Abstract
Objectives
Adults diagnosed with Major Depressive Disorder (MDD) have been found to be characterized by selective attention to negative material and by impairments in their ability to disengage from, or inhibit the processing of, negative stimuli. Altered functioning in the frontal executive control network has been posited to underlie these deficits in cognitive functioning. We know little, however, about the neural underpinnings of inhibitory difficulties in depressed adolescents.
Method
We used functional magnetic resonance imaging in 18 adolescents diagnosed with MDD and 15 age- and gender- matched healthy controls (CTLs) while they performed a modified affective go/no-go task that was designed to measure inhibitory control in the presence of an emotional distractor. Participants were presented with either a happy or a sad face, followed by a go or a no-go target to which they either made or inhibited a motor response.
Results
A group (MDD, CTL) by valence (happy, sad) by condition (go, no-go) analysis of variance indicated that MDD adolescents showed attenuated BOLD response in the right dorsolateral prefrontal cortex (DLPFC) and in the occipital cortex bilaterally, to no-go targets that followed a sad, but not a happy, face.
Conclusions
Adolescents diagnosed with MDD showed anomalous recruitment of prefrontal control regions during inhibition trials, suggesting depression-associated disruption in neural underpinnings of the inhibition of emotional distractors. Given that the DLPFC is associated with the maintenance of goal-relevant information, it is likely that sad faces differentially capture attention in MDD adolescents and interfere with task demands requiring inhibition. Keywords: adolescence; depression; response inhibition; prefrontal cortex; fmri
Keywords: adolescence, depression, response inhibition, fMRI, prefrontal cortex
Individuals with Major Depressive Disorder (MDD) consistently exhibit negative biases across a range of cognitive functioning, including attention, interpretation, and memory. These biases are posited to be directly involved in initiating or sustaining a depressive episode (Joormann, et al., 2007; Joormann & Gotlib, 2010), and as such, have been used as targets of treatment for depression since Beck developed cognitive therapy in the 1960s (Beck, 1976). The mechanisms underlying these biases, however, are not well understood. One mechanism that may contribute to the selective attention to negatively valenced stimuli in depressed individuals is an impaired ability to inhibit the processing of negative material (Joormann, 2004; Joormann, Yoon, & Zetsche, 2007). Researchers have shown that depressed individuals exhibit increased interference from negative stimuli on the emotional Stroop task (Gotlib & McCann, 1984), have difficulty inhibiting the processing of negative stimuli on the negative affective priming task (Goeleven et al., 2006; Joormann & Gotlib, 2010), and are quicker to respond to sad targets in an affective go/no-go task (Erickson et al., 2005; Murphy et al., 1999). Collectively, these findings suggest that the emotional nature of the stimuli used in these tasks – typically emotionally salient negative words or faces – disrupts inhibitory functioning in depressed individuals. That is, depressed individuals have difficulty disengaging from the processing of mood-congruent stimuli. This impairment in disengagement, a form of inhibitory dysfunction, is posited to lead to sustained attention to negative stimuli and to the high levels of rumination characteristic of MDD (Gotlib & Joormann, 2010)
Researchers have now begun to examine the neural correlates of impairments in the ability of depressed individuals to inhibit the processing of mood-congruent material. A number of studies have scanned depressed adults as they complete paradigms involving controlled processing of negatively valenced emotional materials. These tasks include the negative affective priming task (Eugène et al., 2010), the emotional Stroop task (Wagner et al., 2006), and the emotional Go/No-Go task (Elliott et al., 2002). The results of these studies suggest a neural model of inhibitory deficits in depression. Specifically, investigations have found depressed individuals to exhibit anomalous activation, compared to their nondepressed peers, in cognitive control regions, including the rostral anterior cingulate cortex (rACC), medial prefrontal cortex, inferior frontal gyrus (IFG), and the right dorsolateral prefrontal cortex (rDLPFC; e.g., Eugène et al., 2010; Siegle et al., 2007; Wang et al., 2008). Thus, altered function in frontal executive control networks may underlie the pronounced behavioral deficits in inhibiting negatively valenced stimuli that have been documented in depressed individuals. Importantly, these neural regions are involved not only in inhibitory control, but also in the regulation and reappraisal of negative emotion (Ochsner et al., 2002, 2004), further supporting the formulation that deficits in the ability to inhibit negative emotionally-salient material underlie the onset and maintenance of MDD (Joormann, 2010).
Much of our understanding of inhibitory deficits in MDD comes from studies of chronically depressed adults. It is not clear, however, whether these same patterns are present in younger individuals who are early in the course of the disorder. This question is particularly important given that the neural networks subserving inhibitory function and cognitive control continue to develop throughout adolescence (Casey et al., 1997; Eigsti et al., 2006; Luna et al., 2004). In particular, researchers have documented distinct behavioral and neural changes throughout adolescence in individuals’ ability to inhibit the processing of negative stimuli (Cohen-Gilbert & Thomas, 2013; Tottenham, Hare & Casey, 2011; Somerville, Hare, & Casey, 2011). More specifically, behavioral studies have shown that younger adolescents experience greater interference from emotional distractors and exhibit greater difficulty inhibiting the processing of this material than do adults. Similar patterns have been reported in neuroimaging studies: compared with adults, adolescents show increased activation in subcortical, limbic regions and attenuated prefrontal activation during the processing of emotional distractors (Hare et al., 2008). These findings suggest that during adolescence there is relatively immature top-down control over more mature bottom-up emotional processing. It is unclear, however, how this imbalance is manifested in adolescents with depression.
Conducting neuroimaging investigations in depressed adolescents that use negatively valenced material in the context of a cognitive control task is critical to furthering our understanding of the interaction between a negative mood state and cognitive functioning in the developing brain. The Go/No-Go (GNG) task is an ideal paradigm to use in such studies. This task requires participants to respond to a selected go target, presented more than 75% of the time, and to override the prepotent tendency to make a motor response when presented with the less frequent no-go target (Casey et al., 1997). Indeed, depressed and nondepressed adolescents differ behaviorally in their performance on an emotional version of the GNG task. For instance, Kyte, Goodyer, and Sahakian (2005) found that depressed adolescents showed enhanced attention to emotional stimuli, exhibiting fewer errors of commission when presented with no-go sad words. Similarly, Ladouceur et al. (2006) found that depressed adolescents were significantly faster at responding to sad face go trials than were both healthy controls and adolescents with anxiety, despite the absence of group differences in accuracy. In both studies, depressed adolescents failed to inhibit their responses to negative emotional stimuli. In a similar affective GNG task, Han et al. (2012) found that higher symptom severity in depressed adolescents was correlated with faster reaction time to affective targets, including happy, fearful, angry, and sad faces. The neural mechanisms that underlie this aberrant behavioral performance in depressed adolescents remain largely unknown. Elucidating these mechanisms can clarify whether negative attentional biases are due to dysfunctional inhibition of emotionally charged stimuli in the context of a protracted development of neural regions involved in attentional and inhibitory processes. Such findings may further help to explain why the onset of depression occurs most commonly during adolescence (Kessler et al., 2001).
To date, only one study has examined the neural correlates of performance on a GNG task in depressed adolescents. In this study, which used affectively neutral stimuli, Pan et al. (2011) assessed the neural correlates of inhibitory control in adolescents who had attempted suicide, depressed adolescents with no history of attempted suicide, and healthy adolescent controls. Results of their investigation revealed that, compared to their nondepressed counterparts, non-suicidal depressed adolescents had increased activation of the insula and the ACC during both go- and no-go trials. Importantly, however, because this study used non-valenced stimuli, it is not clear how depressed and nondepressed teens differ in neural activation during the inhibition of emotionally salient material. Further, Pan et al. used a block design that did not permit them to separate accurate and inaccurate trials or, equally important, to conduct separate analyses of the go and no-go trials, which is critical to an assessment of inhibitory functioning.
No study has yet examined neural aspects of inhibitory processing during an emotional GNG task in adolescents who are experiencing a major depressive episode. Thus, in the present study, we used an emotion-modified GNG task to examine, in a sample of currently depressed adolescents, the neural correlates of difficulties in the inhibition of negatively valenced emotional material that have been documented in previous studies. In this modified GNG task, an emotional face (sad or happy) is presented before the go or no-go target (a circle or a square). By presenting the emotional stimuli prior to the go or no-go target stimulus, we could vary the presentation of the emotional stimulus and examine whether prolonged exposure to affective stimuli differentially influenced subsequent response inhibition within the same trial.
Based on previous findings of impaired inhibition of emotional content in depressed adolescents and adults (e.g., Kyte et al., 2005; Ladouceur et al., 2006), we predicted that depressed adolescents in the present study, compared with their nondepressed peers, will make more errors of commission in response to no-go targets that are preceded by a sad emotional face, reflecting impaired inhibitory processing due to the interference of negative stimuli. Similarly, based on previous findings of a mood-congruent attentional bias in depressed adolescents and adults (e.g., Gotlib et al., 2004; Williams et al., 1997), we also predicted that depressed adolescents will exhibit longer response latencies to go trials that are preceded by a sad facial expression. Finally, in terms of neural functioning, we predicted that depressed adolescents would be characterized by anomalous activation of prefrontal cortex during inhibition trials.
Methods
Participants
Eighteen depressed (MDD; 15 female) and 15 healthy control (CTL; 10 female) adolescents, ages 12–17 years, participated in the study1. Participants in the depressed group met Diagnostic and Statistical Manual (4th ed; DSM-IV-TR; American Psychological Association, 1994) criteria for MDD as their primary diagnosis. Depressed adolescents were recruited through the Pediatric Mood Disorders Clinic of an American academic department of psychiatry and from the local community; healthy controls were recruited from the surrounding community. Exclusion criteria for all participants included 1) nonfluent English speakers; 2) contraindications to scan (e.g., metal implants, braces, etc.); 3) history of major neurological disorder or illness; and 4) a diagnosis of bipolar I disorder, attention deficit hyperactive disorder, psychotic disorders or current alcohol/drug dependence; and 5) intellectual delay or learning difficulties. For the healthy controls, exclusion criteria also included any past or current Axis I disorder. All participants were offered hourly compensation for their participation in the study.
Procedure
Clinical Assessment
All study participants completed both a parental consent and child assent to participate in the study. At the first laboratory session, adolescents and one parent were administered the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL; Kaufman et al., 1997), a semi-structured clinical interview used to confirm diagnosis in the MDD group and to rule out the possibility of Bipolar I disorder, ADHD and/or drug or alcohol dependency in all participants. The KSADS was also used to assess exclusionary criteria of a current or lifetime Axis I disorder in the control participants. These interviews were conducted by raters with established symptom and diagnostic reliability (κ>0.9). Final diagnostic decisions were made by a board-certified child and adolescent psychiatrist (M.S.). In addition, all participants completed the Children’s Depression Inventory (CDI; Kovacs, 1985), a self-report measure of depressive symptoms, Tanner staging (Marshall & Tanner, 1969, 1970) to assess self-report of pubertal status, and completed demographic questionnaires.
Neuroimaging
Participants who met eligibility criteria returned for a second visit to complete a functional Magnetic Resonance Imaging (fMRI) scan. All scans were conducted on a 3 Tesla GE whole-body scanner (GE Healthcare Systems, Milwaukee, Wisconsin). Foam padding was used to minimize head movement. Twenty-nine axial slices were taken with 4-mm slice thickness. High-resolution T2-weighted fast spin-echo structural images (Repetition Time (TR) = 3000 ms, Echo Time (TE) = 68 ms, ETL = 12, Field of View (FOV) = 22 cm, 192×256) were acquired for anatomical reference. A T2*-sensitive gradient echo spiral in/out pulse sequence was used for functional imaging (TR = 2000 ms, TE = 30 ms, flip angle = 80°, FOV = 22 cm). An automated high-order shimming procedure, based on spiral acquisitions, was used to reduce B0 inhomogeneity. Additional high-resolution images were acquired with an axial 3D FSPGR sequence for T1 contrast (140 slices, 1.3 mm thickness, TR = 6.0 ms, TE = 1.2 ms, TI = 500 ms, flip angle = 11°, FOV = 24 cm, 192×256).
Task
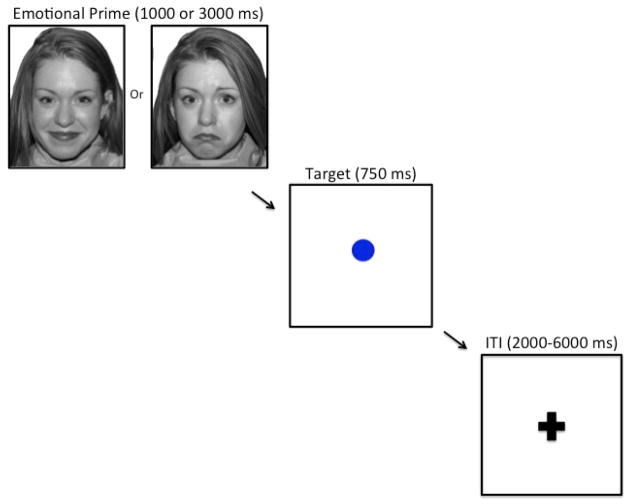
We used a modified affective GNG task to examine the neural correlates of inhibitory control (Figure 1). This task required participants to make a button press when they saw the visual go target (a blue circle) and to inhibit this response when they saw the infrequently presented no-go target (a blue square). The blue circle and blue square were not counterbalanced within or across participants, given that we did not expect that target shape would affect behavioral performance. The go target was presented 75% of the time, thereby creating a prepotent response tendency that must be inhibited in the presence of the no-go target. To be able to assess whether inhibitory function was influenced by the presence of antecent emotional stimuli, we modified the traditional GNG task by presenting either a happy or sad face picture (prime) immediately prior to the go or no-go target. The emotional face prime was presented for 1000 ms (50% of the trials) or 3000 ms (50% of the trials); to increase power, these two durations were collapsed for the behavioral and neural analyses. The go or no-go target that followed the emotional face prime for each trial was presented for 750 ms. Participants were instructed to make a response to the go target as quickly and accurately as possible, and to not respond to the no-go target. Go responses were recorded by pressing a button on an MR-compatible button box in the scanner. All face and target pairs were interspersed with a fixation cross, which was pseudorandomized to maximize the hemodynamic response function, and lasted either 2000, 4000, or 6000 ms. The order of presentation of the face-target pairs was counter-balanced across participants in order to minimize any possible order effects.
Figure 1. Experimental design.
Participants are presented with a picture of a happy or sad face (prime), followed by a go or no-go target that indicates whether the participant should make a button response or inhibit a response. Inter-trial intervals (ITIs) in the form of a variable-duration fixation cross separate each face-target trial.
The novel design of this GNG task avoided important confounds not previously addressed in the literature. Specifically, in previous iterations of the emotional GNG task, emotional stimuli (typically faces) have served as response targets (e.g., Hare et al., 2005). This use of emotional stimuli as response targets implicitly requires that participants label the emotion in order to perform the task correctly. Importantly, however, such labeling has the potential to reduce the salience of the emotional expression (Hariri, Bookheimer, & Mazziotta, 2000; Lieberman, Hariri, Jarcho, Eisenberger, & Bookheimer, 2005), which may in turn decrease the interference experienced in this paradigm by depressed adolescents. Similarly, our task design allowed for the examination of the effect of prolonged emotional stimuli on inhibitory function. Our lab and others have shown that attention biases in depression are most strongly observed when emotional stimuli are presented for 1000 ms or longer (Gotlib, et al., 2004; Joormann & Gotlib, 2007). Thus, using the emotional stimuli prior to the target rather than as the target itself (presented for 750 ms) allowed us to present the emotional stimuli for longer durations in order to examine the effect of emotional stimuli on response inhibition.
The task was presented using E-Prime software Version 2.0. A total of 240 trials were divided across two emotion conditions (happy, sad) and two response conditions (go and no-go). The happy and sad emotional expressions were facial displays from the NimStim picture set (http://www.macbrain.org/resources.htm). Ten actors (5 female, 5 male) were displayed showing happy or sad emotional expressions. We presented happy faces rather than neutral faces to the participants because previous researchers have found that neutral faces can be interpreted as negative by children and adolescents (e.g., Tottenham et al., 2013).
Imaging Data Analysis
Analyses were performed in FSL Version 4.1.6 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), using FEAT (FMRI Expert Analysis Tool). The first four volumes of each participant’s functional scan were discarded to allow for stabilization of longitudinal magnetization. The remaining images were preprocessed using a series of steps. Preprocessing included motion correction to the mean image using MCFLIRT (Motion Correction FMRIB’s Linear Image Registration; Jenkinson, Bannister, Brady, & Smith, 2002), spatial smoothing (Gaussian kernel FWHM = 5 mm), and high-pass temporal filtering (t > 0.01 Hz) (Woolrich, Ripley, Brady, & Smith, 2001). Functional data were linearly registered to a common stereotaxic space by first registering to the in-plane T2 image (6 degrees of freedom) then to the MNI152 T1 2 mm brain (12 degrees of freedom) (Jenkinson & Smith, 2001).
Statistical analysis was conducted using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL. A GLM analysis was performed for each participant, including regressors for each face/target combination (happy/go, happy/no-go, sad/go, sad/no-go) that the participants correctly responded to, as well as two separate regressors for incorrect trials (go incorrect and no-go incorrect), for a total of 6 regressors. Motion correction parameters were included as covariates of non-interest. All four runs were combined in a fixed effects analysis for each participant, then combined in a higher-level mixed effects model to investigate within and between-group differences. Higher-level group analyses were conducted using FSL’s FLAME (FMRIB’s Local Analysis of Mixed Effects State) stage 1 and 2 (Beckmann et al., 2003; Woolrich, 2008; Woolrich, 2004). Within-group Z statistical images for each condition were thresholded at Z > 2.0, corrected for multiple comparisons (p < .05).
Results
Participant Characteristics
Demographic and clinical characteristics of the MDD and CTL participants are presented in Table 1. The two groups of participants did not differ in age, t(31) = 0.59, gender distribution, χ2(1) = 1.24, or pubertal stage, t(30) = 0.85, all ps > 0.05. As expected, adolescents in the MDD group had significantly higher CDI scores than did adolescents in the CTL group, t(31) = 9.03, p < 0.01. Eight of the 18 depressed participants had a comorbid anxiety disorder. Seven of the depressed participants were also taking psychotropic medication for depression at the time of the scan. Importantly, medicated and unmedicated depressed adolescents did not differ on any measure of behavioral response or BOLD signal; therefore, we did not include medication status as a covariate in our analyses.
Table 1.
Demographic Information and Clinical Characteristics
| CTL (n = 15) | MDD (n = 18) | |
|---|---|---|
| Gender (% female) | 67 | 83 |
| Mean Age | 15.32 (1.49) | 15.61 (1.51) |
| Tanner Stage | 4.25 (0.58) | 4.42 (0.52) |
| CDI Score | 4.33 (3.64) | 26.67 (8.04) |
| Comorbid Anxiety (n) | 0 | 8 |
| Psychotropic Medication use (n) | 0 | 7 |
Note. Standard deviations are presented in parentheses. CTL = control participants; MDD = depressed participants; CDI = Children’s Depression Inventory.
Behavioral Data
Latency of Go Responses
Latencies of correct responses to go trials in the scanner were analyzed using a two-way (Group [MDD, CTL] repeated over Valence [happy, sad]) analysis of variance (ANOVA). Mean latencies are presented by group and valence in Table 2. The ANOVA did not yield a significant main effect of group, F (1,31) = 0.10, p = 0.76, η2 = .003 or a significant interaction of group and valence, F(1,31) = 1.84, p = 0.19, η2 = .06. There was, however, a main effect of valence, F(1,31) = 8.84, p < 0.01, η2 = .22: across both groups; participants were significantly faster to respond to targets following happy faces than following sad faces.
Table 2.
Behavioral Performance on the Modified Affective Go/No-Go Task
| CTL (n=15) | MDD (n=18) | |||
|---|---|---|---|---|
| Happy | Sad | Happy | Sad | |
| Mean Reaction Time (ms) | 444.7 (41.8) | 456.6 (39.8) | 452.5 (33.1) | 456.9 (39.3) |
| Go Accuracy (% Correct) | 96.5 (5.2) | 94.7 (5.9) | 92.1 (7.2) | 90.9 (7.2) |
| No-Go Accuracy (%Correct) | 88.3 (12.2) | 88.0 (10.1) | 86.9 (10.7) | 88.2 (10.5) |
Note. Standard deviations are presented in parentheses. CTL = control participants; MDD = depressed participants.
Accuracy of Go Responses and No-Go Inhibitions
Percent of correct responses to go and no-go targets for the MDD and CTL participants for the happy and sad conditions are also presented in Table 2. Two-way (Group [MDD, CTL] repeated over valence [happy, sad]) ANOVAs conducted on the percent of correct go responses did not yield a significant main effect of group, F(1,31) = 3.31, p = 0.08, η2 = .10, or a significant interaction of group and valence, F(1,31) = 0.25, p = 0.62, η2 = .08. There was, however, a significant main effect of valence, F(1,31) = 6.89, p = 0.01, η2 = .18: across both groups, participants were more accurate for go targets following happy faces than for go targets following sad faces. The ANOVA conducted on the percent of correct inhibitions in the no-go condition did not yield significant main effects of group, F(1,31) = 0.03, p = 0.87, η2 = .001, or valence, F(1,31) = 0.12, p = 0.74, η2 = .004 or a significant interaction of group and valence, F(1,31) = 0.35, p = 0.56, η2 = .01.
Imaging data
A group (MDD, CTL) by valence (happy, sad) by condition (go, no-go) ANOVA was conducted using whole-brain data to isolate regions involved in cognitive control, and to examine whether activation in these regions was modulated by group status and by the valence of emotional stimuli. This voxel-wise ANOVA yielded significant three-way interaction effects in two brain regions: a frontal cluster encompassing both the right inferior frontal gyrus (IFG) and right dorsolateral prefrontal cortex (DLPFC; x, y, z coordinates of peak voxel: 60, 2, 26; centralized subpeak: 46, 42, 24: Brodmann’s Area [BA] 6/9; Figure 2); and a cluster encompassing the occipital cortex (x, y, z coordinates of peak voxel: 10, −66, 8; BA 18; Figure 3).
Figure 2.
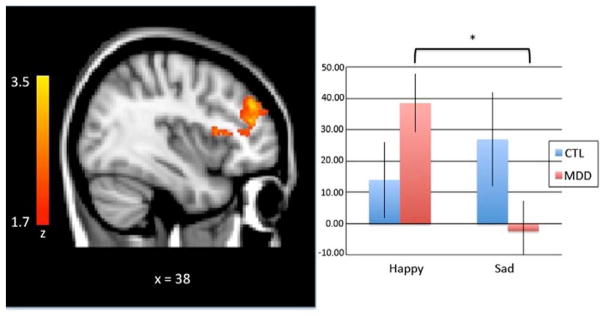
A group (MDD, CTL) by valence (happy, sad) by condition (go, no-go) analysis of variance revealed decreased DLPFC activation during sad face - no-go target trials relative to happy face - no-go target trials in the MDD group; the CTL group showed no such modulation based on emotion type. Activation maps (left) are thresholded at a Z > 2.0 and corrected for multiple comparisons using a cluster-based p < .05. MNI coordinates are indicated for slice distance (in mm). Parameter estimates (showing the amount of signal change measured in arbitrary units) of BOLD signal response were extracted from this functionally-defined ROI and plotted in the bar graph (right).
Figure 3.
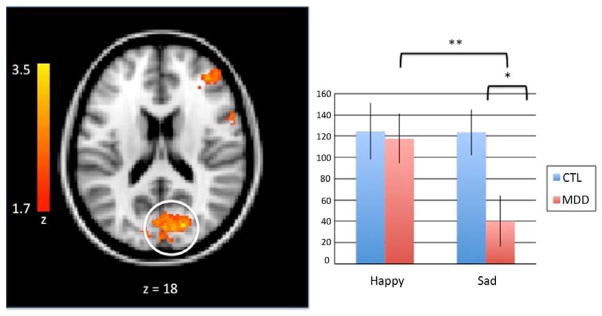
A group (MDD, CTL) by valence (happy, sad) by condition (go, no-go) analysis of variance revealed decreased BOLD signal in the occipital cortex in response to sad face - no-go target trials in the MDD group. Activation maps (left) are thresholded at a Z > 2.0 and corrected for multiple comparisons using a cluster-based p < .05. MNI coordinates are indicated for slice distance (in mm). Parameter estimates (showing the amount of signal change measured in arbitrary units) of BOLD signal response were extracted from this functionally-defined ROI in the occipital cortex (circled) and plotted in the bar graph (right).
Given our focus in this study on inhibitory functioning, we conducted, within clusters arising from the three-way interaction of group, valence and condition, follow-up analyses of no-go trials. Specifically, we examined group by valence interactions. These analyses yielded a main effect of valence in the occipital cortex, F(1,31) = 6.43, p = 0.02, η2 = .17: both groups showed higher levels of activation in this region when inhibiting a no-go target that followed a sad face than when inhibiting a no-go target that followed a happy face. There was no comparable effect in the DLPFC, and no main effect of group in either ROI.
The analyses also yielded a significant interaction of group and valence in the DLPFC, F(1,31) = 2.04, p = 0.01, η2 = .20. Decomposition of multifactor effects indicated that these interactions were due to differences between the MDD and CTL adolescents in neural activation during response inhibition to targets that varied as a function of valence. Specifically, whereas CTL adolescents showed similar levels of activation in the DLPFC while inhibiting a response following the presentation of happy and sad faces, t(14) = 0.87, p > .05, depressed adolescents showed less activation in the DLPFC while they were inhibiting a response to a no-go target that followed a sad face than to a no-go target that followed a happy face, t(17) = 3.2, p < 0.01.
Similar findings for the interaction of group and valence were obtained for no-go trials in the occipital cortex, F(1,31) = 6.15, p = 0.02, η2 = .17. Whereas CTL adolescents showed equivalent levels of activation in the occipital cortex while inhibiting responses to targets following the presentation of happy and sad faces, t(14) = 0.04, p > 0.05, depressed adolescents showed less activation in this region during no-go targets that followed a sad face compared to no-go targets that followed a happy face, t(17) = 3.38, p < 0.01. In addition, depressed adolescents had significantly lower levels of activation in the occipital cortex than did CTL adolescents during no-go targets that followed the presentation of a sad face, t(31)= 2.52, p < 0.05; the two groups did not differ in levels of activation in the occipital cortex during inhibition of responses to targets that followed a happy face, t(14) = 0.04, p > 0.05.
Discussion
The present study was designed to examine difficulties in response inhibition in the presence of emotional distractors in depressed adolescents. In this context, we assessed behavioral and neural aspects of inhibition following the presentation of happy and sad faces in carefully diagnosed depressed adolescents and in age- and gender-matched healthy controls. Depressed and nondepressed adolescents did not differ in their behavioral performance on the task. When we compared depressed and nondepressed adolescents’ neural activation during no-go targets, however, we documented a mood-congruent distraction effect in the DLPFC. CTL participants showed equivalent levels of BOLD response to no-go targets that followed happy and sad faces; in contrast, depressed adolescents exhibited significantly less DLPFC activation when they were inhibiting a response following the presentation of a sad face than following the presentation of a happy face. We found a similar pattern of BOLD response in the occipital cortex. Specifically, the MDD adolescents exhibited less activation in the occipital cortex than did the CTL adolescents in response to no-go targets that followed the presentation of a sad face; in contrast, the two groups did not differ in activation in this region in response to no-go targets that followed the presentation of a happy face. Thus, the depressed adolescents showed anomalous neural responses related to inhibitory functioning on trials involving the presentation of sad faces.
Previous experiments using the more traditional emotional GNG task, which involve making or inhibiting a response to an emotional target, have found mood-congruent behavioral facilitation effects in depressed adolescents (Erickson et al., 2005; Kyte et al., 2005; Ladouceur, 2006). Similar to Han et al.’s (2012) findings, however, we did not find this enhanced attention to negative stimuli in our behavioral data. In fact, several groups of investigators have failed to replicate behavioral results in the scanner, likely due to minor, but important, changes to task designs that are necessary to accommodate scanning environments (e.g., Rubia et al., 2005; Seidman et al., 2006; Smith et al., 2006). We did, however, find differential neural activation in MDD adolescents in regions that are involved in executive function and inhibition, suggesting that neural underpinnings of task performance are more sensitive to group differences than are behavioral outcomes (Halari et al., 2009).
Differential recruitment of the DLPFC by the depressed and nondepressed adolescents in this study indicates that disrupted DLPFC functioning may contribute to the pathogenesis of MDD in both adolescents and adults. Our results add to existing literatures implicating a role for DLPFC dysfunction in depression. Investigators have previously reported dampened resting-state DLPFC responses in depressed adolescents (Halari et al., 2009; Koenigs & Grafman, 2009), decreased DLPFC activation during cognitive information processing in depressed adults (Siegle et al., 2007), and increased DLPFC activation with greater symptom remission in depressed adults (Koenigs et al., 2009). The DLPFC is a key node in the executive network of the brain and is posited to be specifically involved in guiding and maintaining attention toward task-relevant goals in the face of interference (Banich et al., 2009; Miller & Cohen, 2001; Munakata et al., 2011). Whether attenuated activation in the DLPFC in depressed adolescents is due to increased limbic activation to mood-congruent faces in the sad no-go trials, as has been proposed (Monk et al., 2008; Mayberg, 1997), is not clear because the design of our task prohibited the separation of the emotion and cognitive trial epochs. Nevertheless, the current study provides preliminary evidence in support of the possibility that the processing of mood-congruent sad faces interferes with subsequent cognitive control in depressed adolescents. Given findings indicating that depressed individuals experience difficulty disengaging from negative stimuli (Gotlib & Joormann, 2010), we suggest that in the current study, sad faces were especially salient for depressed adolescents and, as a result of increased processing of salient material, the depressed adolescents were less able than their nondepressed peers to effectively recruit the DLPFC during performance of the response inhibition epoch of each trial. Particularly relevant to the role of the DLPFC in the pathogenesis of MDD is its involvement in the regulation and reappraisal of negative emotions (Ochsner et al., 2002, 2004). Thus, dysfunction in this neural region may contribute to the deficits in emotion regulation that have been posited to characterize individuals with MDD (Joormann, 2010).
We also found depression-related differences in activation in the occipital cortex: compared to the CTL adolescents, depressed adolescents showed a greater decrease in activation of this region during no-go trials that were preceded by a sad face. It is noteworthy that activation in this cluster extends from the striate cortex, which is associated with basic visual processing (Somers et al., 1999), and into the extrastriate cortices, which are associated with modulating basic visual processing (Haxby et al., 2000). Although we did not predict this finding, ours is not the first study to report inhibition-related activation in the occipital cortex. For example, in a study of neural correlates of inhibition during a go/no-go task with a relatively large sample, Steele et al. (2013) suggested that activation in the occipital cortex, particularly within the cuneus/precuneus, during no-go conditions is due to decreased recruitment of the default mode network rather than to an inhibition-related increase in this region. In this context, therefore, to the extent that the processing of a no-go target that follows a sad face is likely to be cognitively demanding, depressed adolescents may decrease their recruitment of task-irrelevant regions (e.g., extrastriate cortex) more so than do their nondepressed counterparts, a pattern that may reflect increased effort or attention to the task. Because we did not predict depression-related differences in neural recruitment of the occipital cortex, it is important in future research to replicate these findings and to delineate the role of the occipital cortex in the interaction of depression status, attention, and the processing of mood-congruent information.
We should note three limitations of this study. First, in order to distinguish neural response to go versus no-go targets, we used a rapid event-related design. Inherent in all event-related designs, however, is a randomized inter-trial interval (ITI). Using an ITI in the context of the current GNG task (ranging from 2000–6000 ms) may have interfered with participants’ initiation of a prepotent motor response by making it easier to inhibit a motor response following a no-go target. This decrease in task difficulty due to adaptation of the task to the scanner environment is supported by near-ceiling behavioral accuracy levels for both groups, which may be responsible for the lack of group differences in the behavioral measures in this study.
Second, nearly half of the depressed adolescents in this study were taking one or more psychotropic medications to treat their depression; the effects of these pharmacologic agents on brain function are largely unknown. Importantly, however, although the medicated adolescents obtained higher scores on the CDI than did their unmedicated counterparts (M medicated = 29.43 [7.79]; M unmedicated = 13.04 [11.86]; t(31) = 3.81, p<.01), these two groups of depressed adolescents did not differ on any dependent measure in this study, including response times, errors of omission/commission, and neural activation. Moreover, most of the adolescents who were taking psychotropic medication had limited lifetime exposure (mean of only 5.2 months). Nevertheless, it will be important for future research to examine how different types of medications may alter hemodynamic response functions in depressed individuals in response to the demands of inhibition tasks.
Finally, we should note that we had only 33 participants in this study. Therefore, it will be important in future research to replicate these results and to continue to elucidate the effect of depression severity on inhibitory function in the presence of affective stimuli.
In conclusion the present findings are important in documenting the role of impairment in DLPFC activation during inhibitory functioning in adolescents diagnosed with MDD. Future studies should extend this work to examine the extent to which affective material interferes with cognitive control processes in depressed adolescents. Moreover, it will be important to examine prospectively how disruptions in these control processes may contribute to the onset and maintenance of depression, and to recovery from this disorder. Findings from such research will have important implications for the development and evaluation of new interventions for MDD.
Acknowledgments
This work was supported by an NSF Graduate Fellowship to NLC, by NIMH grants MH101545 to IHG and MH090617 to LCFR, by the National Alliance for Research in Schizophrenia and Affective Disorders Distinguished Investigator Award to IHG and Young Investigator Award to LCFR, and by the Hope for Depression Research Foundation to IHG and LCFR.
Footnotes
We originally recruited 11 additional participants (4 MDD and 5 CTL), but they were excluded from analysis due to motion artifacts, failure to record behavioral responses, or poor and outlying behavioral performance, resulting in a sample size for analysis of 18 MDD and 15 CTL participants.
Contributor Information
Natalie L. Colich, Email: ncolich@stanford.edu.
Lara C. Foland-Ross, Email: lfolandross@stanford.edu.
Caitlin Eggleston, Email: ceggleston@stanford.edu.
Manpreet K. Singh, Email: mksingh@stanford.edu.
Ian H. Gotlib, Email: ian.gotlib@stanford.edu.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- Beck AT. Cognitive therapy and emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Thomas KM. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Development. 2013;84(6):1954–1966. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17(6):478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. American Journal of Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Eugène F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Research: Neuroimaging. 2010;181:30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, Raedt RD, Baert S, Koster EHW. Deficient inhibition of emotional information in depression. Journal of Affective Disorders. 2006;93:149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Neubauer Ye D, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McCann CD. Construct accessibility and depression: An examination of cognitive and affective factors. Journal of Personality and Social Psychology. 1984;47:427–439. doi: 10.1037//0022-3514.47.2.427. [DOI] [PubMed] [Google Scholar]
- Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, et al. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. The Journal of Child Psychology and Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, et al. Selective neurocognitive impairments in adolescents with major depressive disorder. Journal of Adolescence. 2012;35:11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Joormann J. Attention bias in dysphoria: the role of inhibitory processes. Cognition and Emotion. 2004;18(1):125–147. [Google Scholar]
- Joormann J. Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science. 2010;19(3):161–166. [Google Scholar]
- Joormann J, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Yoon KL, Zetsche U. Cognitive inhibition in depression. Applied and Preventive Psychology. 2007;12(3):128–139. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-aged children – present and lifetime version: initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–989. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. Journal of Child Psychology and Psychiatry. 2005;46(9):995–1005. doi: 10.1111/j.1469-7610.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;47(11):1107–15. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience. 2005;8:720–722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Marshall Wa, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall Wa, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, et al. Amygdala and mucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. The American Journal of Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15(10):453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JRC, Segreti A, Akkal D, Hassel S, et al. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(6):602–611. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activaton during inhibition and error detection in medication-naïve adolescents with ADHD. The American Journal of Psychiatry. 2005;162(6):1067–75. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT. Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophrenia Research. 2005;85:58–72. doi: 10.1016/j.schres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naïve children and adolescents with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 2006;163(6):1044–51. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RBH. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences. 1999;96(4):1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L, Hare TA, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, Pearlson G, Kiehl KA. A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behavioural Brain Research. 2013;256:529–36. doi: 10.1016/j.bbr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Frontiers in psychology. 2011;2(39):1–9. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, Goff B. A negativity bias for ambiguous facial-expression valence during childhood: converging evidence from behavior and facial corrugator muscle responses. Emotion. 2013;13(1):92–103. doi: 10.1037/a0029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel HJ, Sauer H, Schlösser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry. 2006;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Research: Neuroimaging. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorder. Chichester, England: Wiley; 1997. [Google Scholar]
- Woolrich MW. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilinear linear modelling for FMRI group analysis using bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]