Presentation
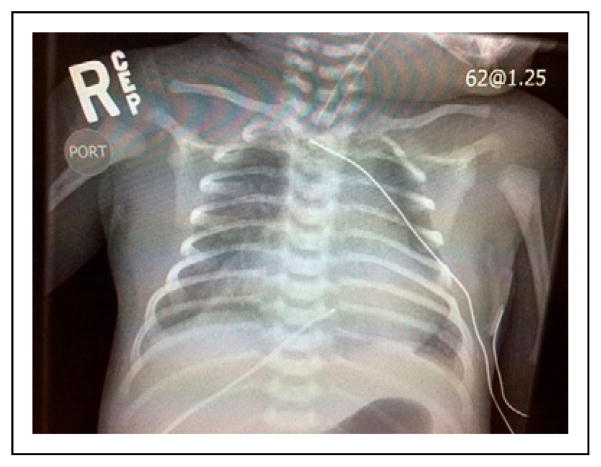
A previously healthy male infant born at 39 weeks’ gestation to a 33-year-old, gravida 1 para 1 mother presents to an outside hospital with respiratory distress at age 10 days. The infant was delivered via cesarean delivery for failure to progress, with Apgar scores of 8 and 9 at 1 minute and 5 minutes, respectively. His nursery stay was uneventful, and he was discharged home on day 2 after birth. During the next several days, he developed feeding difficulty and increased work of breathing. At the outside hospital emergency department, rectal temperature is 98.8°F (37.1°C), heart rate is 170 beats per minute, blood pressure is 105/74 mm Hg, respiratory rate is 78 breaths per minute, and oxygen saturation is 84% on room air. Reported physical examination findings are otherwise unremarkable. Continuous positive airway pressure with pressure support breaths is initiated. Electrocardiography reveals sinus tachycardia. Initial laboratory evaluation reveals the following: pH 6.8; bicarbonate, 7 mEq/L (7 mmol/L); base deficit, −27 mEq/L (−27 mmol/L); lactate, 153 mg/dL (17 mmol/L); anion gap, 29 mEq/L (29 mmol/L); white blood cell count, 27,000/μL (27.0 × 109/L), with normal differential; hematocrit, 57% (0.57); C-reactive protein, 1.0 mg/L (9.5 nmol/L); and glucose, 39 mg/dL (2.2 mmol/L). The patient is intubated. Chest radiography is performed (Fig 1). Dextrose, normal saline, bicarbonate, and broad-spectrum antibiotics are given for presumed sepsis, and the patient transfers to our neonatal intensive care unit for further evaluation and management.
Figure 1.
Chest radiograph of the infant.
On arrival of the infant at our institution, the genetics service is consulted, and metabolic workup is initiated immediately, including measurement of serum ammonia, acylcarnitine profile, plasma amino acids, and urine orotic and organic acids. Ampicillin, cefotaxime, and intravenous fluids are administered. The diagnosis is made after performing a thorough physical examination.
Discussion
One of the most common causes of lactic acidosis and respiratory distress in a neonate is septic shock. However, the report from the transferring hospital was more consistent with an alternative diagnosis for several reasons. First, the laboratory study results were inconsistent with sepsis, including a normal C-reactive protein level and a normal white blood cell differential. C-reactive protein measurement is a highly sensitive test with an outstanding negative predictive value for ruling out infection. Second, the report of elevated blood pressure from the outside hospital was not consistent with sepsis. Septic shock is characterized by a systemic inflammatory response syndrome and hypotension rather than hypertension. Third, the lactic acidosis appeared to be out of proportion to the patient’s clinical status. As a result, we favored a metabolic disorder over sepsis, especially in the setting of hypoglycemia at the outside hospital.
We continued broad-spectrum antibiotics and initiated workup for metabolic disorders at our institution. However, the findings of physical examination on admission were notable for preductal oxygen saturation (97%), postductal oxygen saturation (85%), regular rate and rhythm, 2/6 systolic ejection murmur heard best over the left upper sternal border, normal S1, physiologic split S2, and an S3 gallop. The liver was palpable 1 cm below the costal margin. Radial pulses were prominent bilaterally, but femoral pulses were diminished. Right upper extremity blood pressure was 120/75 mm Hg, and right lower extremity blood pressure was 43/19 mm Hg. Dopamine and prostaglandin E1 infusions were initiated, and a pediatric cardiologist was consulted. Echocardiography revealed severe aortic coarctation, tiny (1-mm) patent ductus arteriosus, and severely depressed biventricular systolic function (Fig 2). Shortly after initiation of dopamine and prostaglandin infusions, the acidosis resolved and lactate level normalized. The patient underwent uncomplicated coarctation repair via left thoracotomy the following day and was discharged home 8 days later.
Figure 2.
A. Echocardiogram showing the aortic arch. There is a discrete coarctation of the aorta and classic posterior shelf (arrow). B. Echocardiogram showing flow acceleration across severe coarctation of the aorta (long arrow) with a tiny ductus arteriosus (short arrow). The descending aorta (DAo) and left pulmonary artery (LPA) are also shown.
Differential Diagnosis
The differential diagnosis of lactic acidosis in the neonate includes distributive shock from systemic infection, inborn errors of metabolism, cardiogenic shock from acquired or congenital heart disease, and hypovolemia, among other less common causes of lactic acidosis. Inborn errors of metabolism include urea cycle defects, organic acidemias, and disorders of amino acid metabolism. Evaluation of serum ammonia, lactate, pH, plasma amino acids, urine organic acids, and acylcarnitine profile are helpful in initial metabolic workup.
The Condition
Coarctation of the aorta is a common cardiac malformation, accounting for approximately 5% of congenital heart defects recognized in infancy, whose causes have not been elucidated. Most cases of aortic coarctation co-occur with other cardiac abnormalities. Severe coarctation of the aorta is a ductal-dependent lesion that may be fatal if not promptly recognized. It is one of the most commonly missed or delayed diagnoses among congenital heart disease. Pulse oximetry screening shortly after birth may not detect coarctation because preductal and postductal oxygenation may be normal. Pulses and 4-extremity blood pressures may also fail to detect coarctation if the ductus arteriosus is still patent. For this reason, pulses and 4-extremity blood pressures may also fail to distinguish coarctation from normal cardiac anatomy during the early transitional period.
Infants with severe coarctation often present during the first days or weeks of life with persistent tachypnea and cardiovascular shock as the ductus arteriosus closes. Neonates with severe coarctation rely on right to left flow across the ductus to bypass the obstruction and supply the descending aorta. Initial presentation may involve differential cyanosis, absent or diminished femoral pulses, decreased blood pressure in the lower extremities, hepatomegaly, presence of a gallop rhythm, and/or shock. Chest radiography may reveal signs of pulmonary venous congestion and left ventricular outflow tract obstruction, including cardiomegaly and increased pulmonary vascular markings (Fig 1). Laboratory evaluation may reveal profound metabolic acidosis if the coarctation is severe. Echocardiography is the definitive diagnostic test for coarctation (Fig 2).
Although functional closure of the ductus arteriosus usually occurs within 24 hours in term neonates, infants with congenital heart disease may have delayed closure, as was the case with our patient. Other significant left-sided obstructive lesions that are ductal dependent for systemic perfusion and often present similarly include hypoplastic left heart syndrome, critical aortic stenosis, and interrupted aortic arch. Other lesions are ductal dependent for pulmonary blood flow (critical pulmonary stenosis or atresia) or to promote mixing (transposition of the great arteries).
Management
The short-term management of co-arctation of the aorta and other ductal-dependent lesions involves use of prostaglandin E1 to open the ductus arteriosus. Any critically ill neonate presenting in shock with lethargy, respiratory distress, and metabolic acidosis should be empirically treated with prostaglandin infusion until cardiac disease is ruled out. Likewise, treatment with broad-spectrum antibiotics and workup for metabolic disease should be initiated, given the similarities in presentation among cardiac, infectious, and metabolic diseases in the neonate. The most common adverse effects of prostaglandin E1 infusion are apnea and hypotension. Infants who are not intubated require especially close monitoring during prostaglandin infusion. Inotropes should be used in the setting of depressed ventricular function.
There are only a few rare cardiac lesions for which prostaglandin infusion is contraindicated. These lesions involve pulmonary venous obstruction or left atrial obstruction, such as obstructed total anomalous pulmonary venous connection. Deterioration after initiation of prostaglandin treatment should prompt suspicion for these conditions, discontinuation of prostaglandin treatment, and emergency echocardiography.
Surgery is the definitive treatment for coarctation that presents in infancy. This operation involves removal of the narrowed portion and aortic anastomosis. Balloon angioplasty is not recommended in infants because of the high rate of recoarctation.
Lessons for the Clinician
Congenital heart disease, metabolic disorder, and sepsis should be in the differential diagnosis for unexplained anion gap metabolic acidosis in all previously healthy neonates.
Prompt initiation of prostaglandin E1 treatment is recommended for neonates who present in extremis, until cardiac disease is ruled out.
Signs and symptoms of coarctation of the aorta may include discrepant 4-extremity blood pressures, differential cyanosis, tachypnea, absent or diminished femoral pulses, murmur, gallop, hepatomegaly, and/or lactic acidosis.
Footnotes
Author Disclosure
Drs Wallenstein, Olson, Peng, Stevenson, Shaw, Palma, and Bain have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
Suggested Reading
- Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents. 8. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Enns GM, Packman S. Diagnosing inborn errors of metabolism in the newborn: laboratory investigations. Neoreviews. 2001;2:e192–e200. [Google Scholar]
- Martin RJ, Fanaroff AA, Walsh MC. Neonatal-Perinatal Medicine. 9. St Louis, MO: Elsevier; 2011. [Google Scholar]