Abstract
Objective
To examine the role of rheumatoid arthritis (RA) flare, remission, and RA severity burden in cardiovascular disease (CVD).
Methods
In a population-based cohort of RA patients without CVD (age ≥30 years; 1987 ACR criteria met in 1988-2007) we performed medical records review at each clinical visit to estimate flare/remission status. The previously validated RA medical Records-Based Index of Severity (RARBIS) and Claims-based Index of RA Severity (CIRAS) were applied. Age- and sex-matched non-RA subjects without CVD comprised the comparison cohort. Cox models were used to assess the association of RA activity/severity with CVD, adjusting for age, sex, calendar year of RA, CVD risk factors, antirheumatic medications.
Results
Study included 525 RA patients and 524 non-RA subjects. There was a significant increase in CVD risk in RA per time spent in each acute flare vs remission (HR 1.07 per 6-week flare; 95% CI 1.01-1.15). The CVD risk for RA patients in remission was similar to the non-RA subjects (HR 0.90; 95% CI 0.51-1.59). Increased cumulative moving average of daily RARBIS (HR 1.16, 95% CI 1.03-1.30) and CIRAS (HR 1.38, 95% CI 1.12-1.70) was associated with CVD. CVD risk was higher in RA patients who spent more time in medium (HR 1.08, 95% CI 0.98-1.20) and high CIRAS tertiles (HR 1.18, 95%CI 1.06-1.31) vs lower tertile.
Conclusions
Our findings show substantial detrimental role of exposure to RA flare and cumulative burden of RA disease severity in CVD risk in RA suggesting important cardiovascular benefits associated with tight inflammation control and improved flare management in RA patients.
Keywords: Rheumatoid arthritis, flare, disease severity, RARBIS, CIRAS, cardiovascular outcomes
Introduction
There is a growing evidence regarding the association of active rheumatoid arthritis (RA) with increased cardiovascular disease (CVD) risk [1, 2] This evidence is based largely on studies using single-point RA activity measures and surrogate cardiovascular end-points, while the impact of fluctuations of RA activity on CVD is much less studied [3]. Some cross-sectional data suggest that active RA is associated with significantly worse profiles of cardiovascular biomarkers compared with remission [4]. Increased vulnerability of atherosclerotic plaque in active RA versus remission has been reported [5]. A recent prospective study suggested decreased CVD mortality in RA patients with low disease activity [6]. Taken together, this might imply significant differences in CVD risk associated with flare versus remission in RA.
Despite the importance of these implications for CVD risk management in RA, this hypothesis has not been explored in long-term studies. The aims of this study were to examine the role of RA flare, remission, and cumulative burden of RA severity in CVD in a population-based cohort of patients with RA, and to determine whether CVD risk in RA during remission is similar to non-RA subjects.
Methods
A population-based inception cohort of Olmsted County, Minnesota residents aged ≥ 18 years (1987 American College of Rheumatology [ACR] criteria for RA met between 1/1/1988 and 12/31/2007) was previously identified and assembled using the Rochester Epidemiology Project (REP). The REP is a population-based medical records-linkage system that allows ready access to the complete (in-patient and out-patient) medical records from all community medical providers [7-9]. For each patient, the earliest date of fulfillment of ≥4 ACR criteria was considered the RA incidence date. For comparison, a cohort of Olmsted County residents without RA was selected by randomly choosing a subject of similar age and sex for each RA patient. Each non-RA subject was assigned an index date corresponding to the incidence date of the RA patient. Only patients aged ≥30 years at RA incidence were included because CVD events in persons aged <30 years are rare, and this age-group is not commonly included in CVD risk score tools (e.g., Framingham). The institutional review boards of Mayo Clinic and Olmsted Medical Center approved this study.
Patients were followed through medical record review until death, migration, or 7/1/2012. Medical records were reviewed by trained nurse-abstractors blinded to the study hypothesis to ascertain CVD risk factors (age, smoking, hypertension, diabetes mellitus and dyslipidemia) and CVD events: coronary heart disease [CHD] (i.e.,myocardial infarction, revascularization procedures, cardiovascular death, angina); heart failure [HF], stroke and intermittent claudication at RA incidence and throughout follow-up, using standardized criteria as previously described [10, 11].
RA flare was defined as any worsening of disease activity leading to initiation/change/increase of therapy or an expression such as “flare up,” “ongoing,” and “active” in the medical records [12-14]. Remission was defined as the absence of disease activity based on either tender joint count (TJC)=0 and swollen joint count (SJC)=0 and erythrocyte sedimentation rate (ESR)≤10 mm/hr, or TJC28≤1 and SJC28≤1 and C-reactive protein ≤10 mg/L [15, 16]. Visits not classified as flare/remission were considered as intermediate activity. Flares and remissions were considered to begin on the first date they were noted and to resolve halfway between the last flare/remission visit and the subsequent visit where the status was changed. An alternate definition, referred to as “acute flare”, was also examined where flares were defined to last ≤6 weeks, and patients were considered to have intermediate disease activity from the 6-week point until the next visit.
The previously validated RA medical Records-Based Index of Severity (RARBIS) and Claims-based Index of RA Severity (CIRAS) were used to estimate RA severity [14, 17, 18]. Both indices were developed using the resources of New England US Veterans Administration (VA) Health System for healthcare database research, and were shown to correlate well with disease activity score (DAS28). The RARBIS was developed by a Delphi-panel process based on ratings by expert rheumatologists. The CIRAS was developed as a linear regression model using RARBIS as a function of different administrative data variables. Both scores were designed to be calculated using data over the most recent year of follow-up. Data were collected at each medical visit to calculate the RARBIS: joint surgeries, erosions, extra-articular manifestations, arthritis flares, morning stiffness, rheumatoid factor (RF), functional status, acute phase reactants and antirheumatic medications. Claims data were used to calculate the CIRAS based on the number of inflammatory marker tests, platelet counts, chemistry panels, rheumatology visits, rehabilitation visits, RF assessment, and Felty's syndrome.
Statistical Methods
The association of each measure of RA activity/severity with CVD was examined using Cox models adjusted for age, sex, calendar year of RA incidence, cardiovascular risk factors and antirheumatic medications. Flare and remission states were modeled using time-dependent covariates, which changed during follow-up at the time when the activity level changed based on the definitions listed earlier.
RARBIS and CIRAS were calculated daily during follow-up using the most recent year of information for each calculation. RARBIS was computed both with and without the optional medication sub-scale. Since the results throughout were similar for both, we have reported the results for RARBIS with medications. Correlations between the scores were computed using Spearman methods. Smoothing spline techniques were used to plot trends in the scores over time.
Both single time-point and time-varying variables were examined for association with CVD outcomes. Single time-point estimates included daily RARBIS and CIRAS scores which were computed for each day using 1 year of prior information on RA activity/severity. Cumulative measures of CIRAS and RARBIS were computed at each day during follow-up using all information up to and including that day. These measures included the daily cumulative average, the cumulative percentage of time in each tertile (low, medium and high) of CIRAS and RARBIS, and the number of years of cumulative time in each tertile. Time-varying measures were included in the Cox model analyses using time-dependent covariates, which were allowed to change daily. Non-linear relationships between each RA activity/severity measure and CVD risk were examined using smoothing splines. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study included 525 RA patients without CVD prior to RA incidence. The mean age at incidence was 54.6 years (standard deviation [SD]: 13.7); 71% female (Table 1). During the mean follow-up of 10.1 years, 129 patients developed CVD (rate: 2.4 events/100 person-years), including 76 patients with CHD, 47 with HF, 26 with stroke and 22 patients with intermittent claudication. Disease activity/severity data were collected for 15,649 medical visits (mean 29.8, Q1 16, Q3 41, range 2-136 visits per patient; rate 3.0 visits/person-year).
Table 1. Characteristics of 525 patients with incident rheumatoid arthritis (RA) in 1988-2007.
| Characteristic | Value |
|---|---|
| Age, years | 54.6 (±13.7) |
| Female sex | 372 (71%) |
| Length of follow-up, years | 10.3 (±5.6) |
| Rheumatoid factor positivity | 359 (68%) |
| Smoking status at RA incidence | |
| Never | 245 (47%) |
| Current | 98 (19%) |
| Former | 182 (35%) |
| Cardiovascular risk factors (ever during follow-up) | |
| Hypertension | 453 (86%) |
| Diabetes Mellitus | 94 (18%) |
| Dyslipidemia | 373 (71%) |
| Medication use (ever during follow-up) | |
| Methotrexate | 342 (65%) |
| Hydroxychloroquine | 340 (65%) |
| Other disease modifying antirheumatic drugs | 151 (29%) |
| Biologics | 107 (20%) |
| Corticosteroids | 419 (80%) |
| RA severity scores at RA incidence: | |
| RARBIS with medications | 3.3 (±1.4) |
| RARBIS without medications | 3.0 (±1.4) |
| CIRAS | 4.5 (±1.9) |
Values in the table are mean (±SD) for continuous characteristics and N (%) for discrete characteristics.
RARBIS = Rheumatoid Arthritis medical Records-Based Index of Severity (RARBIS); CIRAS = Claims-based Index of Rheumatoid Arthritis Severity
Patients were flaring at the time of visits to providers in 2829 (18%) visits and were in remission at the time of 1545 (10%) visits. The median duration of flare was 2.4 months (Q1 1, Q3 5.4 months). Defining flare to start on the visit date indicative of flare and to end halfway between a visit with flare and a subsequent visit without flare, we found no difference in the risk of CVD during flare (hazard ratio [HR] 0.96, 95% confidence interval [CI] 0.46-1.99) or intermediate activity (HR 1.30, 95%CI 0.77-2.20) vs remission adjusted for age, sex and CVD risk factors. Defining acute flare to last only 6 weeks after a visit indicative of flare, there was a suggestion of increased CVD risk during acute flare (HR 1.70, 95%CI 0.62-4.64) and during intermediate activity (HR 1.39, 95%CI 0.80-2.41) vs remission, but these effects did not reach statistical significance. However, when we examined the time spent in acute flare, there was a significant increase in CVD risk per year spent in acute flare (HR 1.87, 95%CI 1.04-3.35) vs remission. As each acute flare was defined to be 6 weeks long, it is useful to report this result per 6 weeks instead of a year; the CVD risk increased by 7% for time spent in each additional acute flare (HR 1.07 per 6-week flare, 95%CI 1.01-1.15). There was no increase in CVD risk for time in intermediate activity vs remission (HR 0.95 per year in intermediate activity, 95%CI 0.84-1.08).
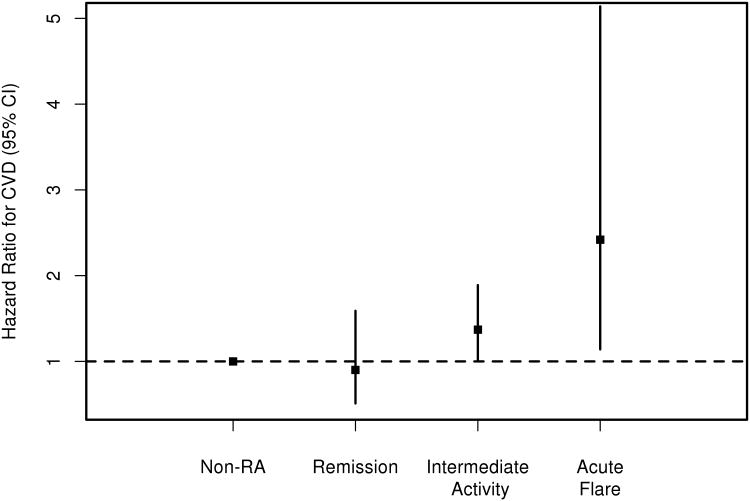
The non-RA cohort included 524 patients without prior CVD whose age (Mean: 54.5 years; SD: 13.5 years) and sex (71% female) were similar to the RA cohort. During a median follow-up of 7.7 years, 77 non-RA subjects developed CVD (rate 1.7 events/100 person-years) including 63 patients with CHD, 60 with HF, 17 with stroke and 14 patients with intermittent claudication. The CVD risk for RA patients in remission was similar to non-RA subjects (HR 0.90, 95%CI 0.51-1.59 adjusted for age, sex, calendar year and cardiovascular risk factors). However, RA patients had an increased CVD risk during intermediate activity (HR 1.37, 95%CI 1.01-1.89) and during acute flare (HR 2.42, 95%CI 1.14-5.14) vs the non-RA subjects, adjusting for age, sex, calendar year and cardiovascular risk factors (Figure 1). Remission was achieved during the first year following RA incidence for 63 patients. The CVD risk for these patients was similar to non-RA subjects (HR 1.05, 95%CI 0.57-1.93), but the CVD risk among those who did not achieve remission during the first year was higher than among non-RA subjects (HR 1.38, 95%CI 1.02-1.86).
Figure 1.
Cardiovascular disease risk depending on the level of rheumatoid arthritis (RA) disease activity in patients with RA as compared to the non-RA subjects adjusted for age, sex, calendar year and cardiovascular risk factors.
When looking at RA disease severity measures, the mean RARBIS with medications and CIRAS at RA incidence were 3.3 (SD 1.4; min 1; max 10) and 4.5 (SD 1.9; min 0.6; max 9.5), which are similar to the scores of 4.4 and 4.38, respectively, as reported in the developmental patient sample (15). The RARBIS and CIRAS in our study were modestly correlated with each other (r= 0.19, p< 0.001). There was no apparent association of RARBIS or CIRAS at RA incidence with CVD (HR 1.06 per 1 unit increase, 95% CI 0.93-1.20, p= 0.41 and HR 1.06, 95% CI 0.95-1.17, p= 0.32, respectively). Similarly, there were no apparent associations with CVD for the CIRAS and RARBIS at 1 year after RA incidence. The highest RARBIS and CIRAS in the first year were marginally associated with higher CVD risk (HR 1.07, 95%CI 0.97-1.17, p=0.16 and HR 1.16, 95%CI 1.01-1.32, p=0.03, respectively). Significant non-linear effects of CIRAS and RARBIS on risk of CVD were not found.
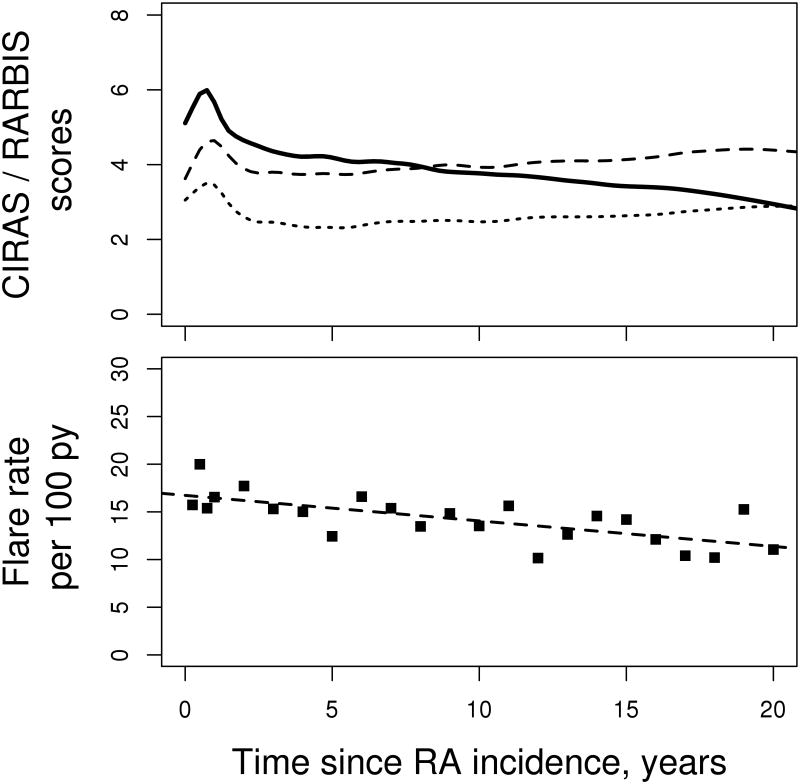
Overall, both CIRAS and RARBIS increased during the first year after index date, potentially reflecting an initial spike of RA activity and initial extensive work-up including laboratory and imaging assessments. Thereafter, CIRAS tended to decrease, but the RARBIS with and without medication subscale remained largely unchanged during the follow-up (Figure 2).Importantly, the flare rate decreased over time by 0.3 per 100 person-years each year (p<0.001) which corresponds to the lack of increase in severity scores after the first year of RA.
Figure 2.
Trends in Claims-based Index of Rheumatoid Arthritis Severity (CIRAS; solid line) Rheumatoid Arthritis medical Records-Based Index of Severity (RARBIS) with medications (dashed line) and RARBIS without medications (dotted line) [upper panel] and flare rate [lower panel] in patients with rheumatoid arthritis (RA).
With regards to cumulative RA severity measures, increase in cumulative moving average of daily CIRAS and RARBIS was associated with increased CVD risk (Table 2). Furthermore, patients who spent more time in medium and high CIRAS, but not RARBIS, tertiles were more likely to have an increased CVD risk than those who spent more time in the lower tertile.
Table 2. Rheumatoid arthritis (RA) activity/severity measures and their association with risk of cardiovascular disease.
| RA activity/severity measure | Variables | Hazard ratio* (95% CI) | P value |
|---|---|---|---|
| Flare/ | Daily Flare | 0.96 (0.46, 1.99) | 0.91 |
| Intermediate/ | Daily Intermediate activity | 1.30 (0.77, 2.20) | 0.33 |
| Remission | Daily Remission | 1 (reference) | |
|
| |||
| Years in Flare | 1.01 (0.89, 1.44) | 0.40† | |
| Years in Intermediate Activity | 0.92 (0.81, 1.04) | ||
| Years in Remission | 1 (reference) | ||
|
| |||
| Acute Flare/ | Daily Acute Flare | 1.70 (0.62, 4.64) | 0.30 |
| Intermediate/ | Daily Intermediate activity | 1.39 (0.80, 2.41) | 0.24 |
| Remission | Daily Remission | 1 (reference) | |
|
| |||
| Years in Acute Flare | 1.87 (1.04, 3.35) | 0.056† | |
| Years in Intermediate Activity | 0.95 (0.84, 1.08) | ||
| Years in Remission | 1 (reference) | ||
|
| |||
| CIRAS | Daily CIRAS | 1.27 (1.11, 1.46) | 0.0005 |
|
| |||
| Daily Cumulative Average CIRAS | 1.38 (1.12, 1.70) | 0.002 | |
|
| |||
| Years in Low CIRAS tertile | 1 (reference) | 0.006† | |
| Years in Medium CIRAS tertile | 1.08 (0.98, 1.20) | ||
| Years in High CIRAS tertile | 1.18 (1.06, 1.31) | ||
|
| |||
| RARBIS with medications | Daily RARBIS with medications | 1.10 (1.01, 1.21) | 0.04 |
|
| |||
| Daily Cumulative Average RARBIS | 1.16 (1.03, 1.30) | 0.01 | |
|
| |||
| Years in Low RARBIS tertile | 1 (reference) | 0.18† | |
| Years in Medium RARBIS tertile | 0.98 (0.90, 1.07) | ||
| Years in High RARBIS tertile | 1.06 (0.99, 1.14) | ||
|
| |||
| RARBIS without medications | Daily RARBIS without medications | 1.15 (1.04, 1.27) | 0.006 |
|
| |||
| Daily Cumulative Average RARBIS | 1.17 (1.04, 1.33) | 0.01 | |
|
| |||
| Years in Low RARBIS tertile | 1 (reference) | 0.052† | |
| Years in Medium RARBIS tertile | 1.11 (1.00, 1.24) | ||
| Years in High RARBIS tertile | 1.07 (1.00, 1.14) | ||
adjusted for age, sex, year of RA, cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, smoking status) and antirheumatic medications (methotrexate, hydroxychloroquine, other disease modifying anti-rheumatic drugs, biologics, corticosteroids)
RARBIS = Rheumatoid Arthritis medical Records-Based Index of Severity (RARBIS); CIRAS = Claims-based Index of Rheumatoid Arthritis Severity
generalized test of a difference between the tertiles
When the association of CHD alone with RA activity/severity status was examined, the results remained similar to those with all CVD outcomes combined. Only CIRAS score and time spent in medium and high CIRAS tertiles vs lower tertile, but not other measures of RA activity/severity, were associated with increased CVD risk (see supplemental table). Similar results were found for HF and stroke when examined separately (data not shown). The association of acute flare (HR 2.45, 95%CI 0.94-6.37, p=0.07) and intermediate activity (HR 1.36, 95%CI 0.94-1.96, p=0.10) with the risk of CHD in RA vs non-RA approached statistical significance while CHD risk in RA remission was similar to the non-RA cohort (HR 0.91, 95%CI 0.45-1.84, p=0.79).
Discussion
The literature evidence on the impact of changes in RA disease activity on CVD is scarce. This study is among the first to show the deleterious role of each acute flare and protective effect of remission on CVD in RA. Indeed, time spent in each acute flare was estimated to increase CVD risk by 7% compared to remission. The risk of CVD was not significantly increased during the flare, but the point estimate was quite high (HR 1.7). These findings suggest that, along with joint damage, there may be substantial cardiovascular damage accumulating with each flare. This observation may potentially explain the disproportional excess of cumulative CVD risk in patients with RA versus their non-RA counterparts as shown previously [19]. Thus, there should be a potential CVD benefit associated with the prevention of each flare, underscoring the importance of tight inflammation control in RA.
Increasing evidence suggests that early and sustained remission may provide significant survival benefits in RA [20-22]. The impact of remission on CVD outcomes is less explored, but likely confers cardiovascular benefit [4, 5, 23,24]. In our study, patients with RA in remission had similar risk of CVD as non-RA subjects, while intermediate activity and acute flare of RA were associated with 1.4- and 2.4-fold increases in the occurrence of CVD (particularly, CHD)compared to the non-RA subjects. Likewise, CVD risk in RA patients who achieved remission during the first year after the disease onset did not differ from the non-RA subjects, while those who did not achieve remission during the first year of RA incidence had a 38%-increase in risk of CVD as compared to the non-RA subjects. These observations support the intricate relationship between inflammation and CVD in RA, suggesting important outcome benefits of strategies that focus on achieving early control of disease activity and remission, such as the treat-to-target strategy, both for joint disease progression and for cardiovascular outcomes.
This study is among the first to report the association of higher cumulative burden of RA severity with CVD occurrence in RA using population-based longitudinal data. Each year spent in the high tertile of CIRAS vs the lower tertile translated into an 18%-increase in CVD risk. This finding echoes the recent study by Arts et al, which showed that RA patients with a consistently high disease activity measured by time-averaged DAS28>5.1 were at substantially increased CVD risk versus those with lower disease activity, although after the correction for confounders the difference between DAS28>5.1 and DAS28<3.2 was attenuated (p=0.074) [25]. They also showed a proportional increase in CVD hazard as the time-averaged DAS28 increased. Concordantly, we found that increases in cumulative moving average of daily CIRAS and RARBIS were associated with increased CVD risk. We had limited DAS28 data, as these have only been recently systematically recorded in our clinic. Examination of 278 visits with DAS28 data suggests good validity of the RARBIS and CIRAS as reflections of disease activity/severity. Correlation between DAS28 and RARBIS was very good (r=0.40; p<0.001), as well as between DAS28 and CIRAS (r=0.19; p=0.008).
Of interest is the finding that the increase in CVD risk over time was observed despite the decreasing flare rate and the lack of increase in RA activity/severity scores after the first year of RA. Taken together, these findings suggest an accrued detrimental role of RA activity and associated damage on CVD outcomes over time, which was widely assumed based on the association of the single-point markers of RA severity and damage (e.g., extra-articular manifestations, joint erosions, RF positivity) with CVD outcomes [10, 26-29], but until now was insufficiently supported by evidence from longitudinal studies. In contrast to significant associations of time-varying measures of RARBIS and CIRAS with CVD, their single-point estimates were not significantly associated with CVD. It is possible that time-varying covariates may be more representative of the disease burden than single-point covariates which cannot capture the extent of long-term disease severity.
A potential limitation of our retrospective study design is that only information coming to medical attention was used to define “flare” and “remission”, as well as to calculate RARBIS and CIRAS. This could result in misclassification of visits into the intermediate activity category for patients who didn 't seek medical attention either during a flare or after the flare resolved. However, we believe the comprehensive resources of the REP likely minimized shortcomings associated with these retrospective data.
RARBIS and CIRAS indices were devised and validated for healthcare database research by medical records review and claims data acquisition, respectively, suggesting their applicability to our data source, as well as to the methods of data collection used herein. RA severity defined by RARBIS and CIRAS may not be generalizable to other measures of RA disease activity/severity or to patient-reported measures. However, RARBIS has been shown to have good construct validity and moderate convergent validity with DAS28 [14, 17], and CIRAS has been shown to have moderate convergent validity with RARBIS [18]. The somewhat lower correlation between RARBIS and DAS28 in our study (r=0.40) vs the developers' sample (r=0.48) is not unexpected and may be explained by differences in patient characteristics, and the common phenomenon that model performance appears to be better in the development data vs external data (i.e., optimism).
We did not explore whether remission was treatment-induced or spontaneous, and whether either type might have a different impact on CVD risk. Although the analyses were adjusted for antirheumatic medication use, we did not analyze the impact of medication exposure on the described associations of RA activity and CVD risk. However, this study is one of the pioneer studies of long-term changes in RA activity and their role in CVD outcomes in RA and seeds ground for future research. Finally, during the period of investigation, the population of Olmsted County was predominantly white. Thus, the results may not be generalizable to nonwhite individuals.
In conclusion, there is a meaningful 7%-increase in CVD risk with the exposure to each acute flare, but not remission, in RA versus the general population highlighting the pivotal role of flares in shaping the risk of CVD in RA. Higher long-term burden of RA severity was associated with significantly increased risk of CVD suggesting accrued detrimental effect of RA activity over time. These findings imply important cardiovascular benefits associated with improved flare management and tight inflammation control in RA.
Supplementary Material
Supplemental table 1. Rheumatoid arthritis (RA) activity/severity measures and their association with risk of Coronary Heart Disease
Acknowledgments
Initial findings of this study were presented at the American College of Rheumatology meeting 2014 [30].
Funding Source: This work was funded by a grant from Roche and by a grant from the National Institutes of Health, NIAMS (R01 AR46849). Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Innala L, Moller B, Ljung L, et al. Cardiovascular events in early rheumatoid arthritis (RA) are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther. 2011;13:R131. doi: 10.1186/ar3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nature reviews. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 3.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–7. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: a cross-sectional comparative study. Ann Rheum Dis. 2011;70:812–7. doi: 10.1136/ard.2010.141523. [DOI] [PubMed] [Google Scholar]
- 5.Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol. 2009;29:1702–8. doi: 10.1161/ATVBAHA.109.190108. [DOI] [PubMed] [Google Scholar]
- 6.Meek IL, Vonkeman HE, van de Laar MA. Cardiovascular case fatality in rheumatoid arthritis is decreasing; first prospective analysis of a current low disease activity rheumatoid arthritis cohort and review of the literature. BMC Musculoskelet Disord. 2014;15:142. doi: 10.1186/1471-2474-15-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 9.Myasoedova E, Crowson CS, Kremers HM, et al. Is the Incidence of Rheumatoid Arthritis Rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myasoedova E, Crowson CS, Nicola PJ, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. J Rheumatol. 2011;38:1601–6. doi: 10.3899/jrheum.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–4. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alten R, Choy EH, Christensen R, et al. Developing a Construct to Evaluate Flares in Rheumatoid Arthritis: A Conceptual Report of the OMERACT RA Flare Definition Working Group. J Rheumatol. 2011;38:1745–50. doi: 10.3899/jrheum.110400. [DOI] [PubMed] [Google Scholar]
- 13.Bingham CO, 3rd, Pohl C, Woodworth TG, et al. Developing a standardized definition for disease “flare” in rheumatoid arthritis (OMERACT 9 Special Interest Group) J Rheumatol. 2009;36:2335–41. doi: 10.3899/jrheum.090369. [DOI] [PubMed] [Google Scholar]
- 14.Sato M, Schneeweiss S, Scranton R, et al. The validity of a rheumatoid arthritis medical records-based index of severity compared with the DAS28. Arthritis Res Ther. 2006;8:R57. doi: 10.1186/ar1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Boers M, Shea B, et al. Minimal disease activity for rheumatoid arthritis: a preliminary definition. J Rheumatol. 2005;32:2016–24. [PubMed] [Google Scholar]
- 16.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–13. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 17.Ting G, Schneeweiss S, Katz JN, et al. Performance of a rheumatoid arthritis records-based index of severity. J Rheumatol. 2005;32:1679–87. [PubMed] [Google Scholar]
- 18.Ting G, Schneeweiss S, Scranton R, et al. Development of a health care utilisation data-based index for rheumatoid arthritis severity: a preliminary study. Arthritis Res Ther. 2008;10:R95. doi: 10.1186/ar2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kremers HM, Crowson CS, Therneau TM, et al. High ten-year risk of cardiovascular disease in newly diagnosed rheumatoid arthritis patients: a population-based cohort study. Arthritis Rheum. 2008;58:2268–74. doi: 10.1002/art.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scire CA, Lunt M, Marshall T, et al. Early remission is associated with improved survival in patients with inflammatory polyarthritis: results from the Norfolk Arthritis Register. Ann Rheum Dis. 2014;73:1677–82. doi: 10.1136/annrheumdis-2013-203339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayakumar K, Norton S, Dixey J, et al. Sustained clinical remission in rheumatoid arthritis: prevalence and prognostic factors in an inception cohort of patients treated with conventional DMARDS. Rheumatology (Oxford) 2012;51:169–75. doi: 10.1093/rheumatology/ker250. [DOI] [PubMed] [Google Scholar]
- 22.Krause D, Schleusser B, Herborn G, Rau R. Response to methotrexate treatment is associated with reduced mortality in patients with severe rheumatoid arthritis. Arthritis Rheum. 2000;43:14–21. doi: 10.1002/1529-0131(200001)43:1<14::AID-ANR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Arts E, Fransen J, Den Broeder A, et al. Sustained clinical remission (DAS28<2.6) protects against cardiovascular disease in rheumatoid arthritis patients. Arthritis Rheum. 2012;64(Supplement 10):1682. [Google Scholar]
- 24.Semb AG, Rollefstad S, Provan SA, et al. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol. 2013;40:359–68. doi: 10.3899/jrheum.120621. [DOI] [PubMed] [Google Scholar]
- 25.Arts EE, Fransen J, den Broeder AA, et al. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis. 2014 Jan 23; doi: 10.1136/annrheumdis-2013-204531. Published online first. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. The increased risk of ventricular diastolic dysfunction and congestive heart failure in patients with rheumatoid arthritis is independent of the duration of the disease. Semin Arthritis Rheum. 2005;35:132–3. doi: 10.1016/j.semarthrit.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Maradit-Kremers H, Nicola PJ, Crowson CS, et al. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 28.Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64:366–70. doi: 10.1016/j.jjcc.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Cutolo M, Kitas GD, van Riel PL. Burden of disease in treated rheumatoid arthritis patients: going beyond the joint. Semin Arthiris Rheum. 2014;43:479–88. doi: 10.1016/j.semarthrit.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Myasoedova E, Chandran A, Ilhan B, et al. The impact of rheumatoid arthritis disease activity on cardiovascular disease risk: what is the role of the flare? Arthritis Rheum. 2014;66(Supplement 11):369. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1. Rheumatoid arthritis (RA) activity/severity measures and their association with risk of Coronary Heart Disease