Abstract
Background
Brain-derived neurotrophic factor (BDNF) is a neurotrophin present in the intestine where it participates in survival and growth of enteric neurons, augmentation of enteric circuits, and stimulation of intestinal peristalsis and propulsion. Previous studies largely focused on the role of neural and mucosal BDNF. The expression and release of BDNF from intestinal smooth muscle and the interaction with enteric neuropeptides has not been studied in gut.
Methods
The expression and secretion of BDNF from smooth muscle cultured from rabbit longitudinal intestinal muscle in response to substance P and pituitary adenylate cyclase activating peptide (PACAP) was measured by western blot and ELISA. BDNF mRNA was measured by rt-PCR.
Key Results
The expression of BNDF protein and mRNA was greater in smooth muscle cells from the longitudinal muscle than from circular muscle layer. PACAP and substance P increased the expression of BDNF protein and mRNA in cultured longitudinal smooth muscle cells. PACAP and substance P also stimulated the secretion of BDNF from cultured longitudinal smooth muscle cells. Chelation of intracellular calcium with BAPTA prevented substance P-induced increase in BDNF mRNA and protein expression as well as substance P-induced secretion of BDNF.
Conclusions & Inferences
Neuropeptides known to be present in enteric neurons innervating the longitudinal layer increase the expression of BDNF mRNA and protein in smooth muscle cells and stimulate the release of BDNF. Considering the ability of BDNF to enhance smooth muscle contraction, this autocrine loop may partially explain the characteristic hypercontractility of longitudinal muscle in inflammatory bowel disease.
Keywords: Neurotrophins, Neuropeptides, Gastrointestinal Tract, Motility, Gut Smooth Muscle, Tachykinins
INTRODUCTION
The regulation of gut function, whether it be motility, secretion or absorption is dependent on the interaction of a variety of biologically active agents, such as neurotransmitters, hormones, neurotrophins, cytokines, etc. delivered to the gut wall through the vasculature, by absorption from the lumen, or secreted by constitutive cells of the gut itself. Not only can these agents directly affect target cells, but it is likely that they can influence the production and secretion of each other. In the present study, we have examined the regulation of the expression and secretion of brain-derived neurotrophic factor (BDNF) from longitudinal smooth muscle cells by two key neurotransmitters of the enteric nervous system, substance P and pituitary adenylate cyclase-activating peptide (PACAP).
BDNF is the most abundant neurotrophin in the brain where it has been shown to be secreted in an activity-dependent manner and to be important to neuronal survival, neurite outgrowth and branching, synaptic plasticity and long term potentiation (LTP) among other functions (reviewed in 1). BDNF is also present in the gut where it is found in smooth muscle of the muscularis externa and gut vasculature (2–8), epithelial and enteroendocrine cells of the mucosa (3, 8–13), and enteric neurons and glia (8–16). In the gut, BDNF has also been shown to have important roles in augmenting the peristaltic reflex by enhancing serotonin (5-HT) and CGRP release from enteroendocrine cells and enteric sensory neurons respectively (9,10), in augmenting calcium responses in enteric neurons elicited by neurotransmitters thereby enhancing synaptic communication within the enteric nervous system (11), increasing colonic myoelectrical activity (17), and enhancing the response of smooth muscle cells to contractile agonists (2, 18). In humans, BDNF increases gut motility, accelerates colonic transit, and increases stool frequency without affecting stool consistency (19–21). BDNF has a key role in interrelationship between the central nervous system and the gut. BDNF produced in gut smooth muscle has a major role in directing the innervation of the gut by vagal sensory neurons and in regulating the survival of vagal sensory neurons where it has been implicated as having a role in satiety (6–7). Additionally, up regulation of BDNF in spinal cord and dorsal root ganglion in response to stress and inflammation plays a role in visceral hypersensitivity and the pathogenesis of inflammatory bowel disease and irritable bowel syndrome (22–26).
Little is known of the regulation of BDNF release from intestinal tissues by neuropeptides; however in other tissues, PACAP and substance P have been shown to influence the production and/or secretion of BDNF (27–36). This interrelationship of PACAP and substance P with BDNF is particularly interesting when applied to the gut because the innervation of the longitudinal muscle of the intestine is composed of two non-overlapping populations of enteric motor neurons. The vast majority are cholinergic excitatory neurons which also contain substance P, and a much smaller population of inhibitory motor neurons which contain vasoactive intestinal peptide, NOS, and PACAP. (37–39). In the present study, we show constitutive expression of BDNF in intestinal smooth muscle, predominately in the longitudinal layer. We also show that PACAP and substance P increase both the expression and release of BDNF from these muscle cells.
MATERIALS AND METHODS
Animals
New Zealand white rabbits (weight: 4–5 lbs) were purchased from RSI Biotechnology, Clemmons, NC and killed by injection of euthasol (100 mg/kg), as approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. The animals were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Tissue preparation, and isolation and culture of smooth muscle cells
The small intestine was dissected out, emptied of contents, and placed on cold smooth muscle buffer (SMB) of the following composition (NaCl 120 mM, KCl 4 mM, KH2PO4 2.6 mM, CaCl2 2.0 mM, MgCl2 0. 6 mM, HEPES (N-2-hydroxyethylpiperazine-N‟ 2-ethanesulfonic acid) 25 mM, glucose 14 mM, and essential amino mixture 2.1% (pH 7.4). Two to three centimeter sections of the intestine were removed from the mid-jejunum to proximal ileum, threaded onto a glass rod, and the longitudinal muscle separated as a sheet from the circular layer by radial abrasion with a KimWipe. The duodenum and distal ileal regions of the intestine were not used in this study.
Smooth muscle cells from intestinal longitudinal and circular muscle layers were isolated by sequential enzymatic digestion of muscle strips, filtration, and centrifugation as described previously (2,40). Muscle strips were incubated for 15 min at 31°C in 30 ml of smooth muscle buffer gasses with 100% oxygen and containing 0.1% collagenase (type II) and 0.1% soybean trypsin inhibitor. The partly digested tissues were washed with 100 ml of enzyme-free smooth muscle buffer and re-incubated for 10 min to allow spontaneous dispersion of muscle cells. Cells were harvested by filtration through 500 µm Nitex mesh and centrifuged twice at 350 g for 10 min.
The dispersed smooth muscle cells were resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM) containing penicillin (200 U mL−1), streptomycin (200 µg mL−1), gentamycin (100 µg mL−1), amphotericin B (2.5 µg mL−1), and 10% fetal bovine serum (DMEM-10). The muscle cells were plated at a concentration of 5 × 105 cells mL−1 and incubated at 37°C. DMEM-10 medium was replaced every 3 days for 2–3 weeks until confluence was attained. The muscle cells in confluent primary cultures were trypsinized (0.5 mg trypsin ml-1), re-plated at a concentration of 2.5 X 105 cells mL−1, and cultured under the same conditions. All experiments were done on cells in first and second passage. In some cases, the cells were plated into 8-well microscope slides for immunostaining and checked for expression of BDNF and for markers of smooth muscle and other cell types. The cultures contained only smooth muscle cells which stain for smooth muscle-specific γ-actin and do not stain for markers of other cells such as enteric neurons, glial cells, or interstitial cell of Cajal as described previously (40). Cultured smooth muscle cells were starved in serum-free medium (DMEM-0) 24-hours before use in experiments to examine expression and release of BDNF.
Experimental protocols
Cultured cells were treated with 10 nM and 100 nM of substance P or PACAP for 24 hrs and 48 hrs. PACAP exists in two forms: the full 38 amino acid form PACAP-38, and a truncated C-terminal form PACAP-27. In preliminary studies, equimolar concentrations of PACAP-27 and PACAP-38 were tested and found to produce identical effects, therefore, the full form of PACAP-38 was used in all studies. Hereafter, PACAP will be used to indicate PACAP-38. For 48 hrs treatment, the medium was changed on the second day and fresh substance P and PACAP was added to the medium. For control experiments, cultures were treated for the same period with DMEM-0. At the end of the experimental periods, the cultured medium was collected and stored at −80°C for subsequent ELISA to detect BDNF release. The cultured cells from the same culture plates were collected by two different methods using Triton X-100-based lysis buffer plus protease and phosphatase inhibitors for subsequent Western blotting or by trypsinization and centrifugation for PCR analysis. Samples were stored at −80°C for subsequent analysis.
The concentrations of PACAP and substance P used in the present study are similar to those used in previous studies of contraction, relaxation, and ligand binding in isolated gut smooth muscle cells (41,42). Isolated muscle cells demonstrate broad concentration response curves where minimal effects are noted at pM concentrations and maximal effects are noted at µM concentrations. The ED50 effects are in the nM range (41,42). While it is not possible to know the exact concentration of these neuropeptides in the vicinity of the smooth muscle cell, following release from enteric neurons or under various physiological or pathophysiological conditions, we have used concentrations which reproduce the physiological action of PACAP and substance P, In addition, these concentrations and durations of incubation were chosen because they are similar to those used in previous studies of BDNF synthesis and secretion by PACAP and substance P in a variety of cultured cell types including airway smooth muscle (27,29,32,33,43–45)
Immunohistochemistry
Intestinal sections from human, rabbit and mouse were immunostained using on-slide technique and smooth muscle cells grown and immunostained on 8-well culture slides. Three cm long intestinal samples (rabbit and mouse) were flushed with SMB and fixed in 4% paraformaldehyde overnight at 4°, incubated in 30% sucrose in 0.1 M PBS solution for 1–3 days at 4°, and embedded in OTC. Cross sections (8 µm) were cut and mounted on gelatin-coated microscope slides. The slides were warmed at 37° for 1 hr and permeabilized with 0.3% Triton X-100 in 0.1 M PBS for 20 min. For cultured cells, the cells on the slide were fixed with 4% paraformaldehyde for 20 min at room temperature. Both preparations were incubated for 1 h at room temperature with blocking solution containing 3% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in PBS-T (0.3% Triton X-100 in 0.1 M PBS, pH 7.4). The sections then were incubated with primary antibody against BDNF (rabbit antibody SC12112, Santa Cruz, CA or sheep antibody AB1513P (Millipore, Billerica, MA) at 1:500 dilution in PBST containing 5% normal donkey serum overnight at 4 °C. After rinsing with 0.1mM PBS, the slides were incubated with Alexa 595 species-specific secondary antibody for 2 h at room temperature and coverslipped in Citifluor. To evaluate for nonspecific binding, control sections were treated as above but without the primary antibody. Specificity of the antibodies was also confirmed by Western blot analysis.
Protein extraction and western blotting
Cultured smooth muscle cells were homogenized with solubilization buffer of the following composition, 50 mM Tris-HCL, 150 mM NaCL, 1 mM EDTA, 1% Triton X-100, 100 mM NaF and containing protease/phosphatase inhibitor cocktail (100 µg mL−1 PMSF, 10 µg mL−1 aprotinin, 10 µg mL−1 leupeptin, 30 mM sodium fluoride and 3 mM sodium vanadate). After sonication for 15 s and centrifugation at 2000 g for 10 min at 4°C, the protein concentrations in the supernatant was determined using a DC protein assay kit from Bio-Rad according to manufacturer’s directions.
Equal amounts of proteins were separated by SDS/PAGE electrophoresis using 10% and 15% (w/v) acrylamide resolving gel. The separated proteins were electrophoretically transferred onto a nitrocellulose membrane, blocked with 5% (w/v) non-fat dried milk/TBS-T (Tris-buffered saline, pH 7.6 plus 0.1% Tween-20) for 1h and incubated for 24 hr at 4°C with BDNF primary antibody (described above) diluted in TBS-T 1% (w/v) non-fat dried milk. After washing, the membrane was incubated with horseradish-peroxidase-conjugated secondary antibody (1: 2000) at room temperature for 1h. The immunoreactive protein was visualized by SuperSignal Femto maximum sensitivity substrate kit.
To ensure equal amount of protein loading, in each blot the same membrane was stripped and re-blotted against a housekeeping protein such as beta actin (AC1978, Sigma, St Louis, MO) or GAPDH (G0110, Santa Cruz, Santa Cruz, CA). Densitometric quantification of protein bands was done using the software FluoroChem 8800. The average band’s intensites were normalized to that of the control of the same lane.
Enzyme-linked immunosorbent assay (ELISA)
BDNF protein in culture medium was measured via a sandwich ELISA using the Promega BDNF Emax immunoassay (Promega Corporation, Madison, WI) according to the manufacturer’s protocol. The samples were acidified to pH < 3.0 with 1 N HCl for 15 min and then neutralized to pH 7.6 prior to use in the ELISA. The antibody was specific for BDNF with less than 3% cross reactivity with NGF, NT-3, NT-4, and no cross reactivity with PACAP, SP, VIP, secretin, and somatostatin. The limit for detection of the ELISA was 4pg/ml and the range was 4 pg/ml to 500 pg/ml. Briefly, ELISA plates were coated with anti-BDNF mAb (1:1000) and incubated overnight at 4°. The next day the plate was washed and blocked with Blocking Buffer (Promega). 100 µl of BDNF standard or sample was added to each well and incubated for two hours at room temperature. The plate was washed and 100 µl anti-BDNF pAb (1:500) was added to each well and incubated at room temperature for two hours. After washing, 100µl of diluted Anti-IgY HRP Conjugate (1:200) was added to each well and developed with TMB solution and 1N HCl. The absorbance at 450 nm was measured using a VICTOR 2 plate reader and the concentration of BDNF in the samples was calculated from the standard curve.
Expression of BDNF by reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from cultured smooth muscle cells using Ultraspec™ RNA isolation kit and treated with TURBO DNase (Ambion, Carlsbad, CA). RNA was reverse transcribed by SuperScript™ II containing 50 mM Tris-HCL, 75 mM KCL, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM deoxynucleoside triphosphate, 2.5 µM random hexamers and 200 units of reverse transcriptase in a 20 ul reaction volume. Quantitative real-time PCR was performed for BDNF with a Taqman probe mixed with PCR Master-Mix for 40 cycles (95 C° for 15 sec, 60 C° for 1 min) on a 7300 real-time PCR system (Applied Biosystems, Grand Island NY). Quantitative real-time PCR of the same sample was performed for β-actin expression as internal control for normalization. Primer sequences for BDNF were TATGAGGGTCGGGCGCCACT (Forward 5’→3’) and TCCCGCCCGACATGTCCACT (Reverse 3’→5’), and for β-actin were CCCTCCATCGTGCACCGCAA (Forward 5’→3’) and CTCGTCTCGTTTCTGCGCCGT (Reverse 3’→5’).
Reactions for real time PCR were done in triplicate and only one PCR product was generated by each primer set. StepOne™ Real-Time PCR System software was used to calculate the fluorescent threshold value. Quantification of gene expression was done using the relative qRT-PCR method. The signal of BDNF transcript in a treatment group was related to that of the control group. Cycle threshold (Ct) values were obtained and the relative fold change in gene expression was calculated as 2−ΔΔCt as described previously (22).
Statistical Analysis
The appropriate statistical tests (ANOVA and Paired t-tests) were carried out using GraphPad (PRISM/GraphPad® Software, La Jolla, CA). A probability of p<0.05 was considered significant. Values are reported as mean ± SEM and n refers to number of animals from which each culture was derived.
RESULTS
Localization of BDNF protein and mRNA in intestinal smooth muscle
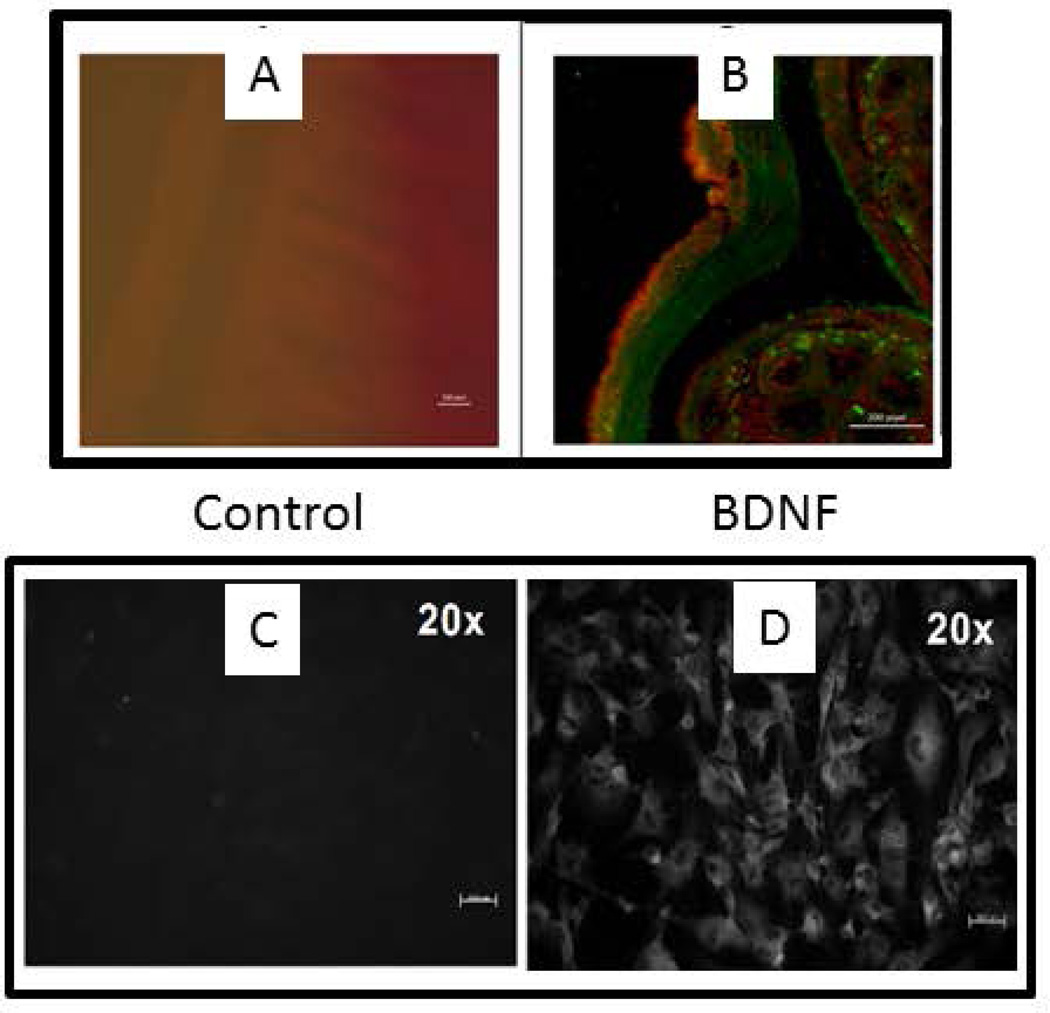
In intestinal tissue slices from human, rabbit and mouse, immunostaining with antibody to BDNF demonstrated the expression of BDNF in the longitudinal muscle layer. Staining of BDNF in the circular muscle layer was much less intense than in the longitudinal muscle layer as illustrated for the rabbit intestine in Figure 1A. A similar pattern of BDNF expression was obtained in intestinal tissues from the human and mouse (Data not shown). To confirm the presence of BDNF in longitudinal SMCs, smooth muscle cells from the longitudinal layer of rabbit intestine were grown in primary culture and immunostained for BDNF (Fig.1B). Control immunostaining with omission of BDNF antibody demonstrated the specificity of staining by the BDNF antibody (Fig. 1A & B).
Figure 1.
Localization of BDNF in GI smooth muscle. (A) Immunostaining of BDNF (red) in longitudinal muscle of rabbit intestine. A1 is control staining where primary antibody is omitted. A2 is costained for BDNF (red) and 5-HT (green). Magnification: 100×. (B) Smooth muscle cells isolated from longitudinal muscle layer, grown in culture, and stained for BDNF. Left panel is control where primary antibody is omitted. Right panel is stained for BDNF.
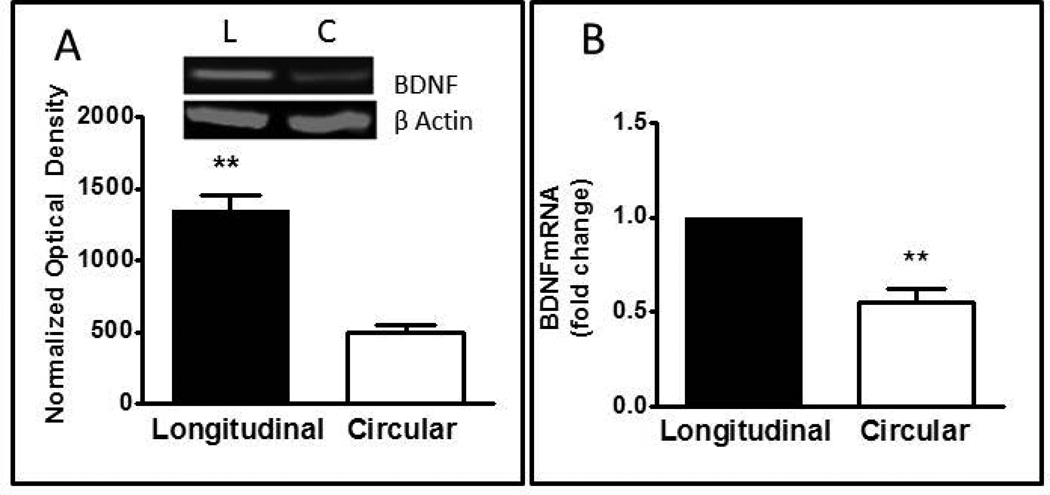
To further confirm the immunohistological BDNF expression, western blot analysis of BDNF was done on total protein extracts from smooth muscle cells from rabbit longitudinal and circular muscle layer. The expression of BDNF protein was detected at the expected molecular weight of mature BDNF (14kDA) in both longitudinal and circular muscle smooth muscle cells (Fig.2A). Comparing the densities of protein bands in the two regions revealed a significantly higher (1.7-fold) expression of BDNF in longitudinal muscle than circular muscle (Fig 2A; p<0.001, n=8), consistent with the immunohistochemical findings.
Figure 2.
Expression of BDNF protein and mRNA in smooth muscle cells of rabbit intestine. (A) Western blot of BDNF protein in homogenates of longitudinal and circular muscle cells. Insert illustrates a typical Western blot of BDNF and β actin. Bar graph illustrates optical density of BDNF blots normalized to β actin. Values are Mean ± SEM; n=8; **= p<0.01 for difference between circular and longitudinal muscle. (B) Quantative rt-PCR of BDNF mRNA in circular and longitudinal muscle cells. Relative expression was calculated by the ΔΔCT method as described in Methods. Values are Mean± SEM; n=5; **= p<0.01 for difference between circular and longitudinal muscle.
Total RNA isolated from longitudinal and circular muscle cells in culture was converted to cDNA and subjected to amplification with specific primers for BDNF and GAPDH. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to measure mRNA levels of BDNF and GAPDH. Consistent with the protein level detected by western blot, BDNF mRNA was expressed in both muscle layers and the expression was significantly less in the circular muscle cells as compared to the longitudinal muscle cells (p<0.01, n=5) (Fig. 2B).
Effect of PACAP and substance P on BDNF expression by longitudinal muscle cells
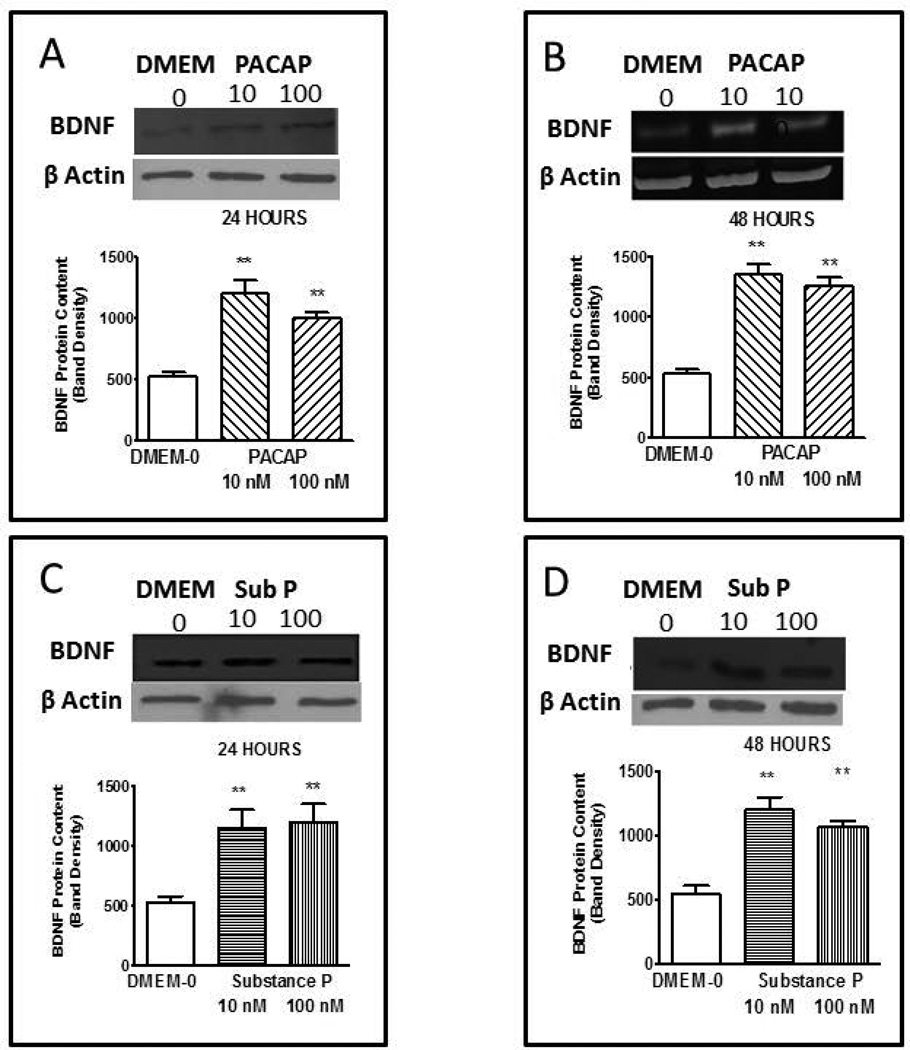
The effect of PACAP on BDNF protein content in primary smooth muscle cells was evaluated after 24 and 48 hrs incubation. Longitudinal smooth muscle cells were treated with PACAP-38 (10 & 100 nM) for 24 and 48 hrs. Both concentrations of PACAP significantly (p<0.001 in each case vs basal DMEM-0) up-regulated BDNF protein expression following 24 hrs (Fig. 3A) and 48 hrs (Fig 3B) of incubation. These concentrations of PACAP (10 and 100 nM) more than doubled the content of BDNF protein content in primary smooth muscle cells under each condition but there was no significant difference in the amount of BDNF induced by the two concentrations doses although there was slightly more BDNF produced at the 48 hr incubation.
Figure 3.
Expression of BDNF synthesis induced by PACAP and substance P. Incubation with longitudinal smooth muscle cells in culture with PACAP at 10 and 100 nM for 24 (A) and 48 (B) hrs or substance P ar 10 and 100 nM for 24 (C) and 48 (D) hrs increased expression of BDNF above control levels obtained with incubation in DMEM-0. Bar graphs illustrate optical density of BDNF blots normalized to β actin. Band density of the control DMEM-0 was 522 ± 38 in arbitrary units. Inserts illustrate typical blots. Values are Mean ± SEM; n=5–7; **= p<0.01 for difference from expression in presence of DMEM-0.
The effect of substance P on BDNF expression was examined in the same manner and following the same protocol as described for PACAP but in separate cultures of longitudinal muscle of the intestine. Longitudinal smooth muscle cells were treated with substance P (10 & 100 nM) for 24 and 48 hrs. Both concentrations of substance P significantly (p<0.005) up-regulated BDNF protein content following 24 hrs (Fig. 3C) and 48 hrs (Fig. 3D) of incubation. Similar to PACAP, both 10 and100 nM substance P more than doubled the expression of BDNF protein content in primary smooth muscle cells under each condition but there was no significant difference in the amount of BDNF induced by the two concentrations at the two time points.
Effect of PACAP and substance P on BDNF secretion in longitudinal smooth muscle cells
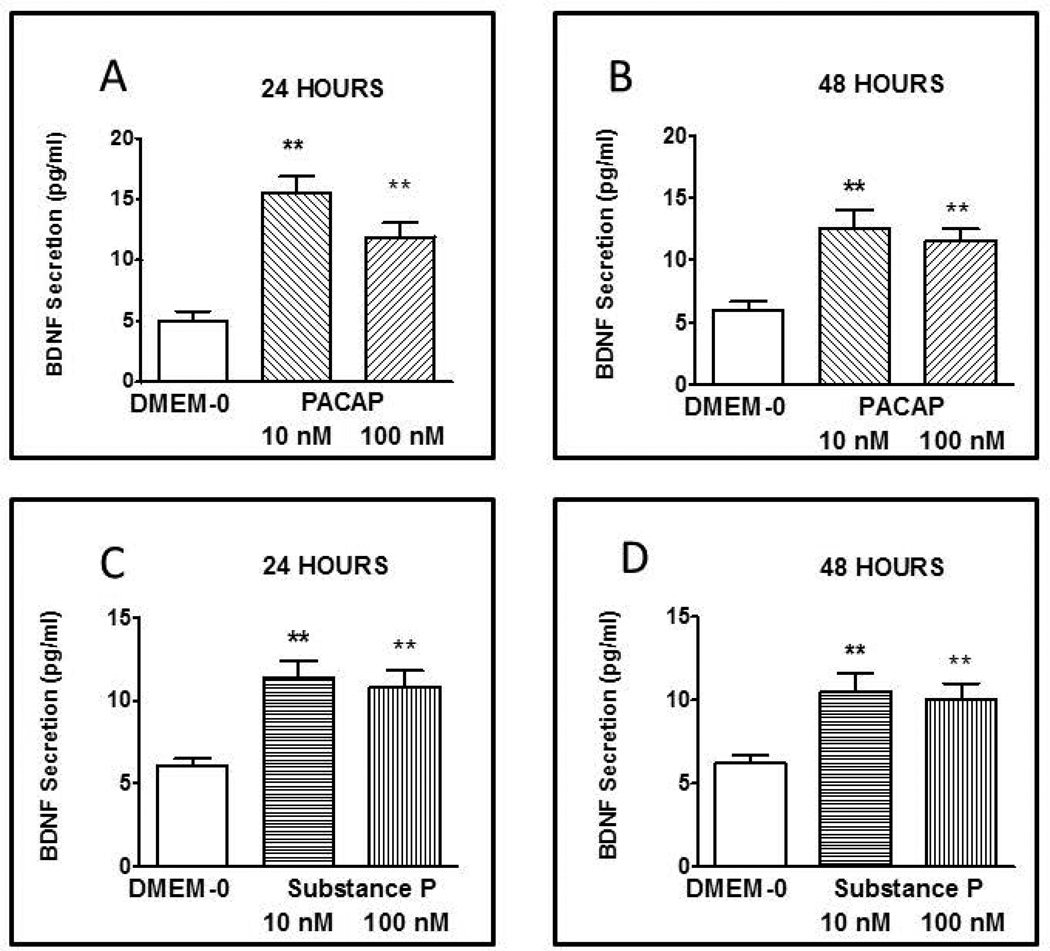
To study the effect of PACAP on the secretion of BDNF, primary longitudinal SMCs cultures were treated with PACAP (10 and 100 nM) for 24 and 48 hrs and the amount of BDNF release into the medium was determined by ELISA. After 24 hours incubation, both 10 and 100 nM PACAP caused a significant elevation in BDNF release above basal levels of 5 ±0.8 pg/ml (Fig. 4A). The increase in BDNF secretion (15.5 ± 1.4 pg/ml) induced by 10 nM PACAP was significantly greater (p<0.05) than the BDNF secretion (11.9 ± 1.2 pg/ml) induced by 100 nM PACAP. After 48 hrs incubation, PACAP (10 and 100 nM) also induced a significant increase in BDNF release (Fig. 4B). Although the levels of BDNF secretion at 48 hrs were less than elicited during 24 hrs incubation at the same concentrations, the difference between BDNF secretion at 24 hrs and 48 hrs was not significantly different.
Figure 4.
Secretion of BDNF induced by PACAP and substance P. Incubation of longitudinal smooth muscle cells in culture with PACAP at 10 and 100 nM for 24 (A) and 48 (B) hrs and with substance P at 10 and 100 nM for 24 (C) and 48 (D) hrs increased secretion of BDNF above control levels obtained with incubation in DMEM-0. BDNF in the culture medium was measured by specific ELISA. Values are Mean ± SEM; n=3–5; **= p<0.01 for difference from expression in presence of DMEM-0.
The effect of substance P on BDNF secretion was examined in the same manner and following the same protocol as described for PACAP but in separate cultures of longitudinal muscle of the intestine. Longitudinal smooth muscle cells were treated with substance P (10 and 100 nM) for 24 and 48 hrs. Both concentrations of substance P significantly increased BDNF secretion above basal levels of 6.1± 0.4 pg/ml following 24 hrs (Fig. 4C) and 48 hrs (Fig. 4D) of incubation. The increased elicited by 10 nM substance P at 24 hours was greater (11.4±1 pg/ml) however there was no significant difference between the concentrations or periods of incubation..
Role of calcium in mediating the effect of substance P on BDNF expression in and secretion from longitudinal muscle cells
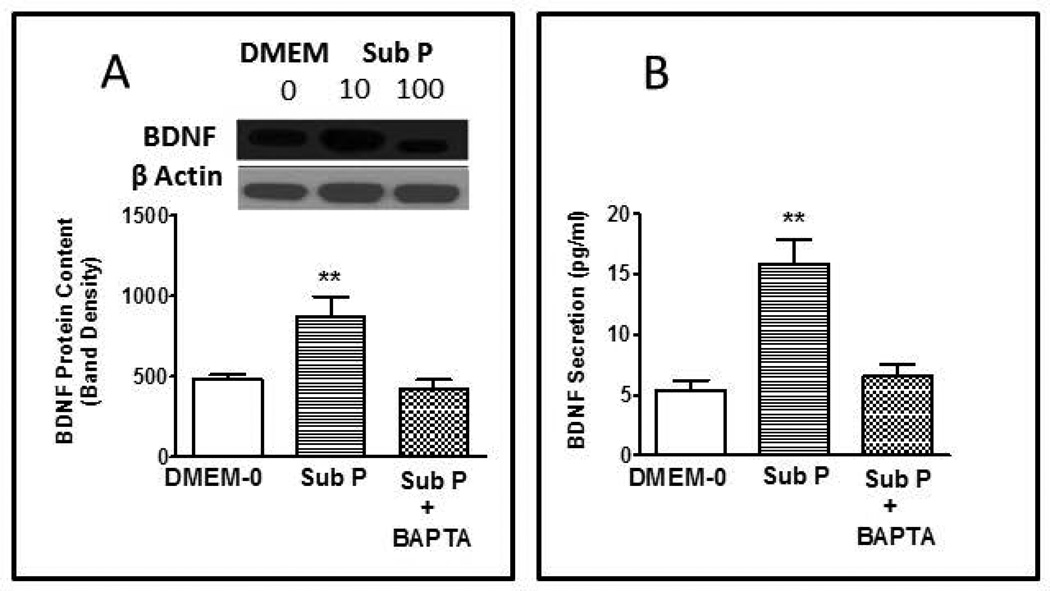
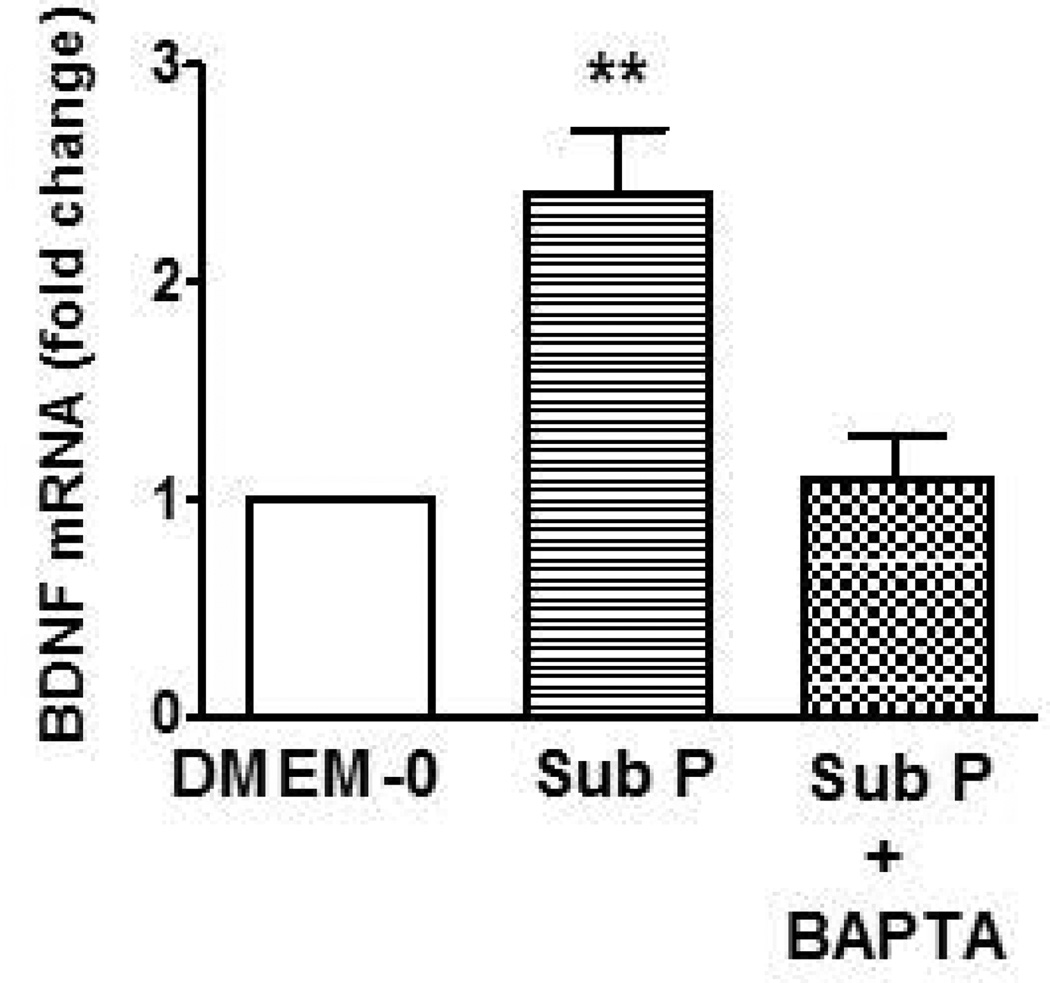
Since there are more substance P/acetylcholine containing neurons innervating the longitudinal muscle layer than there are PACAP containing neurons, the mechanism of substance P-induced effects on BDNF was studied further. Since activation of tachykinin receptors leads to increase in intracellular calcium (Ca2+) in producing contraction, we tested the role of Ca2+ in mediating the effect of substance P on BDNF expression and secretion. Incubation of culture of longitudinal muscle cells with 10 nM substance P for 24 hr in the presence of 1 µM BAPTA-AM (acetoxymethyl ester of 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid) to chelate Ca2+ abolished the ability of substance P to increase BDNF expression as measured by western blot (Fig 5A) as well as by in cell western assay (data not shown). Consistent with the effect of BAPTA on expression of BDNF protein, treatment with 1 µM BAPTA-AM prevented the upregulation of BDNF mRNA expression induced by 10 nM substance P for 24hrs as determined by quantitative RT-PCR (Fig. 6).
Figure 5.
Effect of BAPTA on the expression and secretion of BDNF induced by substance P. Incubation of smooth muscle cells in cultures for 24 hours with the Ca2+ chelating agent BAPTA abolished the increase in BDNF expression (A) and secretion (B) induced by 10 nM substance P. In Western blots (A), optical density of BDNF blots was normalized to β actin. Inserts illustrate typical blot. BDNF secretion (B) was measured in the culture medium by specific ELISA. Values are Mean ± SEM; n=3–5; **= p<0.01 for difference from expression in presence of DMEM-0.
Figure 6.
Effect of BAPTA on BDNF mRNA levels induced by substance P. Incubation of smooth muscle cells for 24 hours with the Ca2+ chelating agent BAPTA abolished the increase in BDNF mRNA levels induced by 10 nM substance P. Relative expression was calculated by the ΔΔCT method as described in Methods. Values are Mean ± SEM; n=3–5; **= p<0.01 for difference from expression in presence of DMEM-0.
In neurons and other cell types, BDNF is secreted by the calcium-dependent regulated pathway rather than by the constitutive pathway. BAPTA-AM was therefore used to test the role of the calcium-dependent regulatory pathway in mediating the substance P-induced secretion of BDNF. Incubation of longitudinal muscle cultures with 10 nM substance P for 24 hr caused a nearly 2-fold increase in BDNF release into the medium. Incubation with 10 nM substance P for 24 hr in the presence of 1 µM BAPTA-AM abolished the release of BDNF in response to substance P (Fig. 5B) indicating that BDNF is released by the regulatory pathway following in increase in Ca2+ induced by activation of tachykinin receptors by substance P.
DISCUSSION
In the present study, we have shown that the neurotrophin BDNF is constitutively expressed by intestinal smooth muscle of rabbit. The expression of BDNF mRNA and protein is much greater in smooth muscle from the longitudinal layer than the circular muscle layer. The neuropeptides substance P and PACAP increased the expression of BDNF mRNA and protein in cultured longitudinal muscle cells and caused the secretion of BDNF from these cells. Both the increase in expression and release of BDNF by substance P are likely to be Ca2+-dependent.
In the wall of the gut, BDNF is present in several sources including mucosa, enteric neurons and glia, and smooth muscle. Most studies have focused on neurally-derived BDNF based on studies from the cell biology of BDNF in central nervous system. In the present study, we have studied the expression and release of BDNF from gastrointestinal smooth muscle. In histological section and in cultures of smooth muscle cells derived from intestinal smooth muscle, BDNF mRNA and protein were much more prevalent in the longitudinal layer. There are very few studies of BDNF in gut smooth muscle however recent studies indicate that BDNF expression in gut smooth muscle is important to the development of vagal sensory innervation (5–7). Interestingly, conditional knockout of BDNF in intestinal smooth muscle of mice resulted in enhanced innervation by vagal sensory neurons. This is reflected in increased intraganglionic laminar endings of these neurons in myenteric ganglia and increased numbers of axon bundles in the longitudinal orientation. r. The increase in axon bundels was found to be specific to those oriented longitudinally because there was no increase in the number of axon bundels in the circular orientation, The authors conclude that BDNF suppresses survival of intraganlaionic laminar endings and axon growth or guidance. The greater extent of the effect on the axons in the longitudinal orientation of the smooth muscle specific BDNF knockout is consistent with the greater expression of BDNF in this layer which we have identified in the present study. It may also explain the lesser innervation of longitudinal muscle than circular muscle by enteric neurons consistent with our observations that BDNF is unique among neurotrophins in inhibiting the outgrowth of neurites from cultured myenteric ganglia of guinea pig (46).
While BDNF is constitutively expressed in longitudinal muscle of gut, the present study demonstrates that its expression is increased by neuropeptides known to be released from enteric neurons innervating the longitudinal muscle. The intrinsic innervation of the longitudinal muscle is much sparser than that of the circular muscle layer and consists mainly of motor neurons derived from the myenteric plexus.
PACAP is expressed in inhibitory motor neurons innervating the longitudinal muscle along with vasoactive intestinal peptide (VIP) and nitric oxide synthase (NOS) (37–39). In the present study, the expression and secretion of BDNF from longitudinal muscle cells was shown to be increased by PACAP. Although not significantly different, the effect of 10 nM PACAP was slightly greater than 100 nM. Incubation for 24 hours with PACAP caused slightly greater expression of BDNF but slightly less release. The latter difference may have been due to some degradation of BDNF over the longer time period. Although this is the first description of the ability of PACAP to increase expression and release of BDNF from gut smooth muscle, a similar relationship has been described in neural tissues. In cortical and hippocampal neurons, sympathetic neurons, and a cultured neuroblastoma cell line, PACAP has been shown to increase BDNF release and expression (27–32,47–49). Where examined in neuronal tissue, the effect is predominately mediated by activation of PAC1 receptors that are more selective for PACAP than VIP (32,47–49). Although neither the nature of the receptor nor the effectiveness of VIP were examined in the present study, studies in mouse and rat longitudinal muscle from ileum and colon suggest that the effects of PACAP in longitudinal muscle are mediated by PAC1 receptors. In PAC1 receptor knockout mice, the smooth muscle effects of both PACAP-27 and PACAP-28 were abolished (50). In preliminary studies (data not shown) identical results were obtained with PACAP-27 and PACAP-38, also suggesting interaction of PACAP with PAC-1 receptors on smooth muscle cells. Thus, it is likely that the effect of PACAP in increasing BDNF expression and release in longitudinal muscle is also mediated by PAC1 receptors. If this is the case, the increase in BDNF synthesis and secretion are much less likely to be stimulated by VIP than PACAP as reflected in the selectivity of the PAC1 receptor.
In the present study we also examined the effect of substance P on BNDF expression and release in intestinal longitudinal smooth muscle. As noted above, the main innervation of the longitudinal muscle is by cholinergic excitatory motor neurons, many of which also contain substance P (38,39). Preliminary studies (data not shown) indicated that BDNF synthesis and secretion is not affected by acetylcholine. This is consistent with the lack of effect of Trk B antagonists and antiserum alone on cholinergic contractions which are augments in the presence of BDNF (2). Substance P increased the expression of BDNF to approximately the same degree as did PACAP. As with PACAP the effect of 10 nM was slightly but not significantly greater than the effect of 100 nM substance P. With regard to the ability to release BDNF, the amount of BDNF released at 24 and 48 hours of incubation were similar to each other but the release was less than elicited by PACAP at 24 hours. The greater innervation of longitudinal muscle by substance P-containing motor neurons would, however, suggest that the stimulation of BDNF release in vivo by substance P would be more relevant. We therefore went on to examine the mechanism of regulation of BDNF expression and release by substance P in greater detail.
Incubation of cultures of intestinal longitudinal smooth muscle cells with the calcium chelating agent BAPTA abolished both the increase in BDNF expression and release. BAPTA also abolished the increase in BDNF mRNA in response to substance P. These findings indicate that an increase in intracellular calcium likely mediated both the substance P-induced effects on BDNF although an increase in Ca2+I was not measured in the present study. In support of this notion, we have shown in previous studies that tachykinins increase Ca2+I in isolated intestinal smooth mscule cells (41). Although the specific tachykinin receptor mediating the effect of substance P was not examined in the present study, this previous study indicated that selective agonists of the NK1, NK2, and NK3 receptor were capable of increasing Ca2+I in isolated intestinal smooth muscle cells. Interestingly, although the signaling pathways mediating the PACAP induced BDNF synthesis and secretion were not investigated in the present study, we have previously shown that PACAP is also capable of increasing Ca2+I in isolated gut smooth muscle cells (42). The secretion of BDNF from intestinal smooth muscle has not been previously reported; however, much is known of the activity-dependent secretion of BDNF from neural tissues. BDNF is secreted from neurons as the precursor, proBDNF, and as the processed mature BDNF form. In is not clear if both are secreted from gut smooth muscle but even in neuronal tissue, the proBDNF form is converted to the mature BDNF by the action of extracellular matrix metalloproteinases and plasmin. The ELISA used in the present study was directed towards mature BDNF but does not distinguish between the pro-and mature forms of BDNF. Activity-dependent secretion of BDNF from cortical and hippocampal neurons as well as from other regions is via the regulatory secretion pathway and requires calcium elevation, usually via calcium influx, which is further enhanced and sustained via calcium-induced calcium release mediated through ryanodine receptors (51–53). It is noteworthy that calcium influx, activation of ryanodine-sensitive receptors and calcium-induced calcium release is the mechanism of agonist induced elevation of intracellular calcium in intestinal longitudinal muscle (54–55). Similarly, the ability of BAPTA to abolish the substance P-induced increase in BDNF mRNA suggests that the effects of substance P on BDNF expression are likely also mediated by an increase in calcium via regulation of the bdnf gene. Although there are multiple promotors for the bdnf gene, it is quite likely that calcium chelation would cause a significant inhibition of transcription since the promoter region of BDNF exon IV contains three Ca2+ response elements : CaRE1, CaRE2 and CaRE3 (51–53). The co-dependence of BDNF secretion and transcription of the bdnf gene on intracellular Ca2+ levels suggests that the increase in intracellular Ca2+ -induced by substance P would not only cause BDNF release but also immediately begin to restore intracellular BDNF levels by increasing production of BDNF mRNA.
Although little is known about the expression of BDNF in gut smooth muscle, the expression by airway smooth muscle and pulmonary artery smooth muscle has been studied in much more detail (33–36,56–59). Expression of BDNF levels in these smooth muscle is also responsive to environmental cues including changes in oxygen level, elevated levels of inflammatory cytokines such as interleukin 1β and TNFα, and the neuropeptide substance P. Interestingly, in airway smooth muscle, substance P-induced BDNF release and secretion was dependent on calcium influx similar to the findings in the present study in longitudinal smooth muscle of the intestine (33,34). In the airways, once released, BDNF has been shown to lead to hyper-responsiveness of the smooth muscle by enhancing calcium influx to neuropeptides, including substance P, and to acetylcholine (33–36,43–45,57). This is also similar to longitudinal smooth muscle of intestine where BDNF enhances the contractile response to neuropeptides and acetylcholine by augmenting the increase in intracellular Ca2+ induced by these agents (2,18).
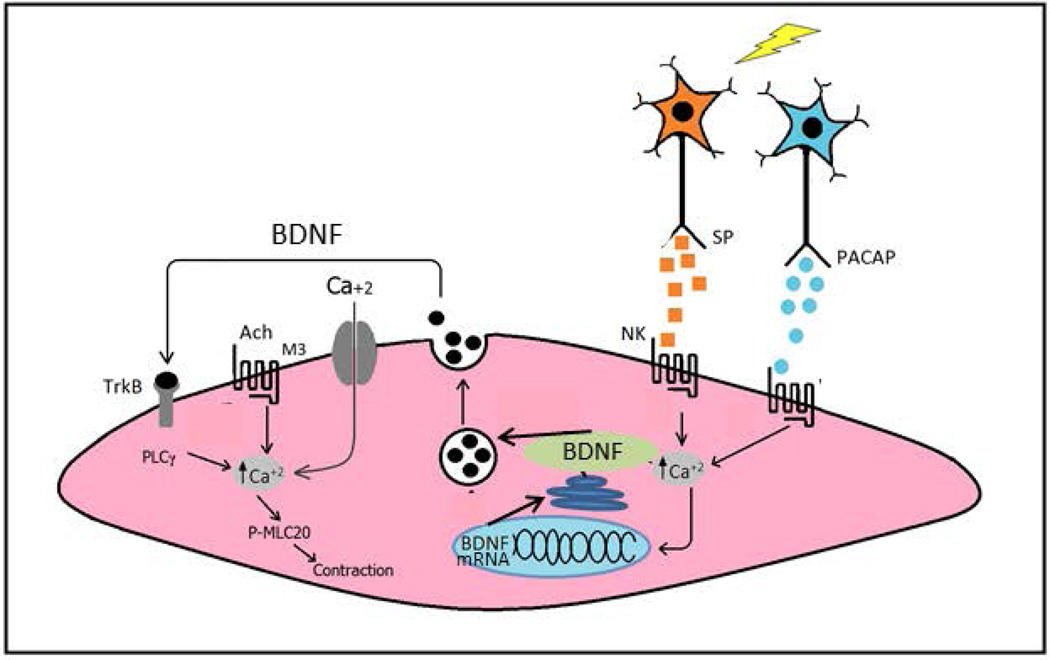
In analogy to the role of BDNF airway smooth muscle (35,36), a model is presented in Figure 7 to explain the role of BDNF in longitudinal muscle of the intestine based on the results of the present study and our recent study (2) of the effects of BDNF on longitudinal muscle contractility. The neurotransmitters PACAP and substance P, released from distinct enteric motor neurons innervating the longitudinal muscle, activates receptors on longitudinal smooth muscle cells leading to increase in intracellular Ca2+. The increase in intracellular Ca2+ causes increase transcription of the bdnf gene, increase in BDNF mRNA, and increase in BDNF protein expression. The increase in intracellular Ca2+ also leads to local secretion of BDNF. BDNF then acts to augment cholinergic muscarinic contraction of longitudinal muscle via activation of specific trk B receptors on longitudinal smooth muscle cells which is mediated by increased activation of PLC-γ (2). Thus BDNF acts in an autocrine manner leading to hypercontractility of the longitudinal muscle of the intestine. Considering that substance P and BDNF are increased in intestinal inflammation it is possible that this autocrine loop leads to the hypercontractility that is characteristic of the response of longitudinal smooth muscle in inflammatory bowel disease (60–63).
Figure 7.
Model of the expression and role of BDNF in intestinal longitudinal muscle. Based on the results of the present study and our recent study (2) of the effects of BDNF on longitudinal muscle of the rabbit we propose the model for the action of BDNF. Release of neurotransmitters pituitary adenylate cyclase-activating peptide (PACAP) and substance P (SP) from enteric neurons activates receptors on longitudinal smooth muscle cells to increase [Ca2+]i. The increase in [Ca2+]i causes increased transcription of the bdnf gene, increase in BDNF mRNA, and increase in BDNF protein expression and secretion. BDNF in turn acts in an autocrine manner to augment cholinergic muscarinic contraction of longitudinal muscle via activation of specific trk B receptors on longitudinal smooth muscle cells which is mediated by increased activation of PLC-γ (2).
KEY MESSAGES.
-
1
Cultures of smooth muscle from the longitudinal muscle layer of rabbit intestine were used to study the synthesis and secretion of the neurotrophin, brain-derived neurotrophic factor (BDNF), which has been shown in other studies to enhance smooth muscle contraction and peristalsis.
-
2
The smooth muscle cells from the longitudinal muscle layer expressed more BDNF than cells from the circular muscle layer. Similarly, mRNA levels were higher in smooth muscle cells from the longitudinal layer than from the circular muscle layer.
-
2
The main neuropeptides released from enteric motor neurons innervating the longitudinal muscle layer, namely substance P and pituitary adenylate cyclase-activating peptide (PACAP), increased the expression and secretion of BDNF from smooth muscle cells. Both expression and secretion of BDNF induced by substance P were dependent on increase in intracellular calcium.
-
4
A model is proposed in which neuropeptide transmitters released from enteric neurons cause increased BDNF expression and release which in turn leads to increased contractile responses. This autocrine loop may in part explain the hypercontractility of longitudinal smooth muscle characteristic of inflammatory bowel disease.
ACKNOWLEDGEMENTS
FUNDING
This work was supported by grant DK34153 (JR Grider) from the National Institute of Diabetes and Digestive and Kidney Diseases. KS Murthy was supported by DK28300, HI Akbarali was supported by DK46367, and M Al-Qudah was supported by a grant from the Jordan University of Science and Technology, Irbid, Jordon. R. Alkahtani was supported by a grant from the Kingdom of Saudi Arabia.
Footnotes
A portion of the these results were reported in preliminary form in an abstract presented at Experimental Biology 2012 (The FASEB Journal 2012;26:1140.6).
DISCLOSURE
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
M A-Q, HIA, KSM and JRG were responsible for design and conception of research.
M A-Q and RA performed the experiments.
M A-Q, KSM and JRG analyzed and interpreted the data.
M A-Q, KSM and JRG drafted the manuscript.
M A-Q, RA, HIA, KSM, and JRG edited, revised and approved the final manuscript.
REFERENCES
- 1.Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nature Reviews; Neuroscience. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 2.Al-Qudah M, Anderson CD, Mahavadi S, Bradley ZL, Akbarali HA, Murthy KS, Grider JR. Brain-derived neurotrophic factor enhances cholinergic contraction of longitudinal muscle of rabbit intestine via activation of phospholipase C. American Journal of Physiology. 2014;306:G328–G337. doi: 10.1152/ajpgi.00203.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox EA. A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Autonomic Neuroscience. 2006;126:9–29. doi: 10.1016/j.autneu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Fox EA, Murphy MC. Factors regulating vagal sensory development: potential role in obesities of developmental origin. Physiology & Behavior. 2008;94:90–104. doi: 10.1016/j.physbeh.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox EA, Biddinger JE. Early postnatal over nutrition: potential roles of gastrointestinal vagal afferents and brain-derived neurotrophic factor. Physiology & Behavior. 2012;106:400–412. doi: 10.1016/j.physbeh.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox EA. Vagal afferent controls of feeding: a possible role for gastrointestinal BDNF. Clinical Autonomic Research. 2013;23:15–31. doi: 10.1007/s10286-012-0170-x. [DOI] [PubMed] [Google Scholar]
- 7.Biddinger JE, Fox EA. Reduced intestinal brain-derived neurotrophic factor increases vagal sensory innervation of the intestine and enhances satiation. Journal of Neuroscience. 2014;34:10379–10393. doi: 10.1523/JNEUROSCI.1042-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. American Journal of Pathology. 1999;155:1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grider JR, Piland BE, Gulick MA, Qiao LY. Brain-derived neurotrophic factor augments peristalsis by augmenting 5-HT and calcitonin gene-related peptide release. Gastroenterology. 2006;130:771–780. doi: 10.1053/j.gastro.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. American Journal Physiology. 2007;292:G429–G437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 11.Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berght P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut. 2008;57:314–322. doi: 10.1136/gut.2007.131839. [DOI] [PubMed] [Google Scholar]
- 12.Johansson M, Norrgård O, Forsgren S. Study of expression patterns and levels of neurotrophins and neurotrophin receptors in ulcerative colitis. Inflammatory Bowel Disease. 2007;13:398–409. doi: 10.1002/ibd.20072. [DOI] [PubMed] [Google Scholar]
- 13.Lucini C, Maruccio L, de Girolamo P, Vega JA, Castaldo L. Localisation of neurotrophin-containing cells in higher vertebrate intestine. Anatomy & Embryology. 2002;205:135–140. doi: 10.1007/s00429-002-0237-x. [DOI] [PubMed] [Google Scholar]
- 14.Guarino N, Yoneda A, Shima H, Puir P. Selective neurotrophin deficiency in infantile hypertrophic pyloric stenosis. Journal Pediatric Surgery. 2001;36:1280–1284. doi: 10.1053/jpsu.2001.25795. [DOI] [PubMed] [Google Scholar]
- 15.Hoehner JC, Wester T, Pahlman S, Olsen L. Localization of neurotrophin and their high-affinity receptors during human enteric nervous system development. Gastroenterology. 1996;110:756–567. doi: 10.1053/gast.1996.v110.pm8608885. [DOI] [PubMed] [Google Scholar]
- 16.Hoehner JC, Wester T, Pahlman S, Olsen L. Alterations in neurotrophin and neurotrophin-receptor localization in Hirschsprung’s disease. Journal Pediatric Surgery. 1996;31:1542–1529. doi: 10.1016/s0022-3468(96)90170-0. [DOI] [PubMed] [Google Scholar]
- 17.Chai NL, Dong L, Li ZF, Du KX, Wang JH, Yan LK, Dong XL. Effects of neurotrophins on gastrointestinal myoelectric activities of rats. World Journal of Gastroenterology. 2003;9:1874–1877. doi: 10.3748/wjg.v9.i8.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Yu Y, Yuan Y, Zuo X, Li Y. Brain-derived neurotrophic factor enhances the contraction of intestinal muscle strips induced by PS and CGRP in mice. Regulatory Peptides. 2012;178:86–94. doi: 10.1016/j.regpep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo X, Li Y. Brain-derived neurotrophic factor accelerated gut motility in slow-transit constipation. Acta Physiologica. 2014;212:226–238. doi: 10.1111/apha.12374. [DOI] [PubMed] [Google Scholar]
- 20.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 119:41–50. doi: 10.1053/gast.2000.8553. 200. [DOI] [PubMed] [Google Scholar]
- 21.Bradley WG BDNF Study group. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 22.Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterology & Motility. 2008;20:928–938. doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu S, Grider JR, Gulick M, Xia CM, Shen S, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Experimental Neurology. 2012;238:209–217. doi: 10.1016/j.expneurol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winston JH, Li Q, Sarna SK. Chronic prenatal stress epigenetically modifies spinal cord BDNF expression to induce sex-specific visceral hypersensitivity in offspring. Neurogastroenterology & Motility. 2014;26:715–730. doi: 10.1111/nmo.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joo YE. Increased expression of Brain-derived neurotrophic factor in irritable bowel syndrome and its correlation with abdominal pain. Journal of Neurogastroenterology and Motility. 2013;19:109–111. doi: 10.5056/jnm.2013.19.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 27.Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP1-38) enhances N-methyl-d-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. Journal of Biological Chemistry. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- 28.Braas KM, Schultz KC, Bond JP, Vizzard MA, Girard BM, May V. Microarray analyses of. Pituitary adenylate cyclase-activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:18566–18570. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarovici P, Cohen G, Arien-Zakay H, Chen J, Zhang C, Chopp M, Jiang H. Multimodal neuroprotection induced by PACAP38 in oxygen-glucose deprivation and middle cerebral artey occlusion stroke models. Journal of Molecular Neuroscience. 2012;48:526–540. doi: 10.1007/s12031-012-9818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frechilla D, Garcia-Osta A, Palacios S, Cenarruzabeitia E, Del Rio J. BDNF mediates the neuroprotective effect of PACAP-38 on rate cortical neurons. Neuroreport. 2001;12:919–923. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 31.Reichenstein M, Rehavi M, Pinhasov A. Involvement of Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors in the mechanism of antidepressant action. Journal of Molecular Neuroscience. 2008;36:330–338. doi: 10.1007/s12031-008-9116-0. [DOI] [PubMed] [Google Scholar]
- 32.Holighaus Y, Weihe E, Eiden LE. STC1 induction by PACAP is mediated through cAMP and ERK1/2 but not PKA in cultured cortical neurons. Journal of Molecular Neuroscience. 2012;46:75–87. doi: 10.1007/s12031-011-9653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meuchel LW, Stewart A, Smelter DF, Abeejo AJ, Thompson MA, Zaidi SIA, Martin RJ, Prakash YS. Neurokinin-neurotrophin interactions in airway smooth muscle. American Journal of Physiology. 2011;301:L91–L98. doi: 10.1152/ajplung.00320.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vohra PK, Thompson MA, Satish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochimica et Biophysica Acta. 2013;1833:2953–2960. doi: 10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash YS, Tompson MA, Meuchel L, Pabelick CM, Mantilla CB, Zaldi S, Martin RJ. Neurotrophins in lung health and disease. Expert Reviews in Respiratory Medicine. 2010;4:395–411. doi: 10.1586/ers.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakash YS, Martin RJ. Brain-derived neurotrophic factor in the airways. Pharmacology & Therapeutics. 2014;143:74–86. doi: 10.1016/j.pharmthera.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Portbury AL, McConalogue K, Furness JB, Young HM. Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreativity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell and Tissue Research. 1995;279:385–392. doi: 10.1007/BF00318496. [DOI] [PubMed] [Google Scholar]
- 38.Brookes SJ, Song ZM, Steele PA, Costa MA. Identification of motor neurons to the longitudinal muscle of the guinea-pig ileum. Gastroenterology. 1992(103):961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- 39.Brookes SJH. Classes of enteric nerve cells in the guinea-pig small intestine. Anatomical Record. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Teng BQ, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. American Journal of Physiology. 1998;275:G342–G351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 41.Hellstrom PM, Murhty KS, Grider JR, Makhlouf GM. Coexistence of three tachykinin receptors coupled to Ca++ signaling pathways in intestinal muscle cells. Journal of Pharmacology and Experimental Therapeutics. 1994;270:236–243. [PubMed] [Google Scholar]
- 42.Murthy KS, Jin J-G, Grider JR, Makhlouf GM. Characterization of PACAP receptros and signaling pathways in rabit gastric muscle cells. American Journal of Physiology. 1997;272:G1391–G1399. doi: 10.1152/ajpgi.1997.272.6.G1391. [DOI] [PubMed] [Google Scholar]
- 43.Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. American Journal of Physiology. 2006;291:L447–L456. doi: 10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- 44.Abcejo AJ, Sathish V, Smelter DF, Aravamudan B, Thompson MA, Hartman WR, Pabelick CM, Prakash YS. Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One. 2012;7:e44343. doi: 10.1371/journal.pone.0044343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sopi RB, Martin RJ, Haxhiu MA, Dreshaj IA, Yao Q, Jafri A, Zaidi SIA. Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and imparement of relaxation in lung parenchyma. American Journal of Physiology. 2008;295:L348–L355. doi: 10.1152/ajplung.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grider JR, Acevedo JR, Bushman TL. Inhibition of neurite growth by BDNF, a neurotrophic factor expressed and secreted by myenteric neurons in culture. Gastroenterology. 1997;112:A739. [Google Scholar]
- 47.Rat D, Schmitt U, Tippmann F, Dewachter I, Theunia C, Wieczerzak E, Postina R, Leuven F, Fahrenholz F, Kojro E. Neuropeptide pituitary adenylate-cyclase activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB Journal. 2011;25:3208–3218. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zink M, Otto C, Zorner B, Zacher C, Schultz G, Henn FA, Gass P. Reduced expression of brain-derived neurotrophic factor in mice deficient for pituitary adenylate cyclase activating polypeptide type-I-receptor. Neuroscience Letters. 2004;360:106–108. doi: 10.1016/j.neulet.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Hammack SE, Cheung J, Rhodes KM, Schultz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekblad E, Jongsma H, Brabet, Bockaertj, Sundler F. Characterization of intestinal receptors for VIP and PACAP in rat and in PAC1 receptor knockout mice. Annals of the New York Academy of Sciences. 2000;921:137–147. doi: 10.1111/j.1749-6632.2000.tb06960.x. [DOI] [PubMed] [Google Scholar]
- 51.Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. International Journal of Physiology Pathophysiology and Pharmacology. 2012;4:188–200. [PMC free article] [PubMed] [Google Scholar]
- 52.Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: implications in brain-related diseases. World Journal of Biological Chemistry. 2014;5:409–428. doi: 10.4331/wjbc.v5.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuczewski N, Porcher C, Lessmann V, Medina I, Gaiarsa JL. Activity-dependent release of BDNF and biological consequences. Molecular Neurobiology. 2009;39:37–49. doi: 10.1007/s12035-009-8050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuemmerle JF, Murthy KS, Makhlouf GM. Agonist-activated, ryanodine-sensitive, IP3-insensitive Ca2+ release channels in longitudinal muscle of intestine. American Journal of Physiology. 1994;266:C1421–C1431. doi: 10.1152/ajpcell.1994.266.5.C1421. [DOI] [PubMed] [Google Scholar]
- 55.Kuemmerle JF, Murthy KS, Makhlouf GM. Longitudinal smooth muscle of the mammalian intestine: A model for Ca2+ signaling by cADPR. Cell Biochemistry and Biophysics. 28:31–44. doi: 10.1007/BF02738308. [DOI] [PubMed] [Google Scholar]
- 56.Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, Best J, Egemnazarov B, Weisel FC, Osswald SL, Schermuly RT, Olschewski A, Seeger W, Weissmann N, Eickelberg O, Fink L. BDNF/TrkB signaling augments smooth muscle proliferation in pulmonary hypertension. American Journal of Pathology. 2012;181:2018–2029. doi: 10.1016/j.ajpath.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 57.Yao Q, Haxhiu MA, Zaidi AI, Liu S, Jafri A, Martin RJ. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. American Journal of Physiology. 2005;289:L307–L314. doi: 10.1152/ajplung.00030.2005. [DOI] [PubMed] [Google Scholar]
- 58.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, Lu J, Fine A, Ai X. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. Journal of Neuroscience. 2011;31:15407–15415. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kemi C, Grunewald J, Ekland A, Hoglund CO. Differential regulation of neurotrophin expression in human bronchial smooth muscle cells. Respiratory Research. 2006;7:18. doi: 10.1186/1465-9921-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Shboul O, Nalli AD, Kumar DP, Zhou R, Mahavadi S, Kuemmerle JF, Grider JR, Murthy KS. Jun kinase-induced overexpression of leukemia-associated Rho GEF (LARG) mediates sustained hypercontraction of longitudinal smooth muscle in inflammation. American Journal of Physiology. 2014;306:C1129–C1141. doi: 10.1152/ajpcell.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. American Journal of Physiology. 2002;282:G226–G232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 62.Shea-Donohue T, Notari L, Sun R, Zhao A. Mechanisms of smooth muscle responses to inflammation. Neurogastroenterology and Motility. 2012;24:802–811. doi: 10.1111/j.1365-2982.2012.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]