Abstract
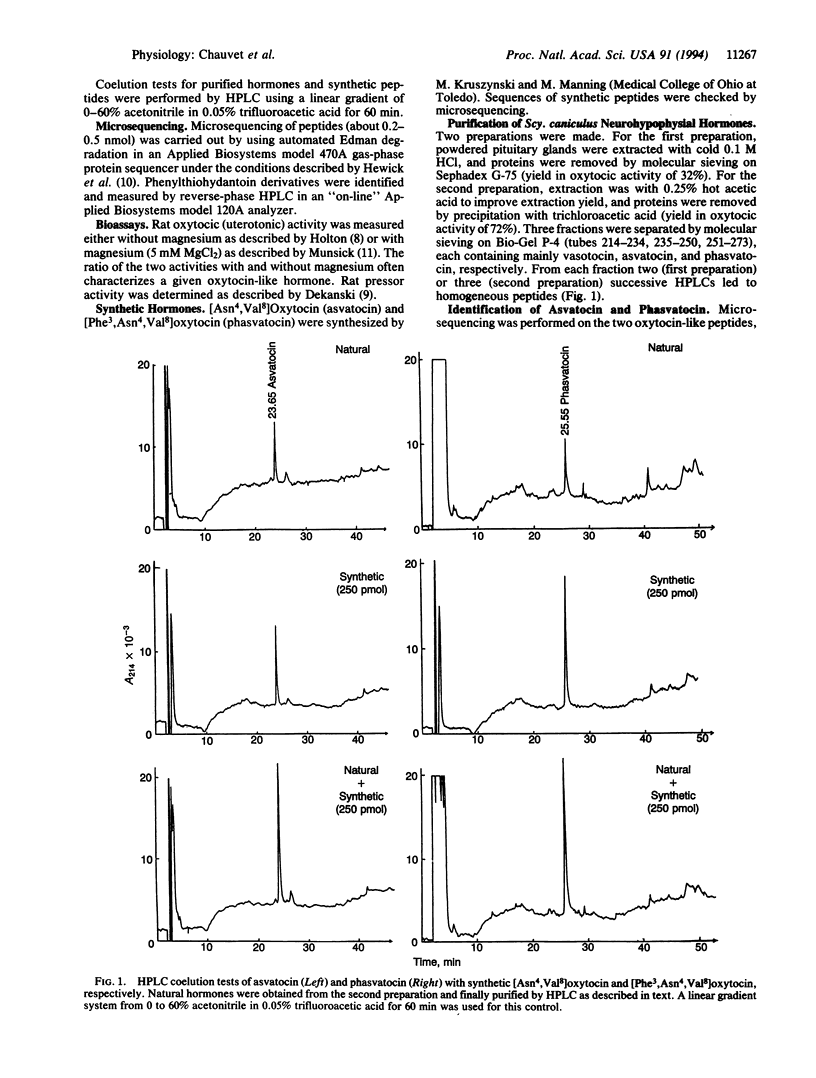
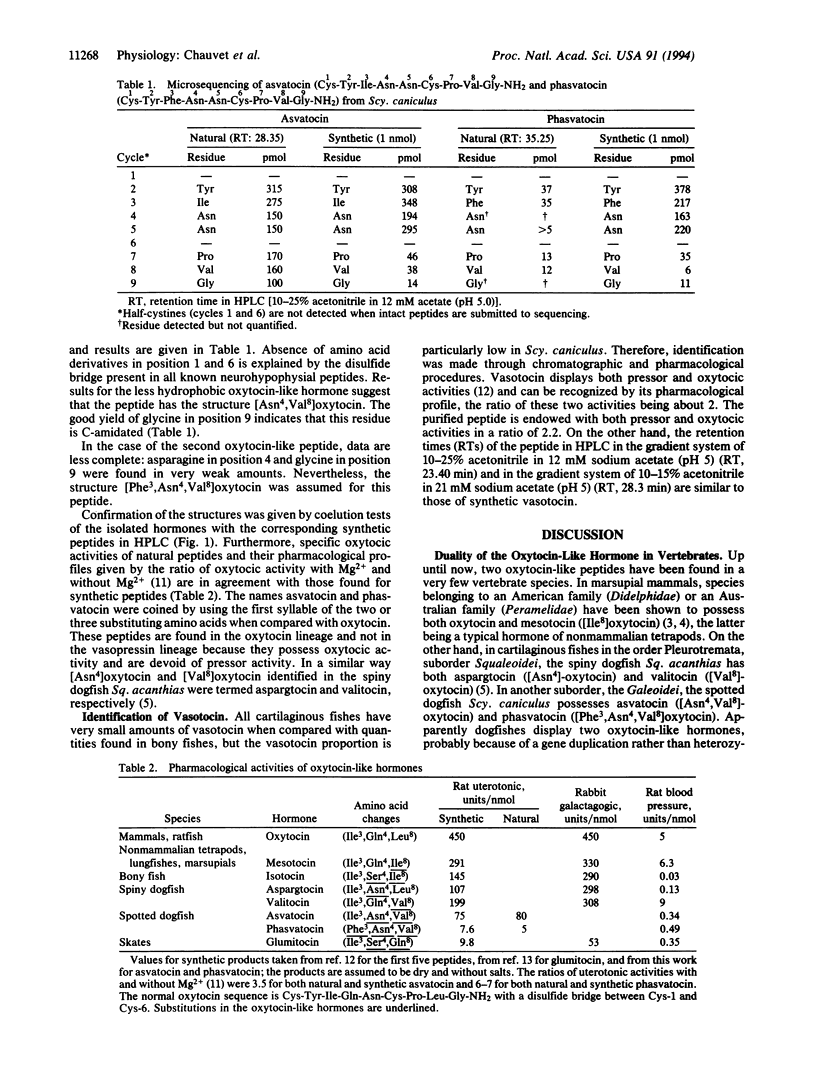
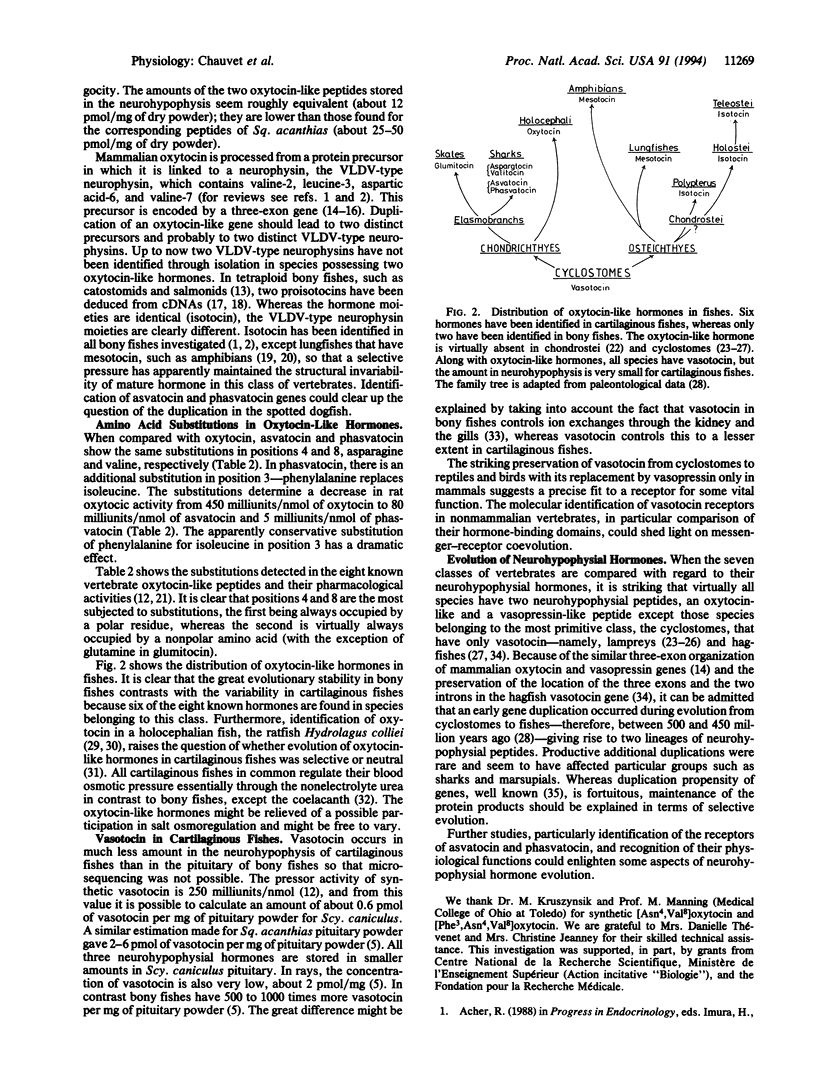
In contrast to most vertebrate species that possess one oxytocin-like hormone and one vasopressin-like hormone, a few groups, such as marsupials or cartilaginous fishes, are endowed with two peptides of either or both types, suggesting possible gene duplications. We have now isolated two oxytocin-like hormones from the pituitary of the spotted dogfish Scyliorhinus caniculus (suborder Galeoidei). Microsequencing as well as chromatographic and pharmacological comparisons with synthetic peptides show that these peptides are [Asn4,Val8]oxytocin (asvatocin) and [Phe3,Asn4,Val8]-oxytocin (phasvatocin). Asvatocin and phasvatocin display oxytocic activity on rat uterus, about 80 and 5 milliunits per nmol, respectively, and virtually no pressor activity on anesthetized rats. They occur in roughly equal molar amounts in the gland; vasotocin is also present in a proportional amount that is lower by about a factor of 20. In addition to the duality, conservative amino acid substitutions are observed in the two oxytocic peptides in positions 4 (Gln-4-->Asn) and 8 (Leu-8-->Val), when compared with oxytocin. Furthermore, replacement of the isoleucine residue found in position 3 of all other oxytocin-like hormones by phenylalanine in phasvatocin is exceptional; it determines a dramatic decrease of the oxytocic activity. Preservation of the C-terminal-amidated nonapeptide pattern in the 12 vertebrate neurohypophysial hormones known to date suggests that both precursors and processing enzymes have coevolved tightly. On the other hand, whereas the great evolutionary stability of the mature hormones (generally observed in vertebrates) suggests a strict messenger-receptor coevolution, the exceptional diversity found in cartilaginous fishes (six oxytocin-like peptides identified out of eight known) might be due to a looseness of selective constraints, perhaps in relationship with their specific urea osmoregulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acher R., Chauvet J., Chauvet M. -T. Molecular evolution of the neurohypophysial hormones: The active peptides of a primitive bony fish Polypterus bichir. FEBS Lett. 1970 Dec 18;11(5):332–335. doi: 10.1016/0014-5793(70)80561-0. [DOI] [PubMed] [Google Scholar]
- Acher R., Chauvet J., Chauvet M. T. A tetrapod neurohypophysial hormone in African lungfishes. Nature. 1970 Jul 11;227(5254):186–187. doi: 10.1038/227186a0. [DOI] [PubMed] [Google Scholar]
- Acher R., Chauvet J., Chauvet M. T., Crepy D. Molecular evolution of neurohypophysial hormones: comparison of the active principles of three bony fishes. Gen Comp Endocrinol. 1968 Dec;11(3):535–538. doi: 10.1016/0016-6480(68)90068-3. [DOI] [PubMed] [Google Scholar]
- Acher R., Chauvet J., Chauvet M. T. Phylogeny of the neurohypophysial hormones. Two new active peptides isolated from a cartilaginous fish, Squalus acanthias. Eur J Biochem. 1972 Aug 18;29(1):12–19. doi: 10.1111/j.1432-1033.1972.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Poulter R. T., Stockwell P. A. Gene duplication in tetraploid fish: model for gene silencing at unlinked duplicated loci. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5575–5579. doi: 10.1073/pnas.75.11.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. W., Jr, Brown S. G. Urea and its formation in coelacanth liver. Science. 1967 Feb 3;155(3762):570–573. doi: 10.1126/science.155.3762.570. [DOI] [PubMed] [Google Scholar]
- Chauvet J., Hurpet D., Michel G., Chauvet M. T., Acher R. Two multigene families for marsupial neurohypophysial hormones? Identification of oxytocin, mesotocin, lysipressin and arginine vasopressin in the North American opossum (Didelphis virginiana). Biochem Biophys Res Commun. 1984 Aug 30;123(1):306–311. doi: 10.1016/0006-291x(84)90413-3. [DOI] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT B. K., HELLER H. THE NEUROHYPOPHYSIAL HORMONES OF BONY FISHES AND CYCLOSTOMES. J Physiol. 1964 Jul;172:74–91. doi: 10.1113/jphysiol.1964.sp007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J., Morley S. D., Heierhorst J., Krentler C., Lederis K., Richter D. Two isotocin genes are present in the white sucker Catostomus commersoni both lacking introns in their protein coding regions. EMBO J. 1989 Oct;8(10):2873–2877. doi: 10.1002/j.1460-2075.1989.tb08435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heierhorst J., Lederis K., Richter D. Presence of a member of the Tc1-like transposon family from nematodes and Drosophila within the vasotocin gene of a primitive vertebrate, the Pacific hagfish Eptatretus stouti. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6798–6802. doi: 10.1073/pnas.89.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hyodo S., Kato Y., Ono M., Urano A. Cloning and sequence analyses of cDNAs encoding vasotocin and isotocin precursors of chum salmon, Oncorhynchus keta: evolutionary relationships of neurohypophysial hormone precursors. J Comp Physiol B. 1991;160(6):601–608. doi: 10.1007/BF00571256. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. DNA and the neutral theory. Philos Trans R Soc Lond B Biol Sci. 1986 Jan 29;312(1154):343–354. doi: 10.1098/rstb.1986.0012. [DOI] [PubMed] [Google Scholar]
- Lane T. F., Sower S. A., Kawauchi H. Arginine vasotocin from the pituitary gland of the lamprey (Petromyzon marinus): isolation and amino acid sequence. Gen Comp Endocrinol. 1988 Apr;70(1):152–157. doi: 10.1016/0016-6480(88)90104-9. [DOI] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- Manning M., Wuu T. C., Baxter J. W., Sawyer W. H. Solid phase synthesis and some pharmacological properties of 4-ser-8-gln-oxytocin (glumitocin). Experientia. 1968 Jul 15;24(7):659–660. doi: 10.1007/BF02138295. [DOI] [PubMed] [Google Scholar]
- Michel G., Chauvet J., Chauvet M. T., Clarke C., Bern H., Acher R. Chemical identification of the mammalian oxytocin in a holocephalian fish, the ratfish (Hydrolagus colliei). Gen Comp Endocrinol. 1993 Nov;92(2):260–268. doi: 10.1006/gcen.1993.1162. [DOI] [PubMed] [Google Scholar]
- Michel G., Chauvet J., Joss J. M., Acher R. Lungfish neurohypophysial hormones: chemical identification of mesotocin in the neurointermediate pituitary of the Australian lungfish Neoceratodus forsteri. Gen Comp Endocrinol. 1993 Sep;91(3):330–336. doi: 10.1006/gcen.1993.1133. [DOI] [PubMed] [Google Scholar]
- Pickering B. T., Heller H. Oxytocin as a neurophyophysial hormone in the holocephalian elasmobranch fish, Hydrolagus collei. J Endocrinol. 1969 Dec;45(4):597–606. doi: 10.1677/joe.0.0450597. [DOI] [PubMed] [Google Scholar]
- Rouillé Y., Chauvet M. T., Chauvet J., Acher R. Dual duplication of neurohypophysial hormones in an Australian marsupial: mesotocin, oxytocin, lysine vasopressin and arginine vasopressin in a single gland of the northern bandicoot (Isoodon macrourus). Biochem Biophys Res Commun. 1988 Jul 15;154(1):346–350. doi: 10.1016/0006-291x(88)90691-2. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Scherer G., Schütz G. Recent gene conversion involving bovine vasopressin and oxytocin precursor genes suggested by nucleotide sequence. Nature. 1984 Apr 5;308(5959):554–557. doi: 10.1038/308554a0. [DOI] [PubMed] [Google Scholar]
- Rurak D. W., Perks A. M. The neurohypophysial principles of the western brook lamprey, Lampetra richardsoni: studies inthe adult. Gen Comp Endocrinol. 1976 Jul;29(3):301–312. doi: 10.1016/0016-6480(76)90042-3. [DOI] [PubMed] [Google Scholar]
- Rurak D. W., Perks A. M. The pharmacological characterization of arginine vasotocin in the pituitary of the pacific hagfish (Polistotrema stoutii). Gen Comp Endocrinol. 1974 Apr;22(4):480–488. doi: 10.1016/0016-6480(74)90024-0. [DOI] [PubMed] [Google Scholar]
- Sausville E., Carney D., Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985 Aug 25;260(18):10236–10241. [PubMed] [Google Scholar]



