. IC-21 cells showed a significant increase in the percentage of positive cells for F4/80, Fc receptor, CD18, CD11c, CD54, CD40, and MMR cultured on all surfaces (Figure 4 and Table 2). However, only on Teflon-AF® surfaces did IC-21 cells display significant increases in CD14 and TLR-4.
J774A.1 and RAW 264.7 cells exhibited the least but mutually very similar significant changes in cell surface marker expression following extended culture. Both cell lines had a statistically significant decrease in CD54, an intracellular adhesion molecule [38], following extended culture on TCPS and PLLA. Both cell lines also experienced a statistically significant increase in MMR on all surfaces after 21 days (Figure 4 and Table 2).
Discussion
Macrophages are a primary cell type responsible for host response to biomaterials, and their phenotype has the potential to shift dramatically. Conflicting, inconsistent data in the archival literature plague any consensus view regarding in vitro assessments of macrophage response to biomaterials [13, 14, 39]. We hypothesize that macrophage sourcing and their culture conditions, including time, contribute to highly variable macrophage responses reported for such studies. Cultured macrophages from different sources have shown varying results [13, 14, 39, 40] and culture time relevant to in vivo development of the foreign body response has not been reported. Therefore, macrophages derived from primary and several secondary sources were compared in long-term 21-day cultures in this study. Mouse bone marrow-derived macrophages were selected as a primary macrophage source as they are commonly used in biomaterials research [26, 41]. Secondary transformed, immortalized murine cell lines RAW 264.7 [1, 2, 42], J774A.1 [3, 43], and IC-21 [44-47] also routinely analyzed in biomaterials studies, were compared. Importantly, these cell types were selected not only for their biomaterials popularity, but also for their variance in oncogenic potential, phenotypic stability, maturation and inflammatory status that may affect their interactions with materials. RAW cells are considered the most monocytic phenotype, followed by J7, and finally IC-21s in a progression toward more macrophage character, and differentiated BMMΦs considered as most macrophage-like [48].
Also, macrophages are reported to alter their response to biomaterials over time in culture [13] but generally most in vitro experiments with macrophages are a few days and their relevance to biomaterials development is questioned. Extended culture of macrophages beyond a few days’ duration is relatively unexplored in the study of in vitro responses to biomaterials, but may more accurately represent macrophage-mediated aspects of in vivo foreign body responses requiring several weeks to develop in vivo [28, 29]. This study therefore cultured macrophages for 21 days to more accurately assess their fidelity to in vivo responses to biomaterials. To provide relevant context, several model biomaterials mimics including poly-L-lactide (PLLA), Teflon-AF®, and tissue-culture polystyrene (TCPS), known to exhibit varying macrophage responses in vitro [13, 14, 48-50], were chosen. Cell morphology and adherent proliferation rates, cytokine/chemokine secretion, and cell membrane marker expression were selected as metrics to compare changes in macrophage response based on cell source, culture time, and culture surface.
During extended 21-day culture in serum-containing media, all macrophages displayed unique adherent morphologies, proliferation rates, and densities (Figures 1 and 2). We have previously reported different monocyte/macrophage morphologies in cultures, with minimal changes in protein expression following short-term (i.e. <3 day) culture on different rigid surfaces [31, 32, 48, 51, 52]. Consistent with prior observations, we observe murine BMMΦ morphology to continually change over the course of 21-day culture in a surface-dependent manner (Figure 1). Transformed secondary murine cell lines (IC-21, J774A.1, and RAW 264.7), however, exhibited consistently rounded morphologies regardless of culture surface (Figure 1). This difference between primary and secondary transformed macrophages may be attributed to loss of contact inhibition by oncongenic transformations of the secondary cell lines and non-equivalence in their more monocytic phenotypes versus differentiated BMMΦ, a point we have emphasized previously [31, 48].
Different cytokine/chemokine secretion were also seen between primary BMMΦ and secondary-derived macrophages. Supernatants from 21-day BMMΦ cell cultures consistently contained MCP-1 and RANTES, while only secondary, immortalized cell lines expressed TNF and MIP-1β. Interestingly, during this study, inflammatory marker IL-6, prevalent at sites of implanted foreign bodies [53], was only present in supernatants obtained from BMMΦ cultures. A previous short-term study detected IL-6 production from the same secondary cell lines using flow cytometry [48]. Our current study extended culture times beyond this previous work, to 21 days. Importantly, at this time point only BMMΦs were able to express detectable levels of IL-6, consistent with,robust expression of IL-6 by BMMΦs compared to the secondary lines in the short-term study [48]. Cytokine concentration data (reported in pg/mL) show very little correlation from surface to surface or across cell types. However, when these data are normalized to cell density per culture surface, they show a general initial burst of expression followed by a relative attenuation, a phenomenon seen previously both in vitro [13] and in vivo [53]. This attenuation could be due to macrophages slowly senescing, which has been shown to increase both with time and cell density [53]. BMMΦ cultures decreased cytokine secretion and then remained at basal levels near Day 7 on all culture surfaces. Secondary IC-21 and RAWs in contrast, reached basal levels and then up-regulated all cytokines thereafter. BMMΦ cultures therefore assert greater equivalence to that reported in vivo for cytokine attenuation [53]. Previous reports also showed that BMMΦs display cultured phenotypes more similar to in vivo responses compared to immortalized monocyte/macrophage cell lines [54].
Unlike Teflon-AF® and TCPS, PLLA hydrolyzes and likely begins to partially degrade over 21 days [37]. Nonetheless, PLLA degradation did not appear to greatly affect either adherent cell morphology (Figure 1) or cell surface receptor expression (Figure 4 and Table 2): these remained relatively consistent over 21 days. However, higher secretion of most cytokines was seen from all cell types on PLLA surfaces at early time points compared to other surfaces (Figure 3). As significant PLLA degradation is unlikely at this early time, observed differences are attributed to distinct PLLA surface chemistry and resulting protein adsorption profiles.
Cell surface markers associated with cell adhesion (CD18, CD11b, CD11c, CD54), stage of maturation differentiation (F4/80, CD14, and Fc receptor), and activation state (CD40, TLR-4, and MMR) were also monitored. Most cell types maintained stable, relatively invariant cell integrin expression (Figure 4 and Table 2). However, variations in the percent positive cells for maturation differentiation indicate inherently different cell maturities from the various cell sources. BMMΦ and IC-21 cells continued to up-regulate expression of maturation markers CD14 and F4/80, and CD14 and Fc, respectively (Figure 4 and Table 2), over 21 days, reflecting progressively distinct differentiation state [35, 48, 55]. Another study, also comparing variations between different macrophage sources, identified unique external receptor expression by each macrophage type [40]. However, consistent with our findings, this identified similarly high CD14 expression by BMMΦ and RAW cells compared to the other cell lines compared [40].
TLR-4, a marker of macrophage M1 activation, decreased in BMMΦ cell cultures, while MMR, a marker of M2 activation increased (Figure 4 and Table 2) [19, 56]. Interestingly, though these changes were not significant, BMMΦs also decreased other M1 markers including CD11c and CD40 [57-59] over 21 days (Figure 4 and Table 2), and significantly increased CD14 in addition to MMR, further supporting this shift towards an M2 phenotype [19, 24, 56, 57]. Cell lines did not follow the BMMΦ phenotypic progression profile, showing inconsistencies between cell sources. Importantly, as tumor-associated macrophages often possess an M2 phenotype, thought to promote tumor growth in vivo [60]. Consistently, the tumor-derived cell lines used in this study may therefore be predisposed towards an M2 phenotype [61]. Nonetheless, all cell cultures in this study display increased MMR expression on all culture surfaces after 21 days of culture (Figure 4 and Table 2), representing a consistent, sustained progression to the M2 phenotype. An increase in MMR expression by all cells, independent of cell source, suggests a potential default or common terminal phenotypic transition at long culture times.
Significantly, the foreign body response is often attributed to M2-mediated inflammatory macrophage responses [19, 22, 23], consistent with M2 shifts during extended cultures. Moreover, after one month implantation in vivo, many biomaterials with highly variable chemistries, hydrophobicities, and compliances such as Teflon, polyurethane, silicone, polyethylene, poly(methyl methacrylate) (PMMA), polyHEMA, Dacron™ polyester, gold, titanium, and alumina all heal essentially the same, producing a fibrous capsule [53, 62, 63]. Though the M2 phenotypic classification is highly over-simplified considering the complex phenotypic continuum that exists in vivo, this general shift is consistent with the common host response experienced by biomaterials at longer times in vivo. Importantly, the 21-day culture period recapitulates an M2 shift independent of macrophage origin or culture surface and may therefore represent a more relevant time frame for in vitro modeling of macrophage in vivo responses to biomaterials.
Conclusions
Macrophage origin, culture times, and biomaterial surfaces all affect macrophage phenotype in cultures. Under identical culture conditions, primary and secondary murine cells respond differently, and cell lines varied with respect to one another in terms of adherent morphology, proliferation, cytokine expression, and cell surface marker expression on three different solid surfaces. No reports document such cell-specific phenotypic variations in vitro over longer culture time frames relevant to maturation of the host foreign body response in vivo. These cell-cell variations likely contribute to inconsistent macrophage responses reported from various in vitro studies on model biomaterials surfaces. Importantly, at long culture times, similar to that required for the foreign body response to develop in vivo, all macrophages, regardless of cell origin or culture surface, shift towards the M2 phenotype, similar to their known shift to an M2 polarization in vivo during the foreign body response. Hence, long culture times may provide a more relevant time frame for modeling certain aspects of macrophage responses to biomaterials found in vivo and may be advantageous to implement in future in vitro culture systems seeking to address macrophage plasticity in biomaterials settings.
Supplementary Material
Acknowledgements
This work was supported by NIH grant EB000894.
References
- [1].Bailey LO, Lippiatt S, Biancanello FS, Ridder SD, Washburn NR. The quantification of cellular viability and inflammatory response to stainless steel alloys. Biomaterials. 2005;26:5296–302. doi: 10.1016/j.biomaterials.2005.01.055. [DOI] [PubMed] [Google Scholar]
- [2].Bailey LO, Washburn NR, Simon CG, Jr., Chan ES, Wang FW. Quantification of inflammatory cellular responses using real-time polymerase chain reaction. Journal of biomedical materials research. 2004;69:305–13. doi: 10.1002/jbm.a.20134. [DOI] [PubMed] [Google Scholar]
- [3].Prabhu A, Shelburne CE, Gibbons DF. Cellular proliferation and cytokine responses of murine macrophage cell line J774A.1 to polymethylmethacrylate and cobalt-chrome alloy particles. J Biomed Mater Res. 1998;42:655–63. doi: 10.1002/(sici)1097-4636(19981215)42:4<655::aid-jbm23>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [4].Lishko VK, Burke T, Ugarova T. Antiadhesive effect of fibrinogen: a safeguard for thrombus stability. Blood. 2007;109:1541–9. doi: 10.1182/blood-2006-05-022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Takebe J, Ito S, Champagne CM, Cooper LF, Ishibashi K. Anodic oxidation and hydrothermal treatment of commercially pure titanium surfaces increases expression of bone morphogenetic protein-2 in the adherent macrophage cell line J774A.1. Journal of biomedical materials research. 2007;80:711–8. doi: 10.1002/jbm.a.30988. [DOI] [PubMed] [Google Scholar]
- [6].Suzuki R, Muyco J, McKittrick J, Frangos JA. Reactive oxygen species inhibited by titanium oxide coatings. Journal of biomedical materials research. 2003;66:396–402. doi: 10.1002/jbm.a.10001. [DOI] [PubMed] [Google Scholar]
- [7].Refai AK, Textor M, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. Journal of biomedical materials research. 2004;70:194–205. doi: 10.1002/jbm.a.30075. [DOI] [PubMed] [Google Scholar]
- [8].Wang YH, Wang WY, Liao JF, Chen CF, Hou YC, Liou KT, et al. Prevention of macrophage adhesion molecule-1 (Mac-1)-dependent neutrophil firm adhesion by taxifolin through impairment of protein kinase-dependent NADPH oxidase activation and antagonism of G protein-mediated calcium influx. Biochem Pharmacol. 2004;67:2251–62. doi: 10.1016/j.bcp.2004.02.020. [DOI] [PubMed] [Google Scholar]
- [9].Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL. Macrophage responses to silk. Biomaterials. 2003;24:3079–85. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- [10].Acharya C, Ghosh SK, Kundu SC. Silk fibroin protein from mulberry and non-mulberry silkworms: cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. Journal of materials science. 2008;19:2827–36. doi: 10.1007/s10856-008-3408-3. [DOI] [PubMed] [Google Scholar]
- [11].Dimitrievska S, Petit A, Ajji A, Bureau MN, Yahia L. Biocompatibility of novel polymer-apatite nanocomposite fibers. Journal of biomedical materials research. 2008;84:44–53. doi: 10.1002/jbm.a.31338. [DOI] [PubMed] [Google Scholar]
- [12].Feyerabend F, Siemers C, Willumeit R, Rosler J. Cytocompatibility of a free machining titanium alloy containing lanthanum. Journal of biomedical materials research. 2009;90:931–9. doi: 10.1002/jbm.a.32151. [DOI] [PubMed] [Google Scholar]
- [13].Jones JA, Chang DT, Meyerson H, Colton E, Kwon IK, Matsuda T, et al. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007;83:585–96. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- [14].Schutte RJ, Parisi-Amon A, Reichert WM. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. Journal of biomedical materials research. 2009;88:128–39. doi: 10.1002/jbm.a.31863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [18].Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, et al. Transcriptional switching in macrophages associated with the peritoneal foreign body response. Immunology and cell biology. 2014;92:518–26. doi: 10.1038/icb.2014.19. [DOI] [PubMed] [Google Scholar]
- [19].Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- [20].Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33:3792–802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [22].McNally AK, DeFife KM, Anderson JM. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol. 1996;149:975–85. [PMC free article] [PubMed] [Google Scholar]
- [23].Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- [24].Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–6. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- [25].Grotenhuis N, Vd Toom HF, Kops N, Bayon Y, Deerenberg EB, Mulder IM, et al. In vitro model to study the biomaterial-dependent reaction of macrophages in an inflammatory environment. Br J Surg. 2014;101:983–92. doi: 10.1002/bjs.9523. [DOI] [PubMed] [Google Scholar]
- [26].Baker DW, Zhou J, Tsai YT, Patty KM, Weng H, Tang EN, et al. Development of optical probes for in vivo imaging of polarized macrophages during foreign body reactions. Acta biomaterialia. 2014;10:2945–55. doi: 10.1016/j.actbio.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wolf MT, Vodovotz Y, Tottey S, Brown BN, Badylak SF. Predicting In Vivo Responses to Biomaterials via Combined In Vitro and In Silico Analysis. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2014.0167. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. Journal of biomaterials science. 2006;17:669–87. doi: 10.1163/156856206777346340. [DOI] [PubMed] [Google Scholar]
- [29].Brodbeck WG, Colton E, Anderson JM. Effects of adsorbed heat labile serum proteins and fibrinogen on adhesion and apoptosis of monocytes/macrophages on biomaterials. J Mater Sci Mater Med. 2003;14:671–5. doi: 10.1023/a:1024951330265. [DOI] [PubMed] [Google Scholar]
- [30].Koenig AL, Gambillara V, Grainger DW. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. Journal of biomedical materials research. 2003;64:20–37. doi: 10.1002/jbm.a.10316. [DOI] [PubMed] [Google Scholar]
- [31].Godek ML, Duchsherer NL, McElwee Q, Grainger DW. Morphology and growth of murine cell lines on model biomaterials. Biomedical sciences instrumentation. 2004;40:7–12. [PubMed] [Google Scholar]
- [32].Godek ML, Sampson JA, Duchsherer NL, McElwee Q, Grainger DW. Rho GTPase protein expression and activation in murine monocytes/macrophages is not modulated by model biomaterial surfaces in serum-containing in vitro cultures. J Biomater Sci Polym Ed. 2006;17:1141–58. doi: 10.1163/156856206778530731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McClary KB, Ugarova T, Grainger DW. Modulating fibroblast adhesion, spreading, and proliferation using self-assembled monolayer films of alkylthiolates on gold. J Biomed Mater Res. 2000;50:428–39. doi: 10.1002/(sici)1097-4636(20000605)50:3<428::aid-jbm18>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [34].Rhoades ER, Orme IM. Similar responses by macrophages from young and old mice infected with Mycobacterium tuberculosis. Mechanisms of ageing and development. 1998;106:145–53. doi: 10.1016/s0047-6374(98)00113-4. [DOI] [PubMed] [Google Scholar]
- [35].Gonzalez-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infection and immunity. 2001;69:1127–33. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dunnett C, Goldsmith C. When and how to do multiple comparisons. In: Buncher CR, Tsay J-Y, editors. Statistics in the Pharmaceutical Industry. 3rd Chapman and Hall/CRC; New York: 2006. pp. 421–52. [Google Scholar]
- [37].Gogolewski S, Jovanovic M, Perren SM, Dillon JG, Hughes MK. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA) J Biomed Mater Res. 1993;27:1135–48. doi: 10.1002/jbm.820270904. [DOI] [PubMed] [Google Scholar]
- [38].Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holt DJ, Grainger DW. Multinucleated giant cells from fibroblast cultures. Biomaterials. 2011;32:3977–87. doi: 10.1016/j.biomaterials.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Berghaus LJ, Moore JN, Hurley DJ, Vandenplas ML, Fortes BP, Wolfert MA, et al. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4. Comparative immunology, microbiology and infectious diseases. 2010;33:443–54. doi: 10.1016/j.cimid.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bueter CL, Lee CK, Wang JP, Ostroff GR, Specht CA, Levitz SM. Spectrum and mechanisms of inflammasome activation by chitosan. J Immunol. 2014;192:5943–51. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Naureckiene S, Edris W, Ajit SK, Katz AH, Sreekumar K, Rogers KE, et al. Use of a murine cell line for identification of human nitric oxide synthase inhibitors. J Pharmacol Toxicol Methods. 2007;55:303–13. doi: 10.1016/j.vascn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [43].Vigo E, Cepeda A, Gualillo O, Perez-Fernandez R. In-vitro anti-inflammatory activity of Pinus sylvestris and Plantago lanceolata extracts: effect on inducible NOS, COX-1, COX-2 and their products in J774A.1 murine macrophages. J Pharm Pharmacol. 2005;57:383–91. doi: 10.1211/0022357055605. [DOI] [PubMed] [Google Scholar]
- [44].Gutting BW, Gaske KS, Schilling AS, Slaterbeck AF, Sobota L, Mackie RS, et al. Differential susceptibility of macrophage cell lines to Bacillus anthracis-Vollum 1B. Toxicol In Vitro. 2005;19:221–9. doi: 10.1016/j.tiv.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [45].Kao WJ, Hubbell JA. Murine macrophage behavior on peptide-grafted polyethyleneglycol-containing networks. Biotechnol Bioeng. 1998;59:2–9. [PubMed] [Google Scholar]
- [46].Daniels AU, Barnes FH, Charlebois SJ, Smith RA. Macrophage cytokine response to particles and lipopolysaccharide in vitro. J Biomed Mater Res. 2000;49:469–78. doi: 10.1002/(sici)1097-4636(20000315)49:4<469::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [47].Bhatia SK, Arthur SD, Chenault HK, Kodokian GK. Interactions of polysaccharide-based tissue adhesives with clinically relevant fibroblast and macrophage cell lines. Biotechnol Lett. 2007;29:1645–9. doi: 10.1007/s10529-007-9465-8. [DOI] [PubMed] [Google Scholar]
- [48].Chamberlain LM, Godek ML, Gonzalez-Juarrero M, Grainger DW. Phenotypic non-equivalence of murine (monocyte-) macrophage cells in biomaterial and inflammatory models. Journal of biomedical materials research. 2009;88:858–71. doi: 10.1002/jbm.a.31930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rodriguez A, Voskerician G, Meyerson H, MacEwan SR, Anderson JM. T cell subset distributions following primary and secondary implantation at subcutaneous biomaterial implant sites. J Biomed Mater Res A. 2008;85:556–65. doi: 10.1002/jbm.a.31562. [DOI] [PubMed] [Google Scholar]
- [50].Nair A, Zou L, Bhattacharyya D, Timmons RB, Tang L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir. 2008;24:2015–24. doi: 10.1021/la7025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Godek ML, Malkov GS, Fisher ER, Grainger DW. Macrophage Serum-Based Adhesion to Plasma-Processed Surface Chemistry is Distinct from That Exhibited by Fibroblasts. Plasma Process Polym. 2006;3:485–97. doi: 10.1002/ppap.200600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Godek ML, Michel R, Chamberlain LM, Castner DG, Grainger DW. Adsorbed serum albumin is permissive to macrophage attachment to perfluorocarbon polymer surfaces in culture. J Biomed Mater Res A. 2008;88A:503–19. doi: 10.1002/jbm.a.31886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schutte RJ, Xie L, Klitzman B, Reichert WM. In vivo cytokine-associated responses to biomaterials. Biomaterials. 2009;30:160–8. doi: 10.1016/j.biomaterials.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Holt DJ, Chamberlain LM, Grainger DW. Cell-cell signaling in co-cultures of macrophages and fibroblasts. Biomaterials. 2010;31:9382–94. doi: 10.1016/j.biomaterials.2010.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schutt C. C14. The International Journal of Biochemistry & Cell Biology. 1999;31:545–9. doi: 10.1016/s1357-2725(98)00153-8. [DOI] [PubMed] [Google Scholar]
- [56].Ma J, Chen T, Mandelin J, Ceponis A, Miller NE, Hukkanen M, et al. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–46. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- [58].Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sinha S, Miller L, Subramanian S, McCarty OJ, Proctor T, Meza-Romero R, et al. Binding of recombinant T cell receptor ligands (RTL) to antigen presenting cells prevents upregulation of CD11b and inhibits T cell activation and transfer of experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2010;225:52–61. doi: 10.1016/j.jneuroim.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. American journal of translational research. 2012;4:376–89. [PMC free article] [PubMed] [Google Scholar]
- [61].Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- [62].Ratner BD. Reducing capsular thickness and enhancing angiogenesis around implant drug release systems. J Control Release. 2002;78:211–8. doi: 10.1016/s0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- [63].Holt DJ, Grainger DW. Host-Implant Response. In: Hollinger JO, editor. An Introduction to Biomaterials. 2nd CRC Press; Boca Raton, FL: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.