Abstract
Small saccades occur frequently during fixation, and are coupled to changes in visual stimulation and cognitive state. Neurophysiologically, fixational saccades reflect neural activity near the foveal region of a continuous visuomotor map. It is well known that competitive interactions between neurons within visuomotor maps contribute to target selection for large saccades. Here we asked how such interactions in visuomotor maps shape the rate and direction of small fixational saccades. We measured fixational saccades during periods of prolonged fixation while presenting pairs of visual stimuli (parafoveal: 0.8 deg eccentricity; peripheral: 5 deg eccentricity) of various contrasts. Fixational saccade direction was biased toward locations of parafoveal stimuli but not peripheral stimuli, ~100–250 ms following stimulus onset. The rate of fixational saccades toward parafoveal stimuli (congruent saccades) increased systematically with parafoveal stimulus contrast, and was suppressed by the simultaneous presentation of a peripheral stimulus. The suppression was best characterized as a combination of two processes: a subtractive suppression of the overall fixational saccade rate and a divisive suppression of the direction bias. These results reveal the nature of suppressive interactions within visuomotor maps and constrain models of the population code for fixational saccades.
Keywords: microsaccades, fixational eye movements, suppression, visuomotor maps, superior colliculus, visual attention
Introduction
Small (< 1–2 deg) saccades occur 1–2 times per second during fixation (Ditchburn & Ginsborg, 1953; Martinez-Conde, Macknik, & Hubel, 2004; Martinez-Conde, Otero-Millan, & Macknik, 2013; Ratliff & Riggs, 1950; Rolfs, 2009; Steinman, Haddad, Skavenski, & Wyman, 1973; Zuber, Stark, & Cook, 1965). The function of these small, fixational saccades for vision has prompted much investigation; they might serve to counter visual fading (Engbert & Mergenthaler, 2006; Martinez-Conde, Macknik, Troncoso, & Dyar, 2006), correct fixation error (Cornsweet, 1956; Engbert & Kliegl, 2004; Engbert & Mergenthaler, 2006; Guerrasio, Quinet, Buttner, & Goffart, 2010), and/or sample visual information at a fine spatial scale (Ko, Poletti, & Rucci, 2010; McCamy, Otero-Millan, Di Stasi, Macknik, & Martinez-Conde, 2014; Otero-Millan, Macknik, Langston, & Martinez-Conde, 2013; Rucci, Iovin, Poletti, & Santini, 2007). The rate and direction of fixational saccades are modulated by sensory processes. For example, the rate of fixational saccades (as well as large saccades) decreases following a visual transient, a phenomenon called “microsaccadic/saccadic inhibition” (Reingold & Stampe, 2002; Rolfs, Kliegl, & Engbert, 2008a; Stampe & Reingold, 2002; Valsecchi & Turatto, 2007). The rate and direction of fixational saccades are also modulated by cognitive processes, including covert shifts of attention (Brien, Corneil, Fecteau, Bell, & Munoz, 2009; Cui, Wilke, Logothetis, Leopold, & Liang, 2009; Engbert & Kliegl, 2003; Galfano, Betta, & Turatto, 2004; Hafed & Clark, 2002; Laubrock, Engbert, & Kliegl, 2005; Laubrock, Kliegl, Rolfs, & Engbert, 2010; Pastukhov & Braun, 2010; Poletti, Listorti, & Rucci, 2013; Rolfs, Engbert, & Kliegl, 2004; 2005) (see Discussion: Fixational saccade direction and attention).
Fixational saccades are motor expressions of neural activity on a continuous visuomotor map. The superior colliculus (SC), for example, topographically encodes the direction and amplitude of saccades (Carello & Krauzlis, 2004; McPeek & Keller, 2004; D. A. Robinson, 1972; Sparks, Holland, & Guthrie, 1976; Wurtz & Goldberg, 1972) and locations of behavioral relevance in the environment, including the locus of attention (Fecteau, Bell, & Munoz, 2004; Ignashchenkova, Dicke, Haarmeier, & Thier, 2003; Lovejoy & Krauzlis, 2010; Müller, Philiastides, & Newsome, 2005). In particular, neurons in the rostral pole of the SC are selective for the direction and amplitude of fixational saccades (Hafed & Krauzlis, 2012; Hafed, Goffart, & Krauzlis, 2009). Nonlinear competitive interactions have been well documented in the responses of SC neurons in a variety of species (Basso & Wurtz, 1997; Hafed & Ignashchenkova, 2013; Li & Basso, 2005; Munoz & Istvan, 1998; Munoz & Wurtz, 1993; Mysore, Asadollahi, & Knudsen, 2010; Vokoun, Huang, Jackson, & Basso, 2014). The responses of a neuron to a stimulus inside its response field (RF) are reduced by the simultaneous presentation of a second stimulus, either also inside the RF (Li & Basso, 2005) or spatially far apart (Basso & Wurtz, 1997; 1998). The nature of these competitive interactions between SC neurons has been modeled using weighted averaging and divisive normalization (e.g., Vokoun et al., 2014). These neural response properties have been shown to underlie a number of behavioral metrics in humans and monkeys, including the rate and direction of large saccades. The rate and direction of fixational saccades, therefore, might provide a complementary characterization of the competitive interactions in visuomotor maps.
We used visual stimulation as an experimental manipulation, and quantified how fixational saccades depended on interactions between pairs of visual stimuli. The task was to maintain fixation on a small central marker. A parafoveal stimulus was presented either alone or simultaneously with a peripheral stimulus. The contrast of the peripheral stimuli (when present) was held constant, while the contrast of the parafoveal stimuli was systemically varied. We measured saccade rate and direction during an epoch 100–250 ms after stimulus onset, i.e., a time interval during which microsaccadic/saccadic inhibition has been reported. We limited our analyses to small saccades (< 2 deg), which we defined as fixational saccades, i.e., saccades that occurred while observers were instructed to fixate centrally. Some of these saccades may have shifted gaze to the parafoveal stimuli. We adopted the term “fixational saccades” to avoid debate on the precise classification of microsaccades and their purpose; our present conclusions do not depend on distinguishing microsaccades from small, exploratory saccades.
We found that fixational saccades were biased toward the parafoveal stimuli, but they were suppressed by the simultaneous presentation of peripheral stimuli. The suppression was best characterized as a combination of two separate processes: a subtractive suppression of the overall fixational saccade rate and a divisive suppression of the direction bias. Specifically, there was a reduction in the overall rate of fixational saccades, similar to previous reports of microsaccadic/saccadic inhibition, when parafoveal stimuli were paired with a peripheral stimulus compared to when parafoveal stimuli were presented alone. This reduction in overall fixational saccade rate was independent of parofoveal contrast and it was modeled as subtractive. In addition, the relative proportion of fixational saccades toward parafoveal stimuli increased with parafoveal contrast, but less so when they were paired with a peripheral stimulus. This process was modeled as divisive. The divisive suppression of the direction bias could not be explained by the change in overall rate. We conclude that the suppression of fixational saccades induced by visual stimulation can be decomposed into two component processes, one subtractive and the other divisive, and we propose a framework in which these computational processes are performed in a visuomotor map like that in the SC.
Materials and Methods
Observers
Eight observers (three females, aged 24–33) with normal or corrected-to-normal vision participated in the study, including one of the authors. Three observers wore glasses while participating in the study. Six were experienced psychophysical observers. Observers provided written informed consent, and the experimental protocol was approved by the University Committee on Activities involving Human Subjects at New York University.
Apparatus, stimuli, and experimental procedure
Observers sat in the dark and were instructed to maintain fixation on a small gray central marker on a dark screen (0.2 deg diameter; 15.2 cd/m2; Weber contrast 1) for the duration of each experimental block, with head positioned on a chin rest to avoid large head movements that might cause artifacts in saccade detection. There was no additional task. Eye movements were recorded (1000 Hz, monocular) with an Eyelink 1000 infrared eye tracker (SR Research Ltd, Ontario, Canada) with a spatial resolution of 0.01 deg from sensor noise and 0.25-0.5 deg average accuracy when using a chin rest. A 9-point (grid) calibration was performed and validated at the start of each experimental block.
Stimuli were brief presentations of white, circular spots on a dark background (7.5 cd/m2). The spots appeared at two eccentricities (0.8 and 5 deg; “parafoveal” and “peripheral”, respectively) and one of the four cardinal locations (above, below, left or right of fixation) (Fig. 1A). There were four trial types: blank, parafoveal-alone, peripheral-alone, and paired parafoveal and peripheral. During the parafoveal-alone trials, a parafoveal stimulus (0.4 deg diameter) was presented at one of the four 0.8 deg locations. During the peripheral-alone trials, a peripheral stimulus (0.6 deg diameter) was presented at one of the four 5 deg locations. During the paired trials, a parafoveal stimulus was presented simultaneously with one of the peripheral stimuli (16 location combinations). Each stimulus was presented for 80 ms, followed by an inter-stimulus interval of 480 ms. A trial epoch (560 ms) was defined as a stimulus presentation plus an inter-stimulus interval, beginning with the onset of the stimulus. Some trials were blank, during which no stimulus was presented, while the fixation marker remained on the screen. Parafoveal stimulus location and contrast varied across trials (luminances: 8.3–75.4 cd/m2; Weber contrasts: 0.1–9), in randomly shuffled order. The peripheral stimuli, when present, were held at a constant contrast (luminance 37.6 cd/m2; Weber contrast 4). Each experimental block consisted of 480 trials (269 s). Each observer completed multiple experimental blocks spanning several days, yielding 13,000–20,000 trials per observer.
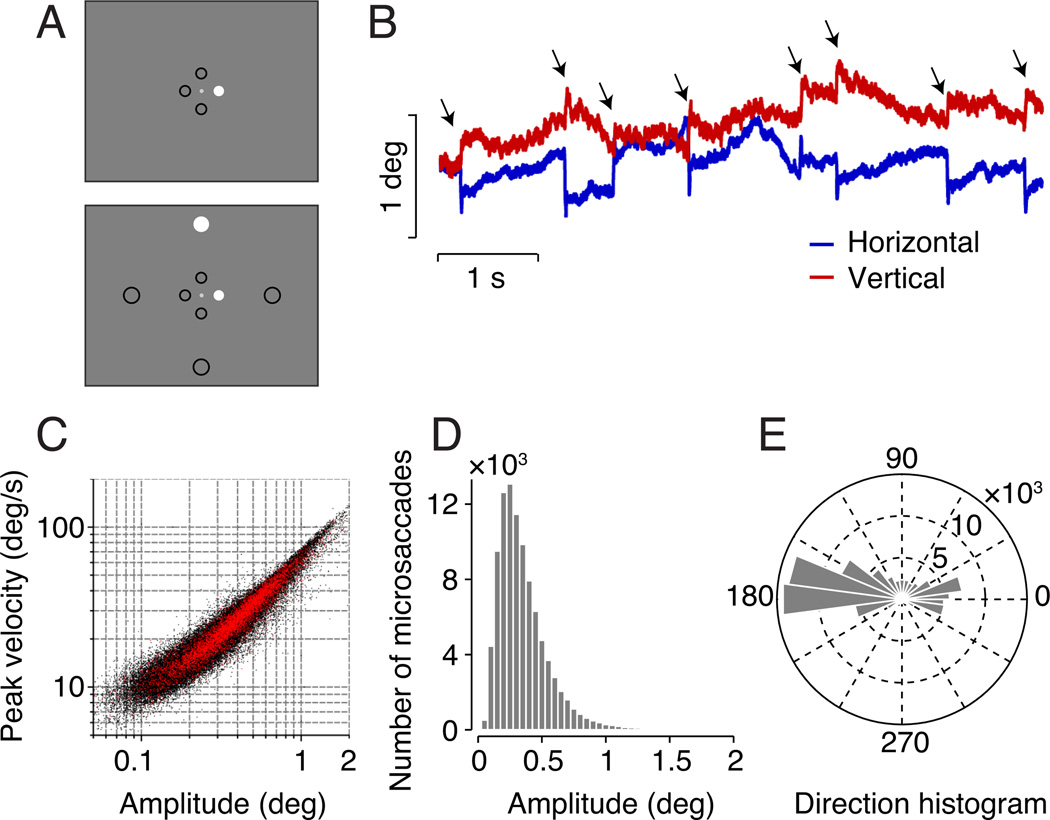
Figure 1.
Stimulus and fixational saccade statistics. A. Parafoveal and peripheral stimuli. Parafoveal stimuli (0.8 deg eccentricity) and peripheral stimuli (5 deg eccentricity) each appeared at one of four cardinal locations (above, below, left, or right of fixation), as denoted by black circles. Black circles did not appear in the actual stimulus. Top, a parafoveal stimulus was presented alone (right parafoveal stimulus shown). Bottom, a parafoveal stimulus was paired with a peripheral mulus (right parafoveal and top peripheral stimulus shown; 16 total possible combinations). Stimuli are schematic and not to scale. B. An example epoch of recorded eye position traces. Blue and red, horizontal and vertical eye positions, respectively. Arrows indicate fixational saccades. C. Main sequence. Peak velocity versus saccade amplitude from all observers (n = 8). Black, all fixational saccades included in the analysis (< 2 deg). Red, congruent fixational saccades during the 100–250 ms time epoch. D. Amplitude distribution of fixational saccades from all observers. E. Direction distribution of fixational saccades from all observers.
Stimuli were generated using MATLAB (Mathworks, MA) and MGL (http://justingarner.net/mgl) on a Macintosh computer and displayed on a 22” flat-screen CRT monitor (Hewlett-Packard p1230; resolution: 1152 × 870; refresh rate: 75 Hz) at a distance of 57 cm. The MGL Eyelink toolbox interfaced with the eye tracker. The monitor provided approximately 39 × 30 deg viewing angle. The display was calibrated and gamma-corrected using a linearized lookup table.
Saccade detection
Raw gaze positions were converted into degrees of visual angle, based on the 9-point (grid) calibration that was performed at the start of each experimental block. Blink intervals were defined according to the Eyelink blink detection algorithm along with samples from 200 ms preceding to 350 ms following each Eyelink-detected blink interval. Sample values during blink intervals were ignored for all subsequent analyses.
Saccades were detected using an established algorithm that compares eye-movement velocity with a threshold (Engbert & Kliegl, 2003; Engbert & Mergenthaler, 2006). The entire eye position trace from each block (after blink removal) was used for setting a saccade-detection velocity-threshold. A threshold criterion for saccade detection was determined based on the 2D (horizontal and vertical) eye-movement velocity during the block. Specifically, we set the threshold to be 7 times the standard deviation of the 2D eye-movement velocity, using a median-based estimate of the standard deviation (Engbert & Kliegl, 2003). A saccade was identified when the eye-movement velocity exceeded this threshold for 7 ms (7 consecutive eye-position samples). We also imposed a minimum intersaccadic interval (defined as the interval between the last sample of one saccade and the first sample of the next saccade) of 10 ms so that potential overshoot corrections were not considered new fixational saccades (McCamy et al., 2012; Møller, Laursen, Tygesen, & Sjølie, 2002). Eye velocity during blink intervals was ignored when calculating the threshold. The saccades identified in this way were manually inspected; 96% of automatically-detected saccades were verified by manual inspection. Saccade detection was unlikely to be affected by pupil size because pupil size changes slowly following stimulus onset (>1 −2 seconds; Wyatt, 2010). We limited our analyses to saccades less than 2 deg, which we defined as “fixational saccades.” We identified ~96,000 fixational saccades across observers (at a rate of 1.18 ± 0.07 Hz, mean ± SEM across observers).
Some fixational saccades might have been missed by the algorithm and were not recovered by manual inspection. This might have biased our results if there were more missed fixational saccades in some conditions versus others. The missed fixational saccades were likely to be small in amplitude and short in duration. Our main analysis was based on fixational saccade rate combined across fixational saccades of all amplitudes. Thus, the fixational saccade rates preferentially reflected fixational saccades of larger amplitudes, which were more reliable. The average amplitude of the detected fixational saccades in the time epoch of interest (100–250 ms after stimulus onset; see next section) did not significantly depend on parafoveal contrast, but did depend on stimulus configuration (parafoveal-only versus parafoveal+peripheral) and observer (three-way ANOVA, main effects of contrast: p = 0.67; stimulus configuration: p < 0.0001; observer: p < 0.0001). This suggested that more saccades might have been missed for some observers because of individual variability in saccade amplitude. However, the individual differences in saccade rates across observers were small (see previous paragraph). It also suggested that there might have been fewer saccades detected for the parafoveal+peripheral condition than for the parafoveal-only condition simply because saccade amplitudes were smaller. However, differences in saccade amplitudes between stimulus configurations were also small (median amplitude for parafoveal-only: 0.33 deg; parafoveal+peripheral: 0.31 deg). Thus, the missed fixational saccades were unlikely to have substantially affected our conclusions.
Eye movements (position samples and fixational saccades) for each experimental block were segmented and assigned to each trial. We defined a trial as containing a blink if a blink interval overlapped for at least 100 ms with the trial interval (from stimulus onset to 480 ms after stimulus onset). These blink trials were excluded from analyses (9.4 ± 5.5% of the overall trials; mean ± standard deviation across n = 8 observers). Trials with 100 ms or more of missing data (for any reason) were also excluded. Using a more stringent or looser criterion for excluding trials (i.e., shorter or longer than 100 ms) yielded similar results, supporting the same conclusions. We also excluded trials containing any large saccades (> 2 deg) from analyses (0.06 ± 0.1% of the overall trials; mean ± standard deviation across n = 8 observers). Analyzing trials containing only saccades < 1 deg or < 30 arcmin, or trials containing saccades of any size yielded similar results, supporting the same conclusions. Finally, we excluded the first 3 trials of each experimental block from analyses. Overall, we analyzed ~11,000–18,000 trials per observer. The range of trials was due to inherent inter-observer variability. The statistical analyses took into account the number of saccades per observer so as not to bias the results by observers with more saccades. Analyzing the data by equalizing the number of trials across observers yielded noisier but similar results, supporting the same conclusions.
Fixational saccade rate, congruent fixational saccade rate, and fraction of congruent fixational saccades
Fixational saccade rate was computed as a function of time for each observer by binning fixational saccades in 5 ms bins as a function of time after stimulus onset, averaging each bin across trials, smoothing the average time course with an 8-point binomial filter (i.e., 40 ms), and finally scaling it to a rate-per-second measure (Fig. 2A).
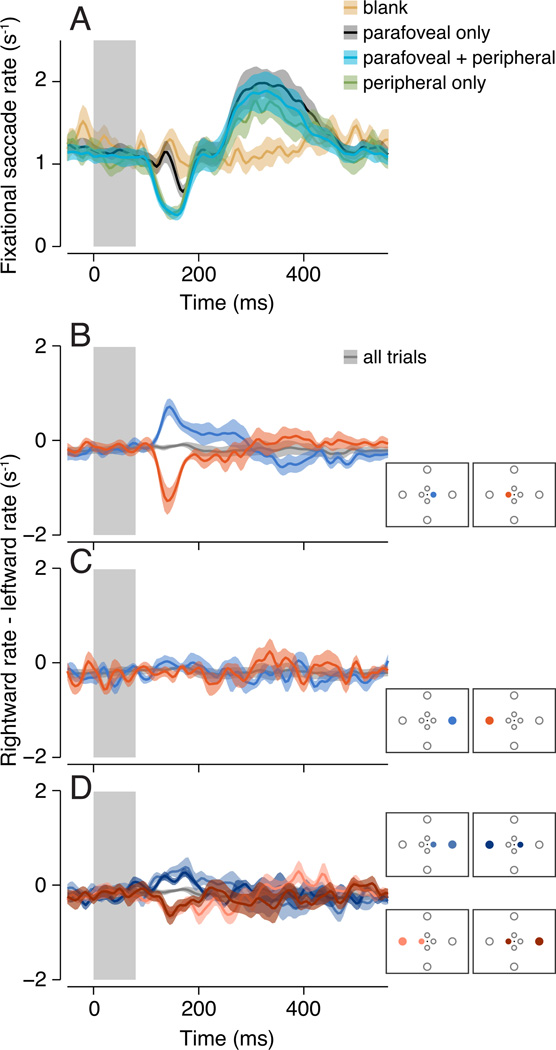
Figure. 2.
Modulation of fixational saccade rate and direction. A. Overall fixational saccade rate as a function of time after stimulus onset. Pale gray rectangle, stimulus presentation. Black, parafoveal stimuli presented alone. Cyan, parafoveal stimuli paired with a peripheral stimulus. Green, peripheral stimuli presented alone. Yellow, blank trials, during which no stimulus was presented. Rate was computed for each observer and then averaged across observers (n = 8). Shaded regions indicate SEM. B–D. Difference between rightward fixational saccade rate and leftward fixational saccade rate, as a function of time after stimulus onset. Same format as panel A. Positive values indicate observers made more rightward fixational saccades than leftward fixational saccade. Gray curves, rate difference across all trials (i.e., baseline bias in fixational saccade direction, identical in all 3 panels). Insets, stimulus configurations color-coded to correspond to each curve in each panel. Note that shaded error bars are similar for conditions in panels B–D, indicating comparable signal-to-noise ratios. B. Rate difference for trials in which right (blue) or left (red) parafoveal stimuli were presented alone. C. Rate difference for trials in which right (blue) or left (red) peripheral stimuli were presented alone. D. Rate differences for trials in which right (two blue curves) or left (two red curves) parafoveal stimuli were paired with peripheral stimuli either on the same (lighter shade curves) or opposite (darker shade curves) side.
The direction of each fixational saccade was computed as the angle between the eye position of the initial sample preceding the fixational saccade and the eye position at maximal displacement from the initial sample. Each fixational saccade was classified as rightward, leftward, upward, or downward depending on its direction (within ±45° around 0°, 180°, 90°, and 270°, respectively). Rates were computed separately for fixational saccades of each direction. Fixational saccade rates computed based on directions defined on narrower angular range (i.e., ±30° around the cardinal directions) yielded similar results, supporting the same conclusions. We defined a fixational saccade as “congruent” if its direction was toward the location the parafoveal stimuli.
In addition to computing saccade rates as a function of time (Fig. 2), summary rate metrics were computed for each stimulus configuration and parafoveal contrast across a time epoch of 100–250 ms (Fig. 3A,C, black and cyan curves), corresponding to the greatest change in fixational saccade direction (Fig. 2B). This was done for both the overall rate and the congruent rate. The overall fixational saccade rate for a particular stimulus configuration and contrast was computed by averaging across trials and across the 100–250 ms time epoch, and then averaging across observers. For each observer, the rate of congruent fixational saccades for each of the four parafoveal locations (i.e., rate of rightward fixational saccades for right parafoveal location, leftward fixational saccades for left parafoveal location, and so on) was similarly computed by averaging across trials and across the 100–250 ms time epoch for each observer. The rates for the four fixational saccade directions were averaged to yield a single congruent rate for each observer and then averaged across observers. Analyzing the data by restricting to only left and right congruent fixational saccades, the predominant fixational saccade directions, yielded similar results, supporting the same conclusions.
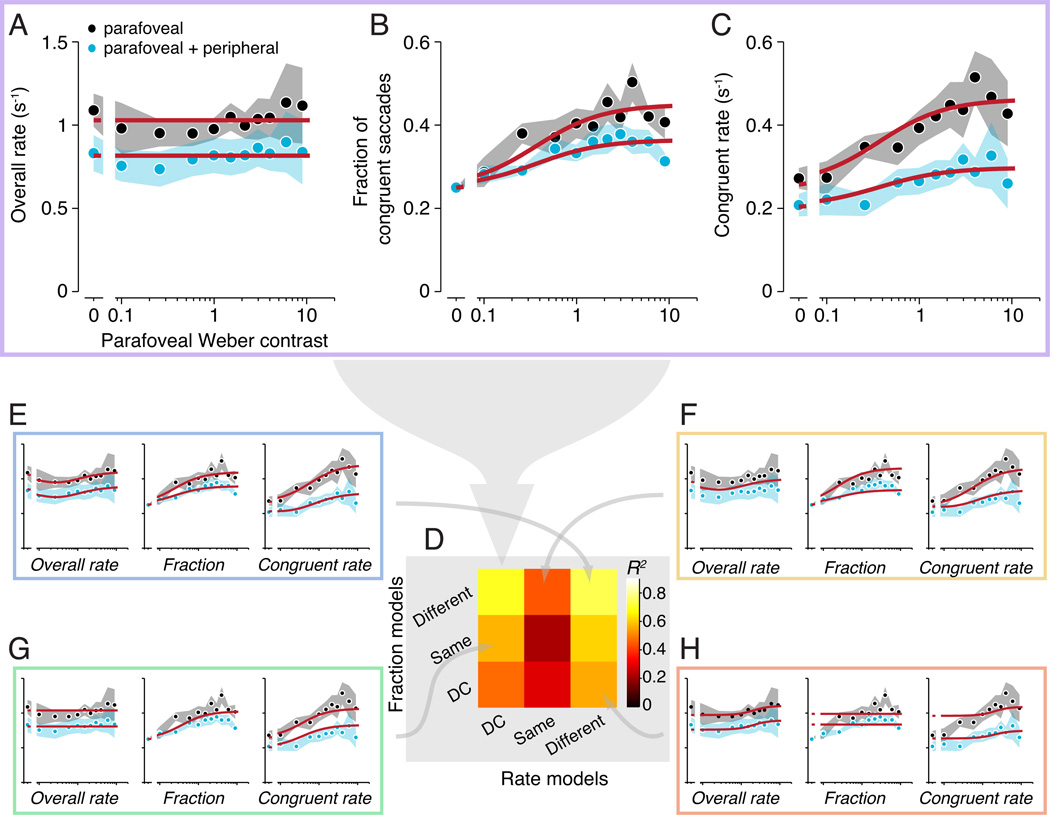
Figure 3.
Suppression of fixational saccades by peripheral stimuli. A–C. Fixational saccade rate during the 100–250 ms time epoch, as a function of parafoveal contrast. Black, parafoveal stimuli presented alone. Cyan, parafoveal stimuli paired with a peripheral stimulus. Circles and shaded regions, mean and SEM across observers. Red curves, best-fitting model (combined DC Rate and Different fraction model; see text). A. Overall fixational saccade rate (any direction) as a function of parafoveal contrast. B. Fraction of congruent fixational saccades as a function of parafoveal contrast. Leftmost points (parafoveal contrast = 0) correspond to a baseline fraction of 1/4. C. Congruent fixational saccade rate as a function of parafoveal contrast. Leftmost points (parafoveal contrast = 0) correspond to baseline congruent rates (overall rates multiplied by 1/4). D. Goodness-of-fit (R2) for every combination of fraction and rate models (3 each, yielding 9 combined models). Colors indicate median R2 (across 1000 permutations of cross validation; see Materials and Methods: Model fitting). Large gray arrow, model shown as the red curves in panels A–C. E–H. Combined model fits. Gray arrows indicate the corresponding model in the matrix. Identical data, axes, and format as panels A–C.
The fraction of congruent fixational saccades (which we abbreviate as “fraction”) was also computed as a function of parafoveal contrast (Fig. 3B). For each observer, we estimated the number of congruent fixational saccades for each of the parafoveal locations across the 100–250 ms time epoch and across trials, and divided that by the total number of fixational saccades (of any direction) from the same time epoch and same trials. The fractions in the four fixational saccade directions were then averaged for each observer and averaged across observers.
The baseline overall rate was defined as the fixational saccade rate when the parafoveal contrast was zero (blank and peripheral-alone conditions; Fig. 3A, leftmost points). The baseline fraction was defined to be the fraction of congruent fixational saccades in the limit as the parafoveal contrast went to zero (Fig. 3B, leftmost points corresponding to contrast 0). By this definition, the baseline fraction in main version of the analysis (directional saccades defined to be ±45° around the cardinal directions) was 0.25, reflecting the four directions. The baseline congruent rate was defined analogously as the rate of congruent fixational saccades in the limit as the parafoveal contrast went to zero (Fig. 3C, leftmost points). It was computed by multiplying the measured baseline overall rate by the baseline fraction (0.25).
For most analyses, we combined across peripheral locations for each parafoveal location because differences between the peripheral locations were small. Some analyses were performed by treating the different peripheral locations separately. In those analyses, for each parafoveal location, a peripheral location was referred to as “same” if it was at the same polar angle as the parafoveal stimulus (e.g., a right peripheral stimulus paired with a right parafoveal stimulus), “opposite” if it was 180° away from the parafoveal location (e.g., a left peripheral stimulus paired with a right parafoveal stimulus), or “orthogonal” if it was 90° away from the parafoveal location (e.g., a up or down peripheral stimulus paired with a right parafoveal stimulus).
Return saccades
Fixational saccades tended to be followed shortly by a subsequent fixational saccade in the opposite direction (Suppl. Fig. 1A). The larger the initial fixational saccade the more likely it was to be followed by a return saccade (Suppl. Fig. 1B). This suggests many fixational saccades occurred to correct the current fixation error introduced by previous fixational saccades or drift, consistent with previous reports (Cornsweet, 1956; Engbert & Kliegl, 2004; Otero-Millan et al., 2011b; Poletti & Rucci, 2010). Therefore, the average gaze position did not move toward the parafoveal stimulus as its contrast increased.
A complementary multiple regression analysis quantified the congruent fixational saccade rates while discounting return saccades, some of which might have been averaged in with the stimulus-evoked fixational saccades in the main analysis (see Supplementary Materials). The regression analysis accounted for the fixational saccades on each trial with a combination of the stimulus on that trial and the eye position preceding the fixational saccade, thus ensuring that the return saccades did not confound the interpretation of the results. The results of this regression analysis supported the same conclusions (Suppl. Fig. 2).
Model fitting
We quantified the nature of suppression by the peripheral stimuli on the parafoveal stimuli. The number of congruent fixational saccades depended on the overall rate of fixational saccades and the direction of those fixational saccades; a change in the rate of congruent fixational saccades could therefore reflect either or both of these factors (Fig. 4). The suppressive effect on these two processes was quantified by fitting models to describe the overall rate, congruent rate, and the fraction simultaneously. There were two curves (each as a function of parafoveal contrast) for each of these quantities, corresponding to the two stimulus configurations: parafoveal-alone and parafoveal+peripheral. The equations for the models are outlined in Table 1.
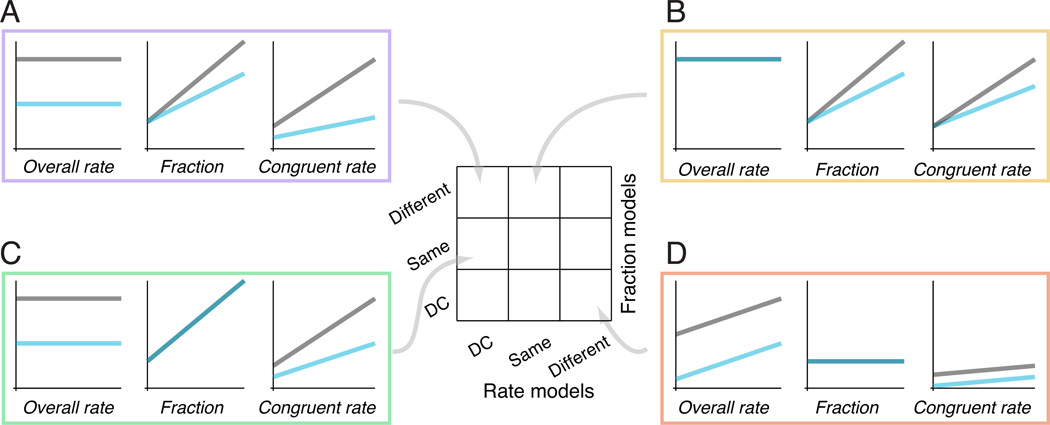
Figure 4.
Schematic showing that the suppression of congruent rate (right panels in A– D) could reflect two independent processes: a suppression of the overall fixational saccade rate (left panels) and a change in the fraction of congruent fixational saccades (middle panels) as a function of parafoveal contrast. Center matrix, all possible combinations of fraction and rate models. Gray arrows indicate corresponding model in the matrix. A. DC Rate and Different Fraction. Gray, parafoveal stimulus alone. Cyan, parafoveal stimuli paired with a peripheral stimulus. A subtractive suppression of the overall rate (left), and an increasing fraction as a function of parafoveal contrast (with a shallower slope for the parafoveal+peripheral condition; middle), correspond to a subtractive+divisive suppression of the congruent rate (right). B. Same Rate and Different Fraction. No suppression of the overall rate, and an increasing fraction as a function of parafoveal contrast (with a shallower slope for the parafoveal+peripheral condition), correspond to a purely divisive suppression of the congruent rate. C. DC Rate and Same Fraction. A subtractive suppression of the overall rate, and an increasing fraction as a function of parafoveal contrast (same for both stimulus configurations), correspond to a subtractive+divisive suppression of the congruent rate. D. Different Rate and DC Fraction. A subtractive suppression of the overall rate, and a constant fraction as a function of parafoveal contrast, correspond to a purely subtractive suppression of the congruent rate.
Table 1.
Equations used for fitting congruent rate, overall rate, and fraction of congruent fixational saccades. Subscripts 1 and 2 indicate parafoveal-only and parafoveal+peripheral conditions, respectively. f1(c) and f2(c), fraction of congruent fixational saccades as a function of parafoveal contrast c. Ro1(c) and Ro2(c), overall rate of fixational saccades. Rc1(c) and Rc2(c), rate of congruent fixational saccades. Rc1,2(c) = Ro1,2(c)f1,2(c) for each combination of rate and fraction models.
| Type of data | DC Model | Same Model | Different Model | ||
|---|---|---|---|---|---|
| Fraction of congruent fixational saccades |
f1(c) = b1 f2(c) = b2 |
f2(c) = f1(c) |
|
||
| 2 parameters: (b1, b2) |
3 parameters: (f, n, f) |
4 parameters: (f1, f2,n,f) |
|||
| Congruent Rate |
Rc1(c) = 1f1(c) Rc2(c) = 2f2(c) |
Rc1(c) = Rcm/ (cm + Rm) + Rc2(c) = Ro2(c)f2(c) = Rc1(c)f2(c)/f1(c) |
Rc1(c) = R cm/ (cm + Rm) + Rc2(c) = Ro2(c)f2(c) = Rc1(c)f2(c)/f1(c) + f2(c) |
||
| Overall Rate |
Ro1(c) = 1 Ro2(c) = 2 |
Ro1 (c) = Rc1(c)/f1(c) Ro2 (c) = Ro1(c) |
Ro1 (c) = Rc1(c)/f1(c) Ro2(c) = Ro1(c) + |
||
| 2 parameters: (1, 2) |
4 parameters: (R, m, R,) |
5 parameters: (R, m, R, ,) |
|||
DC Fraction model, parameters b1 and b2 specify f1 and f2, respectively. Same Fraction model, parameters fn, f correspond to the gain, exponent, and semi-saturation, respectively, of a Nara-Rushton function (shared for f1 and f2). Different Fraction model, f1, and f2 correspond to the gain of f1 and f2, respectively; n, f correspond to the exponent, and semi-saturation (shared for f1 and f2). DC Rate model, parameters 1 and 2 specify the overall rates. Same Rate model and Difference Rate models, parameters R, m, R, and correspond to the gain, exponent, semi-saturation, and baseline, respectively, of the Nara-Rushton function that specify one of the congruent rate curves Rc1(c). The other three curves, Rc2(c), (Ro1(c), and Ro2(c), are specified relative to that function. Same Rate model, Ro1(c), = Ro2(c). Different Rate model, Ro1(c) and Ro2(c) differ by a constant.
The fraction of congruent fixational saccades provided an estimate for how fixational saccade direction depended on the stimulus. The fraction might be constant as a function of parafoveal contrast (“DC Fraction” model) or might increase with parafoveal contrast. It might increase similarly for conditions with or without peripheral stimuli (i.e., no suppression; “Same Fraction” model) or do so differently for the two stimulus configurations (“Different Fraction” model). The DC Fraction model contained two parameters: f 1,2(c) = b1,2, where f 1,2(c) was the fraction as a function of parafoveal contrast for the two stimulus configurations, and b1,2 were parameters that were fit to the data. The Same Fraction model was specified with a Naka-Rushton function: f1,2(c) = fCn/, where f was the asymptotic fraction, f and n determined the semi-saturation contrast and the steepness of the curve. The baseline bf corresponded to the average fraction of fixational saccades in each direction for c = 0; it was fixed at 0.25 in the main version of the analysis, reflecting the baseline fraction when averaged across the 4 directions. The value bf was allowed to vary for other versions of the analysis (e.g., that involved only the horizontal fixational saccades), because the baseline fraction was not necessarily 0.25. In the Different Fraction model, we used the same Naka-Rushton function but using two separate gain parameters f1and f2 for the two curves, while fixing the other two parameters n and f between the two curves. The DC Fraction model and the Same Fraction model were each a subtype of the Different Fraction model.
Similarly, the overall fixational saccade rate might or might not change systematically as a function of the stimulus. We defined three models to describe the overall fixational saccade rate: a “DC Rate” model, in which the overall fixational saccade rate was constant as a function of parafoveal contrast, a “Same Rate” model, in which the overall fixational saccade rate varied with parafoveal contrast but were the same for two stimulus configurations, and a “Different Rate” model, in which the variation in rate differed by a constant offset between the two stimulus configurations. The DC Rate and Same Rate models were each a subtype of the Different Rate model. Because the congruent rate was simply the overall rate multiplied by the fraction of congruent fixational saccades, the model predictions for the congruent rates were computed from the model predictions for the overall rate and for the fraction. Specifically, for each model, Rc1,2(c) = R o1,2(c) f 1,2(c), where R o1,2(c) and Rc1,2(c) were the overall and congruent fixational saccade rates, respectively, as a function of parafoveal contrast for the two stimulus configurations (see Table 1).
The models were fit simultaneously to 6 curves: congruent rate, overall rate, and fraction (averaged across observers) as a function of parafoveal contrast, for each of the two stimulus configurations (parafoveal-alone and parafoveal+peripheral). Although the congruent rate, overall rate, and fraction depended on one another, we used all 6 rather than 4 curves to constrain the fits, because it ensured that squared errors between measurements and predictions were equally distributed across all three sets of quantities. For example, prediction for the congruent rate might have been obtained by only minimizing the summed squared error for the overall rate and the fraction, and then multiplying the predicted overall rate by the predicted fraction. However, this might have produced a poor prediction for the congruent rate compared to directly minimizing its squared error, because squared errors produced from fits to other two quantities were multiplied rather than summed. For the main version of the analysis, the fits excluded the fraction at parafoveal contrast = 0, because these data points were fixed at 0.25 by definition (see two paragraphs above).
Each of the fraction models was paired with each of the rate models (Table 1), yielding a total of 9 possible combined models. For example, the DC Fraction model (with two parameters, b1, b2), when paired with the Different Rate model (with 5 parameters, R, m, R, ,), yielded a total of 7 parameters for the combined model. For each model, we used a constrained nonlinear optimization routine (fmincon function in MATLAB) to numerically solve for the values of the free parameters that minimized the summed squared error between the predicted and measured quantities (Fig 3, red curves).
A split-halves cross validation procedure assessed the goodness of fit for each of the 9 combined models (Fig. 3D). The data were partitioned into training and test sets, each containing half of the data. Because observers had unequal numbers of trials, the trials for each observer were randomly split into training and test halves, each of which was separately combined across observers. For each of the 9 combined models, we estimated the best-fitting model parameters to the congruent rate, overall rate, and fraction obtained from the training data, and used those parameters to predict the congruent rate, overall rate, and fraction obtained from the test data. The predicted quantities were compared with the measured quantities from the test data to yield a goodness-of-fit measure R2 (coefficient of determination, or percentage of variance explained by the fit) for each set of test curves (congruent rate, overall rate, fraction). The R2 for the three sets of curves were averaged to yield a single R2. We opted to use the average R2 rather than a single combined R2, because the curves had different overall variances and the combined R2 would have overweighted the curves with larger variances. The procedure was repeated 1000 times, each with a different random repartition of the data, yielding a distribution of R2 for each of the models. We determined whether model m1 described the data statistically better than another model m2 by evaluating the proportion of R2 differences between the two models (R2m1 − R2m2) that was greater than 0. A p value was determined as the fraction of R2 differences that was smaller than 0.
Results
We studied how fixational saccades depended on spatial locations and contrasts of pairs of visual stimuli. Observers maintained fixation, while parafoveal and peripheral stimuli were flashed briefly at regular intervals at each of four cardinal locations (Fig. 1A). A parafoveal stimulus was presented either alone (Fig. 1A top) or simultaneously with one of the peripheral stimuli (Fig. 1A bottom). On some trials, no stimulus was presented, or the peripheral stimuli were presented alone, which served as baseline conditions. Eye movements were recorded to detect saccades following each stimulus presentation (Fig. 1B).
The detected saccades were characterized by the typical linear relationship between peak velocity and amplitude on a log-log plot (Fig. 1C) (Zuber et al., 1965). Most of the saccades were small in amplitude (Fig. 1D; 98.4% within 1 deg, and 99.9% within 2 deg), consistent with the range reported in the literature for fixational saccades (Engbert, 2006; Martinez-Conde et al., 2013), and confirming stable and accurate fixation across observers. The amplitude distribution of our fixational saccades matched the amplitude distribution of microsaccades reported previously (e.g., Fig. 1a in Martinez-Conde et al., 2013). We limited our analyses to saccades less than 2 deg, which we defined as fixational saccades. Analyses performed by restricting to only saccades less than 1 deg, or less then 30 arcmin, or by using all saccades yielded similar results, supporting the same conclusions. Most of the fixational saccades (~80%) were along the horizontal direction (Fig. 1E), as sometimes reported in literature (Engbert & Kliegl, 2003; Laubrock et al., 2005; Rolfs et al., 2005).
Fixational saccade rate and direction as a function of time
Fixational saccade rate evolved systematically as a function of time after the stimulus onset (Fig. 2A). Fixational saccade rate increased at 250–400 ms (peaking around 300 ms) after the onset of a stimulus, before returning to baseline at around 500 ms. When a peripheral stimulus was presented, fixational saccade rate also decreased about 150 ms after stimulus presentation (Fig 2A, green and cyan curves). The average saccade rate between 100–250 ms was significantly lower for the parafoveal+peripheral condition (p < 0.001; randomization test) and for the peripheral-alone condition (p < 0.001; randomization test) compared to the blank condition. This rate modulation time course, with an early rate decrease below baseline followed by a later rate increase, was consistent with that widely reported in previous studies (Engbert & Kliegl, 2003; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2004; 2008a). In comparison, when parafoveal stimuli were presented alone, the early decrease in fixational saccade rate was small (Fig. 2A, black curve; average rate difference = 0.08 Hz at 100–250 ms for parafoveal-only versus blank conditions, p = 0.02). Fixational saccade rate for the blank trials did not show an early decrease or a late increase. Although one concern was that the fixed inter-trial interval might have modulated fixational saccade rate due to the expectation of a predictable upcoming stimulus (Hafed, Lovejoy, & Krauzlis, 2011; Pastukhov & Braun, 2010), such modulation, even if substantially larger than what was observed, would be unlikely to affect the interpretation of our results as it would be present across all trial types.
Across all trials observers made somewhat more leftward than rightward fixational saccades (Fig. 2B–D, negative value indicated by the gray curves), consistent with the leftward direction bias observed in the overall fixational saccade direction histogram (49% of all saccades were leftward versus 32% rightward; Fig. 1E). This relative difference in rate remained constant as a function of time after stimulus onset, and served as a baseline for measuring the modulation of fixational saccade direction as a function of stimulus location.
When parafoveal stimuli were presented alone, observers made more fixational saccades in the direction of the parafoveal stimuli at around 100–250 ms (Fig. 2B). That is, when the parafoveal stimuli were presented on the right, the rate difference became positive relative to the baseline (Fig 2B, blue curve; p < 0.001, randomization test), indicating that observers preferentially made more fixational saccades to the right than to the left. Conversely, when the parafoveal stimuli were presented on the left, the rate difference became negative relative to baseline in the same time epoch, indicating that observers preferentially made more fixational saccades to the left (Fig. 2B, red curve; p < 0.001, randomization test). In comparison, when only peripheral stimuli were presented, observers did not systematically make more fixational saccades in the direction of peripheral stimuli (Fig. 2C; right peripheral stimulus versus baseline: p = 0.17; left peripheral stimulus versus baseline: p = 0.08).
Although the peripheral stimuli were ineffective in evoking fixational saccades toward them, they nonetheless suppressed direction-specific fixational saccades evoked by the parafoveal stimuli. When parafoveal stimuli were paired simultaneously with a peripheral stimulus, observers made more fixational saccades in the direction of the parafoveal stimuli (Fig. 2D), but the relative increase in direction-specific fixational saccades was smaller compared to when the parafoveal stimuli were presented alone (Fig. 2B). In addition, the direction bias was similar regardless of the relative location of the peripheral stimuli (same and opposite shown in Fig. 2D; orthogonal was similar but not shown).
A similar relationship was observed for upward and downward fixational saccades, though the modulation was smaller than that of horizontal fixational saccades, consistent with the observation that there were very few vertical fixational saccades compared to horizontal fixational saccades (Fig. 1E).
We defined a fixational saccade as “congruent” if its direction was toward the location of the parafoveal stimuli. Congruent fixational saccades tended to occur ~100–250 ms after stimulus onset (Fig. 2B,D; though some observers continued to exhibit an increased congruent fixational saccade rate until 350 ms after stimulus onset), around the same interval during which the overall fixational saccade rate decreased when peripheral stimuli were present (Fig. 2A). The overall rate of fixational saccades increased at a later time epoch after stimulus onset (Fig. 2A; ~300–400 ms), but most of those fixational saccades were not biased toward the location of the stimuli (parafoveal or peripheral). Instead, there was an increase in the rate of fixational saccades in the direction opposite to the parafoveal stimuli during the later time epoch, evident as a reversal of the rate difference around 300–400 ms (Fig. 2B). These fixational saccades might reflect return saccades that corrected fixation error introduced by the initial congruent fixational saccades (see Supplementary Materials: Return saccades). Some of these fixational saccades might have also been evoked by the stimulus, consistent with some previous reports (Galfano et al., 2004; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2004) that fixational saccades tended to be directed opposite the location of an attention cue (see Discussion: Fixational saccade direction and attention). We focused our analyses on the congruent fixational saccades during the earlier time epoch of 100–250 ms.
Fixational saccade rate and direction as a function of parafoveal contrast
The presentation of the peripheral stimuli suppressed the overall fixational saccade rate compared to when the parafoveal stimuli were presented alone (Fig. 3A). When the parafoveal stimuli were presented alone, the overall fixational saccade rate remained relatively constant as a function of the parafoveal contrast. When a peripheral stimulus was simultaneously presented, the overall fixational saccade rate was suppressed relative to the baseline rate of blank trials (Fig. 3A, cyan curve below black point at contrast 0). The decrease in the overall rate was similar for peripheral stimuli presented alone (Fig. 3A, leftmost cyan point) and for increasing levels of parafoveal contrast (contrast 0 versus highest contrast, p = 0.48, randomization test; rate versus contrast across observers, Pearson’s r = 0.05, p = 0.64). This suggests that the effect of the peripheral stimuli on the overall fixational saccade rate was approximately subtractive, leading to a constant decrease in fixational saccade rate at all parafoveal contrasts.
The rate and fraction of congruent fixational saccade depended systematically on parafoveal contrast. The rate of congruent fixational saccades increased with parafoveal contrast when the parafoveal stimuli were presented alone (Fig. 3C, black). Given that the rate of all fixational saccades remained constant with contrast, this indicated that observers made proportionally more fixational saccades toward the parafoveal stimuli with increasing parafoveal contrast. This was reflected by an increasing fraction of congruent fixational saccades (or simply, “fraction”) with parafoveal contrast (Fig. 3B, black). The baseline fraction, by definition, was 0.25 (Fig. 3B, leftmost point corresponding to contrast 0; see Materials and Methods: Fixational saccade rate, congruent fixational saccade rate, and fraction of congruent fixational saccades). The rate of congruent fixational saccades was suppressed when the parafoveal stimuli were paired with a peripheral stimulus (Fig. 3C, cyan). This suppression in rate was greater for increasing contrast. The small rate decrease evident at contrast 0 corresponded to 1/4 of the decrease in the overall rate of fixational saccades. The fraction also was suppressed when peripheral stimuli were presented with parafoveal stimuli (Fig. 3B), suggesting that observers not only made fewer fixational saccades, but also directed a smaller proportion of those saccades toward the parafoveal stimuli.
We also computed the relative fixational saccade rates, after subtracting the baseline fixational saccade rates for each observer before combining across observers. For our principal analyses, we focused on the raw fixational saccade rates, which were unadjusted for the baseline rates, because it made the modeling and fitting procedure easier. We confirmed that the variation in congruent fixational saccade rate as a function of parafoveal contrast was similar with or without adjusting for baseline, supporting the same conclusions.
We hypothesized that the processes of rate and direction were dissociable, and that the suppression of the congruent rate caused by the peripheral stimuli reflected a suppression on both processes (illustrated schematically in Fig. 4). The congruent fixational saccade rate at a particular parafoveal contrast depended on the overall rate of fixational saccades and the fraction of those fixational saccades that were congruent. The fraction provided an estimate for the amount of direction bias evoked by the stimuli, independent of the overall rate. The resulting suppression of the congruent rate might be purely subtractive (i.e., constant decrease in rate with parafoveal contrast; Fig. 4D), purely divisive (i.e., the amount of decrease proportional to rate; Fig. 4B), or a mix of the two (Fig. 4A,C).
Changes in the overall fixational saccade rate and fraction of congruent fixational saccades could combine in different ways to produce the changes in the congruent rate. A subtractive suppression of the overall rate would result in a subtractive component in the suppression of the congruent rate (Fig. 4A,C,D). An increasing fraction with parafoveal contrast would result in a divisive component in the suppression of the congruent rate (Fig. 4C), leading to a larger decrease in congruent rate with contrast. In addition, and critically, a suppression of the fraction would lead to a greater divisive suppression of the congruent rate (Fig. 4A,B; also compare 4A to 4C). Because the type of suppression on the congruent rate could be similar with or without a suppression of the fraction, testing for the suppression of the fraction would provide the key in distinguishing whether peripheral stimuli divisively interact with the effect of parafoveal stimuli in biasing fixational saccade direction, independent of their effect on the rate.
The suppressive effect of the peripheral stimuli was quantified by fitting models to the overall rate, congruent rate, and the fraction simultaneously (see Materials and Methods: Model fitting; Table 1). The models tested for the existence of both the suppression of the overall rate and the suppression of the fraction, thereby isolating the effect of the peripheral stimuli on each process. The models were fit to the measurements, averaged across observers, as a function of parafoveal contrast and corresponding to the two stimulus configurations (parafoveal-alone and parafoveal+peripheral). Three models for the overall rate (“DC”, “Same”, “Different” Rate models) and three models for the fraction (“DC”, “Same” and “Different” Fraction models) were tested, resulting in a total of 9 possible combined models. We assessed the goodness of fit for these models by performing split-halves cross validation (Fig 3D).
The data were best fit by a combination of the DC Rate model and Different Fraction model (Fig. 4A; Fig. 3A–C, red curves), indicating that the results were characterized by a suppression of both the overall rate and the fraction of congruent fixational saccades. In addition, there was no evidence that the overall fixational saccade rate varied as a function of parafoveal contrast. In one model instantiation, the overall rate was constant as a function of parafoveal contrast (Fig. 3A; “DC Rate” and “Different Fraction”). In another instantiation, the overall rate was allowed to vary as a function of parafoveal contrast (Fig. 3E; “Different Rate” and “Different Fraction”). The overall rate, in this case, was constrained by the curves for the congruent rate and the fraction, both of which increased monotonically with contrast (Table 1); but the overall rate itself might vary non-monotonically with contrast. Both of these models fit the data well (Fig. 3A – C versus 3E), and there was no statistical difference between these two models (p = 0.13). Since the DC Rate model was simply a subtype of the Different Rate model, we opted for the simpler model with fewer parameters.
We also tested categories of models that did not include a suppression of the overall rate (Fig. 3D, middle column, “Same Rate” models; Fig. 4B), that did not include a suppression of the fraction (Fig. 3D, middle row, “Same Fraction” models; Fig. 4C), and that had a constant fraction with contrast (Fig. 3D, bottom row, “DC Fraction” models; Fig. 4D). All fit the data poorly. The models that did not include any suppression of the overall rate were worst (Fig. 3D, middle column; Fig. 3F), indicating that the suppression of the overall rate accounted for a large portion of the overall variance. The models that had a constant fraction also did poorly (Fig. 3D, bottom row; Fig. 3H). The models that used the same fraction curve for the two stimulus configurations (Fig 3D, middle row; Fig 3G) did better than the above two model categories, indicating that the suppression of the fraction, if present, might have accounted for a relatively small portion of the overall variance (Fig. 3B, black versus cyan curves). This was expected because the fraction, by definition, was a bounded, unit-less measurement that differed from the rate measures, and had a smaller range in which the curves could vary. Nonetheless, a model with suppression of the fraction of the congruent fixational saccades yielded a statistically significant advantage over a model without (compare Fig. 3A – C with Fig. 3G; p = 0.007, randomization test), indicating significant suppression of the fraction (Fig. 3B, black versus cyan curves). Thus, these results indicate that peripheral stimuli induced a suppression of both the overall rate and the fraction of congruent fixational saccades. Neither change alone was sufficient to account for the data.
While we collapsed across peripheral locations for each parafoveal location in our principal analyses, there were some small but statistically significant differences between congruent rate modulations for the different peripheral locations relative to parafoveal locations (see Materials and Methods: Fixational saccade rate, congruent fixational saccade rate, and fraction of congruent fixational saccades). But these differences did not affect our conclusions. Specifically, parafoveal stimuli paired with a “same” peripheral stimulus evoked a higher congruent fixational saccade rate than those paired with a “opposite” or with a “orthogonal” peripheral stimulus (same versus opposite: p = 0.007; same versus orthogonal: p = 0.018; randomization test). Nonetheless, congruent rate for parafoveal stimuli paired with a “same” peripheral stimulus was still significantly suppressed compared to that for parafoveal stimuli presented alone (p < 0.001, randomization test). Congruent rate was not statistically different between parafoveal stimuli paired with opposite or with orthogonal peripheral stimuli (p = 0.23, randomization test). Additionally, model fits performed separately on each relative peripheral location yielded similar results as those obtained by combining across peripheral locations, supporting the same conclusions.
Altogether, these results revealed that visual stimuli affected fixational saccade rate and direction in different ways. Observers directed a greater proportion of fixational saccades toward the location of the parafoveal stimuli with increasing contrast, even as the overall rate of fixational saccades remained constant with parafoveal contrast. Thus, the effect of parafoveal contrast could be best characterized as increasing the direction bias without affecting the overall rate, leading to more congruent fixational saccades with parafoveal contrast. When the parafoveal stimuli were paired with peripheral stimuli, both direction bias and overall rate were suppressed, leading to a divisive+subtractive suppression of the congruent rate (Fig. 3C, red curves). This was evident as a decrease in the congruent rate with increasing parafoveal contrast, plus an additional, constant amount of suppression of congruent rate at all contrasts.
The suppression of the congruent rate (Fig. 3C) could be explained by three processes. First, the suppression of the overall rate was subtractive (Fig. 3A, red curves). Second, the fraction of congruent fixational saccades increased monotonically with parafoveal contrast (Fig. 3B), which by itself would have led to a divisive suppression of the congruent rate. Third, and importantly, the fraction was also suppressed by the peripheral stimuli (Fig. 3B, red curves), amplifying the divisive suppression of the congruent rate.
Discussion
We measured how fixational saccades depend on the spatial locations and contrast of pairs of visual stimuli while the observers’ only task was to maintain central fixation. Similarly to previous findings (Engbert, 2006; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2005), we found that the directions of fixational saccades were biased toward the parafoveal stimuli shortly following the presentation of a parafoveal stimulus (~100–250 ms post stimulus onset). We also replicated the finding that saccade rate (irrespective of direction) was suppressed during this time interval (Engbert & Kliegl, 2003; Laubrock et al., 2005; Rolfs et al., 2008a; Valsecchi & Turatto, 2007; Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008).
The main finding of this study is that when a peripheral stimulus was presented simultaneously with the parafoveal stimulus, the rate of congruent fixational saccades was suppressed, and this suppression increased with the contrast of the parafoveal stimulus. We quantified the nature of the suppression by separately modeling the overall fixational saccade rate and the fraction of congruent fixational saccades. Suppression of the congruent rate depended on the combination of two separate processes. 1) The overall rate of fixational saccades (irrespective of their direction) was suppressed following a stimulus. This change in rate remained constant with increasing parafoveal contrast and was modeled as subtractive. 2) The fraction of congruent saccades (out of all saccades) increased with parafoveal contrast, but less so when a peripheral stimulus was also presented. The influence of adding the peripheral stimulation was modeled as divisive. The stronger suppression of the congruent saccade rate with increasing parafoveal contrast was explained by the combined influences of both the subtractive and divisive processes but not by either alone.
Fixational saccade direction and attention
A large number of studies have documented a close interaction between fixational saccades and spatial attention (Brien et al., 2009; Engbert & Kliegl, 2003; Galfano et al., 2004; Hafed & Clark, 2002; Laubrock et al., 2005; 2010; Rolfs et al., 2004; 2005). A spatial attentional visual cue has been found to bias fixational saccade direction toward or away from the cue. The use of both central endogenous cues (Engbert & Kliegl, 2003; Laubrock et al., 2005) and peripheral exogenous cues (Brien et al., 2009; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2005) has been investigated. Attention was not directly manipulated in our experiment, so it is difficult to relate our results directly to previous results involving attention manipulation. Nonetheless, the stimuli may have evoked reflexive (exogenous) shifts of attention, and our results are qualitatively consistent with the timing and direction reported in attention studies involving exogenous attention cues (Engbert, 2006; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2005).
In our results, observers made congruent fixational saccades only to parafoveal but not peripheral stimuli, unlike many previous reports involving peripheral attention cues (Brien et al., 2009; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2005). In our experiment, unlike most attention experiments, observers were not required to make perceptual judgements of peripheral stimuli. Rather, observers were instructed to keep stable fixation, which encouraged them to maintain attention centrally; focused attention can render negligible the exogenous effect of irrelevant stimulus onsets in the periphery (Grubb, White, Heeger, & Carrasco, 2014; Tse, Sheinberg, & Logothetis, 2002). Consistent with this interpretation, one study also reported a lack of direction bias evoked by uninformative peripheral flashes (Tse et al., 2002). Conversely, another study reported that higher peripheral attention loads were associated with a larger direction bias (Pastukhov & Braun, 2010). Consequently, our results complement the view that fixational saccades indicate covert orienting of spatial attention (Engbert & Kliegl, 2003; Hafed & Clark, 2002).
Attention studies involving exogenous cues have frequently reported a late cue-incongruent effect (e.g., 300–500 ms), with more fixational saccades directed opposite the cued location (Brien et al., 2009; Galfano et al., 2004; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2004; 2005). We also observed a similar increase in the rate of fixational saccades in the direction opposite to the parafoveal stimuli following the increase in the congruent rate (Fig. 2B). These cue-opposite fixational saccades have been interpreted as reflecting inhibition of return (Posner & Cohen, 1984), in which fixational saccades are biased away from previously activated motor programs or attended locations (Brien et al., 2009; Galfano et al., 2004), or as reflecting overcompensation in inhibiting the automatic oculomotor capture by peripheral stimuli (Rolfs et al., 2004). Another possible interpretation is that most of these fixational saccades were return saccades that corrected fixation error introduced by the initial congruent fixational saccades, since consecutive fixational saccades tended to be opposite in direction (see Supplementary Materials: Return saccades). However, some attention studies have shown that cue-opposite fixational saccades could occur even without preceding cue-congruent fixational saccades (Galfano et al., 2004; Hafed & Clark, 2002; Laubrock et al., 2005; Rolfs et al., 2004), suggesting some fixational saccades might not have been fixation correcting.
Fixational saccade rate modulation
Previous studies have reported a systematic rate modulation after stimulus onset, with an early (~150 ms) decrease followed by a late (~350 ms) increase in rate (Engbert & Kliegl, 2003; Laubrock et al., 2005; Rolfs et al., 2008a; Valsecchi & Turatto, 2007; Yuval-Greenberg et al., 2008). It has been hypothesized (e.g., Hafed & Ignashchenkova, 2013) that this rate modulation is triggered by visual transients, not attention, because a similar rate modulation was observed with a task that did not require attention shifts (Engbert & Kliegl, 2003) or for tasks that had different attention requirements (e.g., exogenous versus endogenous; Laubrock et al., 2005). This suggests that changes in fixational saccade rate and direction reflect separate processes (Hafed & Ignashchenkova, 2013; Laubrock et al., 2005; Pastukhov & Braun, 2010). Our results complement that interpretation. We found that parafoveal stimuli increased the direction bias without affecting the overall rate in the 100–250 ms time epoch (Fig. 2B; Fig. 3A,B). In a later time epoch (~300–400 ms), although fixational saccade rate increased (Fig. 2A), there was little or no bias in fixational saccade direction toward the stimuli.
The early decrease in saccade rate has been termed “microsaccadic/saccadic inhibition” (Reingold & Stampe, 2002; Rolfs et al., 2008a; Stampe & Reingold, 2002; Valsecchi & Turatto, 2007) and has been documented for small fixational saccades as well as for large, voluntary/exploratory saccades after visual transients. It was proposed that the microsaccadic/saccadic inhibition might reflect a fast, reflex-like response of the oculomotor system to sudden changes in the visual scene (Engbert, 2012; Reingold & Stampe, 2002), which might serve to facilitate the processing of new visual information (Rolfs et al., 2008a). The physiological implementation of microsaccadic/saccadic inhibition is a matter of debate (see Possible neural basis below; Engbert, 2006; Hafed, 2011; Hafed & Ignashchenkova, 2013; Reingold & Stampe, 2002; Rolfs et al., 2008a; Stampe & Reingold, 2002). One model (Hafed & Ignashchenkova, 2013) has proposed that stimulus onsets might act like stop signals in classic saccadic countermanding tasks (Logan & Cowan, 1984; Verbruggen & Logan, 2009). According to this model, the early decrease in rate reflects cancelled fixational saccades, whereas the late increase in rate reflects a synchronized saccadic rhythm due to phase resetting. Additionally, the early, directional fixational saccades, like those we observed, reflected movements that the stimulus failed to cancel, perhaps because of spatially congruent activity caused by the visual stimulus in visuomotor maps like the SC (Hafed & Ignashchenkova, 2013).
We found that peripheral stimuli led to a subtractive suppression of fixational saccade rate,but it remains to be seen if and to what extent the suppression depends on the location of the stimuli. The critical test to distinguish between global and peripheral suppression, which has yet to be performed, is whether pairs of parafoveal stimuli might suppress fixational saccades toward one or the other of the two stimuli. In our results, when parafoveal stimuli were presented alone, fixational saccade rate remained relatively similar to the blank condition over the 100–250 ms time epoch (Fig. 3A, black). We speculate that fixational saccade rate during parafoveal-alone conditions can be explained by two factors: 1) any visual transient, regardless of eccentricity, triggered widespread (global) suppression, and 2) parafoveal stimuli caused a bias in fixational saccade direction toward the stimulus, which increased the likelihood of a fixational saccade. These two factors (global suppression plus parafoveal direction bias) would have resulted in little net change in fixational saccade rate during the 100–250 ms time epoch when parafoveal stimuli were presented alone (Fig. 3A). The time course of the fixational saccade rate also revealed that parafoveal stimuli led to a small and delayed rate suppression when they were presented alone (Fig. 2A), consistent with previous findings on saccadic inhibition caused by foveal stimuli (e.g., Rolfs et al., 2008a).
It also remains to be tested whether the size of the subtractive shift in fixational saccade rate would depend systematically on the contrast of the peripheral stimuli and/or the luminance and size of the fixation target. Previous results indicated that microsaccadic/saccadic inhibition was sensitive to the luminance of the visual transient (Rolfs et al., 2008a; Stampe & Reingold, 2002), but we tested only one peripheral contrast in the current experiment. Other studies have shown that the absolute rate of fixational saccades depends on the luminance and size of the fixation target (McCamy, Najafian Jazi, Otero-Millan, Macknik, & Martinez-Conde, 2013; Poletti & Rucci, 2010; Steinman, 1965).
Possible neural basis
Behavioral evidence shows that small, fixational saccades and large, exploratory saccades have similar properties (Otero-Millan et al., 2013; Otero-Millan, Troncoso, Macknik, Serrano-Pedraza, & Martinez-Conde, 2008; Rolfs, Laubrock, & Kliegl, 2006; 2008b; Zuber et al., 1965), and neurophysiological evidence indicates that both of these classes of saccades are controlled by the same neural circuitry (Goffart, Hafed, & Krauzlis, 2012; Hafed et al., 2009; Hafed & Krauzlis, 2012; Krauzlis, Basso, & Wurtz, 2000; van Gisbergen, Robinson, & Gielen, 1981). One key structure in the neural circuitry is the SC, which topographically encodes saccade locations in the contralateral visual hemifield (D. A. Robinson, 1972). Saccade direction and amplitude are continuously represented in the visuomotor map (D. A. Robinson, 1972), down to the smallest saccades. Neurons near the rostral pole of the SC selectively increase their activity for small saccades, and caudal neurons increase their activity analogously for large saccades (Hafed et al., 2009; Hafed & Krauzlis, 2012; Krauzlis et al., 2000; van Gisbergen et al., 1981). In addition, rostral SC neurons maintain tonic activity during fixation (Dorris, Paré, & Munoz, 1997; Hafed & Krauzlis, 2012; Krauzlis et al., 2000; Munoz & Wurtz, 1993). Like large saccades (Lee, Rohrer, & Sparks, 1988; Mcilwain, 1991), the representation for the amplitude and direction of a small, fixational saccade is hypothesized to be based on a population code, with direction and amplitude represented as the center of a mass of a “hill” of activity on the visuomotor map (Hafed, 2011; Hafed & Ignashchenkova, 2013). Analogous visuomotor maps are found in the lateral intraparietal area (Colby & Goldberg, 1999) and the frontal eye fields (Schall & Thompson, 1999); our behavioral results might be entirely explained by SC activity or by a combination of activity in all three maps.
We propose a framework for how a visuomotor population code like that in the SC might explain the results of our experiments. During fixation, the activity was centered on the foveal (rostral) region of the map. Parafoveal stimuli near fixation may have shifted the center of mass of the hill of activity, leading to a bias in the direction of fixational saccades toward the parafoveal locations. The shift may have been greater for larger parafoveal contrasts, resulting in an increase in the congruent fixational saccade rate. The peripheral stimuli in our experiment should have activated peripheral (caudal) locations in the map. The activity may have been insufficient to trigger a large saccade to the corresponding visual field location, but enough nonetheless to suppress activity near foveal (rostral) region of the map.
We observed that the fraction of congruent fixational saccades (i.e., the direction bias) evoked by parafoveal stimulation was suppressed by simultaneous presentation of peripheral stimuli. This interaction likely reflected suppressive interactions within a visuomotor map, possibly the SC. A number of physiological studies have quantified the nature of competitive interactions among neural populations within the SC (Basso & Wurtz, 1997; Li & Basso, 2005; Munoz & Istvan, 1998; Mysore et al., 2010; Vokoun et al., 2014). Population responses in the SC to paired visual stimulation are sub-additive, transitioning anywhere between averaging to winner-takes-all (Li & Basso, 2005; Vokoun et al., 2014). This activity has been shown to be well described by divisive normalization (Mysore et al., 2010; Vokoun et al., 2014), a computation ubiquitous in the visual system and throughout cortex (Busse, Wade, & Carandini, 2009; Carandini & Heeger, 2012). The readout of activity within the SC has been modeled to account for the location and latency of large saccades (Findlay & Walker, 1999; Godijn & Theeuwes, 2002; Kopecz, 1995; Marino, Trappenberg, Dorris, & Munoz, 2012; Trappenberg, Dorris, Munoz, & Klein, 2001; Vokoun et al., 2014). Our results here suggest that fixational saccades could depend on a similar computation. The suppression by peripheral stimuli might have interacted divisively with the responses evoked by parafoveal stimuli, increasing for larger parafoveal contrasts. Thus, the shift in the hill of activity caused by the parafoveal stimuli near fixation would have been suppressed by the peripheral stimuli.
We also observed that peripheral stimuli caused a subtractive suppression of the overall rate of fixational saccades. The precise mechanism for the triggering of fixational saccades, contributing to fixational saccade rate, remains unclear. Several proposals have been put forward (Hafed, 2011; Hafed & Ignashchenkova, 2013; Otero-Millan, Macknik, Serra, Leigh, & Martinez-Conde, 2011a; Rolfs et al., 2008a). One plausible explanation of our results is that the amplitude of activity at the center of mass of the visuomotor map was reduced by peripheral stimulation (Rolfs et al., 2008a). Perhaps in line with this proposal, pharmacological inactivation of the rostral SC has been shown to result in a decrease of fixational saccade rate (Hafed et al., 2009). Another possibility is that the amplitude of activity at the center of mass was largely unchanged, but that the threshold for evoking a saccade was increased (Hafed, 2011).
Besides saccade preparation, the SC is hypothesized to implement a “priority” or “salience” map (Corbetta et al., 1998; Kustov & Robinson, 1996), which also selects saccade targets (Carello & Krauzlis, 2004; McPeek & Keller, 2004) and the locus of attention in the absence of a saccade (Fecteau et al., 2004; Ignashchenkova et al., 2003; Lovejoy & Krauzlis, 2010; Müller et al., 2005). We probed interactions in visuomotor maps using visual stimulation as inputs and small, fixational saccades as outputs. Similar computations might also govern interactions caused by other types of input perturbations (e.g., attention, large saccade preparation), and other output behavioral metrics (e.g., perceptual sensitivity, saccade latency).
Supplementary Material
Fixational saccades depended on interactions between pairs of visual stimuli
Direction of saccades biased toward parafoveal stimuli with increasing contrast
Direction and rate suppressed by simultaneously presented peripheral stimuli
Subtractive suppression of saccade rate and divisive suppression of direction bias
Results characterize competitive interactions within visuomotor maps
Acknowledgments
Supported by NIH grant R01-EY019693 (D.J.H.), EU Marie Curie outgoing international fellowship (S.Y.G) and the Weizmann Institute Advancing Women in Science award (S.Y.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature. 1997;389(6646):66–69. doi: 10.1038/37975. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. Journal of Neuroscience. 1998;18(18):7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien DC, Corneil BD, Fecteau JH, Bell AH, Munoz DP. The behavioral and neurophysiological modulation of microsaccades in monkeys. J. Eye Mov. Res. 2009;3:1–12. [Google Scholar]
- Busse L, Wade AR, Carandini M. Representation of concurrent stimuli by population activity in visual cortex. Neuron. 2009;64(6):931–942. doi: 10.1016/j.neuron.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nature Reviews Neuroscience. 2012;13(1):51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43(4):575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annual Review of Neuroscience. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cornsweet TN. Determination of the stimuli for involuntary drifts and saccadic eye movements. J. Opt. Soc. Am. 1956;46(11):987–988. doi: 10.1364/josa.46.000987. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilke M, Logothetis NK, Leopold DA, Liang H. Vision Research. Vision Research. 2009;49(2):228–236. doi: 10.1016/j.visres.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. The Journal of Physiology. 1953;119(1):1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. Journal of Neuroscience. 1997;17(21):8566–8579. doi: 10.1523/JNEUROSCI.17-21-08566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R. Microsaccades: A microcosm for research on oculomotor control attention, and visual perception. Progress in Brain Research. 2006;154:177–192. doi: 10.1016/S0079-6123(06)54009-9. [DOI] [PubMed] [Google Scholar]
- Engbert R. Computational Modeling of Collicular Integration of Perceptual Responses and Attention in Microsaccades. Journal of Neuroscience. 2012;32(23):8035–8039. doi: 10.1523/JNEUROSCI.0808-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Research. 2003;43(9):1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades Keep the Eyes' Balance During Fixation. Psychological Science. 2004;15(6):431–436. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Engbert R, Mergenthaler K. Microsaccades are triggered by low retinal image slip. Proc. Natl. Acad. Sci. U. S. A. 2006;103(18):7192–7197. doi: 10.1073/pnas.0509557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. Journal of Neurophysiology. 2004;92(3):1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences. 1999;22(4):661–74. doi: 10.1017/s0140525x99002150. discussion 674–721. [DOI] [PubMed] [Google Scholar]
- Galfano G, Betta E, Turatto M. Inhibition of return in microsaccades. Experimental Brain Research. 2004;159(3):400–404. doi: 10.1007/s00221-004-2111-y. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. Journal of Experimental Psychology Human Perception and Performance. 2002;28(5):1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: evidence from superior colliculus inactivation. Journal of Neuroscience. 2012;32(31):10627–10636. doi: 10.1523/JNEUROSCI.0696-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, White AL, Heeger DJ, Carrasco M. Interactions between voluntary and involuntary attention modulate the quality and temporal dynamics of visual processing. Psychonomic Bulletin & Review. 2014 doi: 10.3758/s13423-014-0698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Buttner U, Goffart L. Fastigial Oculomotor Region and the Control of Foveation During Fixation. Journal of Neurophysiology. 2010;103(4):1988–2001. doi: 10.1152/jn.00771.2009. [DOI] [PubMed] [Google Scholar]
- Hafed ZM. Mechanisms for generating and compensating for the smallest possible saccades. European Journal of Neuroscience. 2011;33(11):2101–2113. doi: 10.1111/j.1460-9568.2011.07694.x. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Research. 2002;42(22):2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Ignashchenkova A. On the Dissociation between Microsaccade Rate and Direction after Peripheral Cues: Microsaccadic Inhibition Revisited. Journal of Neuroscience. 2013;33(41):16220–16235. doi: 10.1523/JNEUROSCI.2240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Krauzlis RJ. Similarity of superior colliculus involvement in microsaccade and saccade generation. Journal of Neurophysiology. 2012;107(7):1904–1916. doi: 10.1152/jn.01125.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;323(5916):940–943. doi: 10.1126/science.1166112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Lovejoy LP, Krauzlis RJ. Modulation of Microsaccades in Monkey during a Covert Visual Attention Task. Journal of Neuroscience. 2011;31(43):15219–15230. doi: 10.1523/JNEUROSCI.3106-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nature Neuroscience. 2003;7(1):56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Ko H-K, Poletti M, Rucci M. Microsaccades precisely relocate gaze in a high visual acuity task. Nature Neuroscience. 2010;13(12):1549–1553. doi: 10.1038/nn.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecz K. Saccadic reaction times in gap/overlap paradigms: A model based on integration of intentional and visual information on neural, dynamic fields. Vision Research. 1995;35(20):2911–2925. doi: 10.1016/0042-6989(95)00066-9. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. Journal of Neurophysiology. 2000;84(2):876–891. doi: 10.1152/jn.2000.84.2.876. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384(6604):74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, Kliegl R. Microsaccade dynamics during covert attention. Vision Research. 2005;45(6):721–730. doi: 10.1016/j.visres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Kliegl R, Rolfs M, Engbert R. When do microsaccades follow spatial attention? Attention, Perception & Psychophysics. 2010;72(3):683–694. doi: 10.3758/APP.72.3.683. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332(6162):357–360. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. Journal of Neuroscience. 2005;25(49):11357–11373. doi: 10.1523/JNEUROSCI.3825-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91(3):295. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nature Neuroscience. 2010;13(2):261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino RA, Trappenberg TP, Dorris M, Munoz DP. Spatial interactions in the superior colliculus predict saccade behavior in a neural field model. Journal of Cognitive Neuroscience. 2012;24(2):315–336. doi: 10.1162/jocn_a_00139. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience. 2004;5(3):229–240. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades Counteract Visual Fading during Fixation. Neuron. 2006;49(2):297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Otero-Millan J, Macknik SL. The impact of microsaccades on vision: towards a unified theory of saccadic function. Nature Reviews Neuroscience. 2013;14(2):83–96. doi: 10.1038/nrn3405. [DOI] [PubMed] [Google Scholar]
- McCamy MB, Najafian Jazi A, Otero-Millan J, Macknik SL, Martinez-Conde S. The effects of fixation target size and luminance on microsaccades and square-wave jerks. Peer J. 2013;1(23):e9. doi: 10.7717/peerj.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCamy MB, Otero-Millan J, Di Stasi LL, Macknik SL, Martinez-Conde S. Highly Informative Natural Scene Regions Increase Microsaccade Production during Visual Scanning. Journal of Neuroscience. 2014;34(8):2956–2966. doi: 10.1523/JNEUROSCI.4448-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCamy MB, Otero-Millan J, Macknik SL, Yang Y, Troncoso XG, Baer SM, et al. Microsaccadic efficacy and contribution to foveal and peripheral vision. Journal of Neuroscience. 2012;32(27):9194–9204. doi: 10.1523/JNEUROSCI.0515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain JT. Distributed spatial coding in the superior colliculus: a review. Visual Neuroscience. 1991;6(1):3–13. doi: 10.1017/s0952523800000857. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nature Neuroscience. 2004;7(7):757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. Journal of Neurophysiology. 1998;79(3):1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. Journal of Neurophysiology. 1993;70(2):559–575. doi: 10.1152/jn.1993.70.2.559. [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl. Acad. Sci. U. S. A. 2005;102(3):524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore SP, Asadollahi A, Knudsen EI. Global Inhibition and Stimulus Competition in the Owl Optic Tectum. Journal of Neuroscience. 2010;30(5):1727–1738. doi: 10.1523/JNEUROSCI.3740-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller F, Laursen ML, Tygesen J, Sjølie AK. Binocular quantification and characterization of microsaccades. Graefe. Arch. Clin. Exp. Ophthalmol. 2002;240(9):765–770. doi: 10.1007/s00417-002-0519-2. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Langston RE, Martinez-Conde S. An oculomotor continuum from exploration to fixation. Proc. Natl. Acad. Sci. U. S. A. 2013;110(15):6175–6180. doi: 10.1073/pnas.1222715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Macknik SL, Serra A, Leigh RJ, Martinez-Conde S. Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Annals of the New York Academy of Sciences. 2011a;1233(1):107–116. doi: 10.1111/j.1749-6632.2011.06177.x. [DOI] [PubMed] [Google Scholar]
- Otero-Millan J, Serra A, Leigh RJ, Troncoso XG, Macknik SL, Martinez-Conde S. Distinctive Features of Saccadic Intrusions and Microsaccades in Progressive Supranuclear Palsy. Journal of Neuroscience. 2011b;31(12):4379–4387. doi: 10.1523/JNEUROSCI.2600-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. Saccades and microsaccades during visual fixation, exploration, and search: Foundations for a common saccadic generator. Journal of Vision. 2008;8(14):21–21. doi: 10.1167/8.14.21. [DOI] [PubMed] [Google Scholar]
- Pastukhov A, Braun J. Rare but precious: Microsaccades are highly informative about attentional allocation. Vision Research. 2010;50(12):1173–1184. doi: 10.1016/j.visres.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Poletti M, Rucci M. Eye movements under various conditions of image fading. Journal of Vision. 2010;10(3):6.1–18. doi: 10.1167/10.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Listorti C, Rucci M. Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Current Biology. 2013;23(17):1691–1695. doi: 10.1016/j.cub.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. Attention and Performance X: Control of Language Processes. 1984;32:531–556. [Google Scholar]
- Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. Journal of Experimental Psychology. 1950;40(6):687. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in voluntary and reflexive saccades. Journal of Cognitive Neuroscience. 2002;14(3):371–388. doi: 10.1162/089892902317361903. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Research. 1972;12(11):1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Rolfs M. Microsaccades: Small steps on a long way. Vision Research. 2009;49(20):2415–2441. doi: 10.1016/j.visres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Microsaccade Orientation Supports Attentional Enhancement Opposite a Peripheral Cue: Commentary on Tse, Sheinberg, and Logothetis (2003) Psychological Science. 2004;15(10):705–707. doi: 10.1111/j.0956-7976.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Crossmodal coupling of oculomotor control and spatial attention in vision and audition. Experimental Brain Research. 2005;166(3–4):427–439. doi: 10.1007/s00221-005-2382-y. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Kliegl R, Engbert R. Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision. 2008a;8(11):5–5. doi: 10.1167/8.11.5. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Laubrock J, Kliegl R. Shortening and prolongation of saccade latencies following microsaccades. Experimental Brain Research. 2006;169(3):369–376. doi: 10.1007/s00221-005-0148-1. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Laubrock J, Kliegl R. Microsaccade-induced prolongation of saccadic latencies depends on microsaccade amplitude. J. Eye Mov. Res. 2008b;1(3):1–8. [Google Scholar]
- Rucci M, Iovin R, Poletti M, Santini F. Miniature eye movements enhance fine spatial detail. Nature. 2007;447(7146):851–854. doi: 10.1038/nature05866. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annual Review of Neuroscience. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Holland R, Guthrie BL. Size and distribution of movement fields in the monkey superior colliculus. Brain Research. 1976;113(1):21–34. doi: 10.1016/0006-8993(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Stampe DM, Reingold EM. Influence of stimulus characteristics on the latency of saccadic inhibition. Progress in Brain Research. 2002;140:73–87. doi: 10.1016/S0079-6123(02)40043-X. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Effect of target size, luminance, and color on monocular fixation. J. Opt. Soc. Am. 1965;55(9):1158–1164. [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181(4102):810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. Journal of Cognitive Neuroscience. 2001;13(2):256–271. doi: 10.1162/089892901564306. [DOI] [PubMed] [Google Scholar]
- Tse PU, Sheinberg DL, Logothetis NK. Fixational eye movements are not affected by abrupt onsets that capture attention. Vision Research. 2002;42(13):1663–1669. doi: 10.1016/s0042-6989(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Turatto M. Microsaccadic response to visual events that are invisible to the superior colliculus. Behavioral Neuroscience. 2007;121(4):786–793. doi: 10.1037/0735-7044.121.4.786. [DOI] [PubMed] [Google Scholar]
- van Gisbergen JA, Robinson DA, Gielen S. A quantitative analysis of generation of saccadic eye movements by burst neurons. Journal of Neurophysiology. 1981;45(3):417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience and Biobehavioral Reviews. 2009;33(5):647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokoun CR, Huang X, Jackson MB, Basso MA. Response Normalization in the Superficial Layers of the Superior Colliculus as a Possible Mechanism for Saccadic Averaging. Journal of Neuroscience. 2014;34(23):7976–7987. doi: 10.1523/JNEUROSCI.3022-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. Journal of Neurophysiology. 1972;35(4):575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ. The human pupil and the use of video-based eyetrackers. Vision Research. 2010;50(19):1982–1988. doi: 10.1016/j.visres.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient Induced Gamma-Band Response in EEG as a Manifestation of Miniature Saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Cook G. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science. 1965;150(3702):1459–1460. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.