Abstract
Objective:
To systematically review temporal changes in perioperative safety of carotid endarterectomy (CEA) in asymptomatic individuals in trial and registry studies.
Methods:
The MEDLINE and EMBASE databases were searched using the terms “carotid” and “endarterectomy” and “asymptomatic” from 1947 to August 23, 2014. Articles dealing with 50%–99% stenosis in asymptomatic individuals were included and low-volume studies were excluded. The primary endpoint was 30-day stroke or death and the secondary endpoint was 30-day all-cause mortality. Statistical analysis was performed using random-effects meta-regression for registry data and for trial data graphical interpretation alone was used.
Results:
Six trials (n = 4,431 procedures) and 47 community registries (n = 204,622 procedures) reported data between 1983 and 2013. Registry data showed a significant decrease in postoperative stroke or death incidence over the period 1991–2010, equivalent to a 6% average proportional annual reduction (95% credible interval [CrI] 4%–7%; p < 0.001). Considering postoperative all-cause mortality, registry data showed a significant 5% average proportional annual reduction (95% CrI 3%–9%; p < 0.001). Trial data showed a similar visual trend.
Conclusions:
CEA is safer than ever before and high-volume registry results closely mirror the results of trials. New benchmarks for CEA are a stroke or death risk of 1.2% and a mortality risk of 0.4%. This information will prove useful for quality improvement programs, for health care funders, and for those re-examining the long-term benefits of asymptomatic revascularization in future trials.
Carotid endarterectomy (CEA) in asymptomatic individuals was introduced in the 1970s for long-term prevention of ipsilateral stroke1 and now comprises the bulk of those undergoing endarterectomy in the United States.2 However, the 10-year absolute risk reduction in stroke or death risk in the largest and most recent trial, the Asymptomatic Carotid Surgery Trial 1 (ACST-1), was only 4.6%, demonstrating small absolute benefit of CEA.3,4
On modern medical therapy, ipsilateral ischemic stroke rates have fallen,5 with 3 recent cohort studies demonstrating annual ipsilateral ischemic stroke risks in patients with 50%–99% asymptomatic stenosis of 0.3%–0.8%,6–8 less than the 0.96% risk in ACST.4 If CEA is to prove effective in the future, one of the prerequisites is that perioperative outcomes must become safer not only in trials, but in wider clinical practice.
The aim of this systematic review was to examine temporal trends in perioperative safety of CEA in asymptomatic individuals with 50%–99% internal carotid artery stenosis. When compared with symptomatic patients,9 the benefits of asymptomatic revascularization are less pronounced, and without marked improvements in operative safety, asymptomatic revascularization faces becoming a historical footnote. This analysis will provide valuable information for patients, clinicians, and those designing trials, and establish a benchmark for those funding reimbursement for safe surgery.
METHODS
Search strategy and selection criteria.
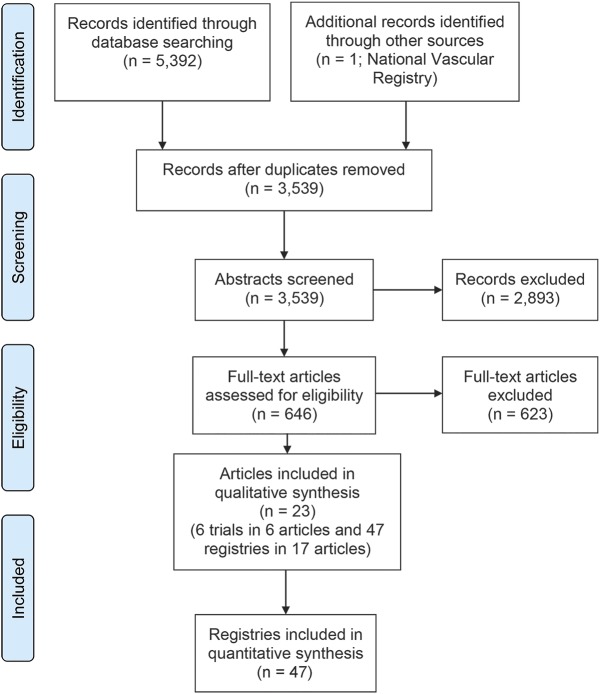
Two independent reviewers (A.B.M., M.I.Q.) performed a systematic literature review on August 23, 2014, according to Preferred Reporting Instructions for Systematic Reviews and Meta-Analyses guidelines.10 The MEDLINE and EMBASE databases were searched using key words “carotid” and “endarterectomy” and “asymptomatic” to identify all published studies from 1947 to August 23, 2014. No filters or limits were applied. Disagreements were settled by an independent, experienced reviewer (A.T.). The search process is summarized in figure 1.
Figure 1. Preferred Reporting Instructions for Systematic Reviews and Meta-Analyses diagram illustrates search results.
Articles published in any language were included if they satisfied the following criteria: registered research trials or surgical registries; analyzable data for a subset of patients with 50%–99% carotid stenosis and no recent ipsilateral or contralateral symptoms; and extractable incidence of 30-day perioperative stroke or death. Articles were excluded if they satisfied any one of the following 6 criteria: (1) studies exclusively concerning carotid angioplasty and stenting; (2) patients undergoing surgery for recurrent or nonatherosclerotic carotid disease; (3) patients undergoing staged contralateral carotid or combined cardiac procedures; (4) review articles, editorials, letters, case reports, case series, animal studies, and studies investigating the morphology or histology of atheromatous carotid plaques; (5) studies reporting data for fewer than 100 asymptomatic procedures, as the expected differences in perioperative risk were of the order of 1%, and thus cannot be precisely determined from small studies11; or (6) studies spanning more than a decade. One study12 reporting registry data from July 1976 to November 1993 was excluded as operations over this 17-year period were summated, the opposite of the purpose of this review.
Data extraction.
Articles were title, abstract, and full text screened. In the absence of sufficient data, authors were contacted by e-mail for further information. Two databases were compiled for analysis: research trials and clinical registries (tables e-1 and e-2 on the Neurology® Web site at Neurology.org, respectively). Data were extracted for one artery per patient where possible, to avoid clustering. Quality assessment of research trials followed CONSORT principles13 (table e-3), which were adapted for clinical registries (table e-4). Perioperative stroke or death, measured as 30-day incidence, was chosen as the primary composite endpoint as it reflects the outcomes the procedure is designed to prevent. It is the conventional safety endpoint for CEA in international guidelines.14,15 The secondary endpoint was all-cause mortality (death), an endpoint less sensitive to reporting bias, as it does not require a neurologist.9 Information on myocardial infarction was available but its definition has changed over time16 and only 3 trials reported data. Endpoints were adjudicated locally and not centrally by the authors of this study. The extraction of event frequencies was performed as follows: the denominator was the actual number of CEAs performed, not the number randomized (i.e., a per-protocol analysis). The numerator was the number of events that followed surgery to 30 days.
Statistical analysis.
An independent statistician (A.J.F.) performed the statistical analysis. For each study, the number of 30-day perioperative events and the total number of procedures was reported during the trial period. Given this format of the data, the mean 30-day incidence of perioperative events was assigned to the midpoint of the study recruitment period. Trials and registries were considered separately. Analysis of the former was limited to visual inspection of a scatterplot because of the low number of studies (figures 2 and 3). In the case of clinical registry data, a logistic meta-regression random-effects model for the intercept was conducted with 30-day incidence as the outcome and time as the explanatory covariate (figures 4 and 5). A random-effects meta-regression was adopted, aimed at estimating the effect of time on the average perioperative outcome with the rest of the variability between studies assumed to be overall random. A Bayesian approach was adopted by conducting the analysis in JAGS version 3.4.0 as detailed in the data supplement.17
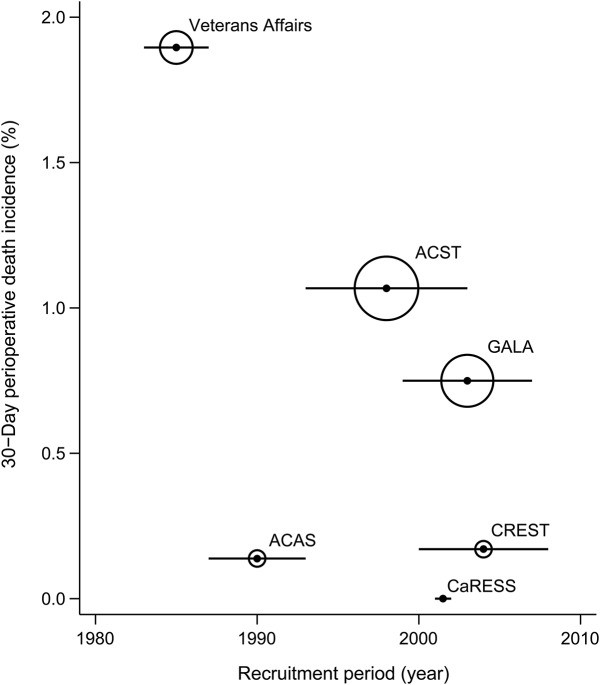
Figure 2. Temporal trends in 30-day perioperative stroke or death incidence in clinical trials.
Horizontal bar corresponds to recruitment period and size of bubble proportional to precision (reciprocal of variance) of results. ACAS = Asymptomatic Carotid Atherosclerosis Study; ACST = Asymptomatic Carotid Surgery Trial; CaRESS = Carotid Revascularization Using Endarterectomy or Stenting Systems; CREST = Carotid Revascularization Endarterectomy versus Stenting Trial; GALA = General Anaesthesia Versus Local Anaesthesia for Carotid Surgery.
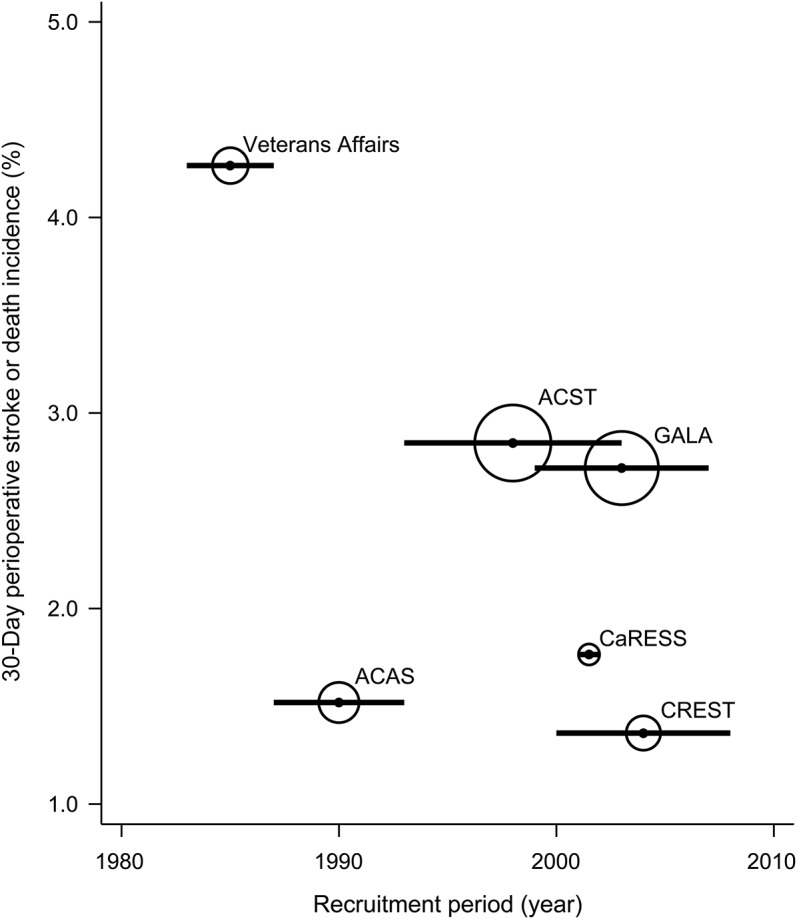
Figure 3. Temporal trends in 30-day perioperative death incidence in clinical trials.
Horizontal bar corresponds to recruitment period and size of bubble proportional to precision of results. ACAS = Asymptomatic Carotid Atherosclerosis Study; ACST = Asymptomatic Carotid Surgery Trial; CaRESS = Carotid Revascularization Using Endarterectomy or Stenting Systems; CREST = Carotid Revascularization Endarterectomy versus Stenting Trial; GALA = General Anaesthesia Versus Local Anaesthesia for Carotid Surgery.
Figure 4. Temporal trends in 30-day perioperative stroke or death incidence in clinical registries.
Meta-regression using random effects model with bubble size proportional to study precision. There was a statistically significant decrease in 30-day perioperative stroke or death incidence over the period 1991–2013, odds ratio 0.94 (95% credible interval 0.93–0.96, p < 0.001), translating into a 6% year on year mean risk reduction. The limits of the 95% credible interval and prediction interval have been drawn respectively in red and in blue. These data do not include preprocedural arteriography-related events.
Figure 5. Temporal trends in 30-day perioperative death incidence in clinical registries.
Meta-regression using random effects model with bubble size proportional to study precision. There was similarly a statistically significant decrease in the 30-day incidence of mortality over the period 1991–2013, odds ratio 0.95 (95% credible interval 0.92–0.97, p < 0.001), translating into a 5% year on year mean risk reduction. The limits of the 95% credible interval and prediction interval have been drawn respectively in red and in blue. These data do not include preprocedural arteriography-related events.
RESULTS
Excluded studies.
No data from the ongoing Stent-Protected Angioplasty in Asymptomatic Carotid Artery Stenosis vs Endarterectomy (SPACE2),18 Asymptomatic Carotid Surgery Trial 2 (ACST-2),19 and European Carotid Surgery Trial 2 (ECST-2) trials were available on request. Data from Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) were excluded because this was a trial specifically for high risk for endarterectomy patients.20 Data from 4 randomized controlled trials were not included because fewer than 100 CEAs in asymptomatic patients were performed during each study21–24 (range 7–84), leaving 6 research trials.3,4,25–28 Forty-seven registries were reported in 17 separate publications.29–45
Included study characteristics.
There were 6 eligible research trials comprising 4,431 endarterectomies, with recruitment between 1983 and 2008. There were 47 eligible clinical registries comprising 204,622 endarterectomies performed between 1991 and 2013. The event rates and characteristics of the trial and registry papers are detailed in tables e-1 and e-2, respectively.
Four out of 6 research studies were conducted in North America and 2 in Europe. Five were randomized controlled trials and the sixth was a high-quality cohort study.27 The number of endarterectomies ranged from 170 to 1,405 per study. Operator experience ranged from a minimum of 2 years' experience25 to 50 CEAs per year with a <3% stroke or death rate.27 The demographics of patients in all the trials were similar with an average age of 64–72 years and 65%–70% of participants were male. Medical treatment consisted of aspirin alone in Veterans Affairs; aspirin and discussion of risk factors in Asymptomatic Carotid Atherosclerosis Study (ACAS); aspirin, statins, and antihypertensives to varying degrees in ACST and Carotid Revascularization Endarterectomy versus Stenting Trial (CREST); and was not stated in General Anaesthesia Versus Local Anaesthesia for Carotid Surgery (GALA) and Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS). Inclusion criteria ranged from 50% to 99% stenosis in Veterans Affairs to 75%–99% stenosis in CaRESS and were not stated in GALA. Asymptomatic status was defined as “absence of cerebral infarction” in Veterans Affairs, “absence of cerebrovascular events or symptoms referable to the contralateral cerebral hemisphere within the previous 45 days” in ACAS, and “without stroke, transient cerebral ischemia or other relevant neurologic symptoms in the past 6 months” in ACST-1 and CREST and was not further specified in GALA or CaRESS. Endpoint adjudication was by an independent neurologist in all cases and 4 out of 6 studies had a blinded endpoint committee. Four studies were funded through government or charitable grants, with one industry-sponsored study and one with a mixture of both.
Of the 47 clinical registries, 17 were based in the United States, 11 in Germany, 4 each in Canada and the United Kingdom, 3 in Sweden, 2 in Australia, and a single dataset each from Austria, Finland, Hungary, Italy, Norway, and Switzerland. They comprised a mixture of vascular society, reimbursement claims, Veterans Affairs registries, and regional audits. The number of endarterectomies ranged from 113 to 17,692 per registry. There were no data on operator experience, patient demographics, medical treatment, or funding. Only one study had a small (7%) proportion of patients reviewed by an independent neurologist postprocedure.34 Inclusion criteria ranged from “no documented history of ipsilateral cerebral ischemia”33 to “no ipsilateral hemispheric symptoms in the past 6 months”38 and in 6 cases were not stated. Exclusion criteria were variable. In Medicare claims registries, it was assumed the data were from consecutive patients for reimbursement purposes; however, this was not necessarily the case for other registries.
Temporal trends in clinical trials.
The scatterplot in figure 2 illustrates that 30-day incidence of stroke or death shows a trend toward reduction over the last 30 years, from 4.3% reported in Veterans Affairs to <1.8% in CaRESS and CREST. However, this is reliant on the validity of the results from Veterans Affairs and ACAS. Without further data points, the significance of this trend is uncertain. Furthermore, in early trials such as Veterans Affairs and ACAS, 0.4%–1.2% of patients had stroke or death as a result of routine diagnostic arteriography, before the introduction of duplex. These arteriographic complications are not included in the 30-day outcomes but are recorded in the data supplement.
For perioperative death alone (figure 3), a similar visual trend was apparent, with a reduction from 1.9% in Veterans Affairs to <0.2% in CaRESS and CREST. This was also dependent on the results from Veterans Affairs and ACAS. Without further data points, the significance of this trend is also uncertain. Again in Veterans Affairs and ACAS, an additional 0%–0.2% of patients had a preprocedural death related to diagnostic arteriography.
Temporal trends in clinical registries.
There was a statistically significant decrease in 30-day perioperative stroke or death incidence over the period 1991–2013, odds ratio (OR) 0.94 (95% credible interval [CrI] 0.93–0.96, p < 0.001), translating into a 6% mean annual decrease in risk, relative to the previous year. There was high heterogeneity (I2 = 78, 95% CrI 70%–85%), as might be expected from registry data. The expected incidence of stroke or death for 1992 was 3.9% (95% CrI 3.0%–4.9%), with a 95% prediction interval (PrI) of 1.9%–7.7%. The respective figure for the last reported timepoint of 2013 was 1.2% (95% CrI 1.0%–1.5%), with a 95% PrI of 0.6%–2.4%. These data do not include preprocedural arteriography-related events.
Similarly, there was a statistically significant decrease in the 30-day incidence of mortality over the period 1991–2013, OR 0.95 (95% CrI 0.92–0.97, p < 0.001), translating into a 5% mean annual risk reduction. There was high heterogeneity despite the use of the hard endpoint of death, I2 = 57% (95% CrI 18%–77%). The mean incidence of death in 1992 was 1.3% (95% CrI 0.9%–1.8%), with a 95% PrI of 0.6%–2.5%. The respective figure for the last reported time point of 2013 was 0.4% (95% CrI 0.3%–0.5%), with a 95% PrI of 0.2%–0.8%. These data do not include preprocedural arteriography-related events.
Heterogeneity across studies.
The distribution of the posterior median contribution to the Cochran Q of each individual study revealed that heterogeneity was due to 5 studies: 4 for death or stroke32,37,40,43 (Vikatmaa et al.40: Italy data from VASCUNET [2007–2009]) and 136 for death only incidence. Sensitivity analysis was performed by excluding data from these studies. In this scenario, heterogeneity was substantially reduced, as expected; however, the OR estimates showed no significant difference from the base case analysis.
DISCUSSION
The results of this analysis demonstrate that the average 30-day incidence of stroke or death has decreased by almost two-thirds both in large-volume international registries and clinical trials over the last 30 years, from approximately 4% to less than 1.5% by 2013. A significant temporal improvement in operative safety was seen in registry data, but was less certain in trial data, due to limited data. As the 95% prediction intervals overlap in 1992 and 2013 for registry studies, it is clear that the results of some of the best performing centers in 1992 are equivalent to the worst performing centers in 2013. However, on average, there has been a significant temporal improvement in operative safety. It should be borne in mind that there was an additional risk of around 1% of a preprocedural stroke or death in early studies due to the need for routine diagnostic catheter arteriography. The temporal trends for death alone were nearly identical with a reduction in incidence from >1% to <0.5% over the last 30 years.
There was high heterogeneity in the registry results for stroke or death and also for the more robust endpoint of death alone. These may reflect selection bias, operator experience, adjudication, and reporting bias in these lower quality studies. In the future, explicit descriptions of asymptomatic status, method of calculation of stenosis, baseline medical therapy, endpoint adjudication, procedural technique, and adjunctive medications used perioperatively should be provided to explore heterogeneity in more detail. Notably, some better performing centers have reported outcomes pre-2000 that were equivalent to worse performing centers in 2013.
No temporal analysis has previously been performed in asymptomatic patients. This study analyzed research trial and registry data separately for average risk asymptomatic patients for CEA over the last 30 years. This approach allowed comparison of results from selected patients in expert centers with results from wider clinical practice, to provide information of what can be achieved alongside what is actually being achieved. Similar temporal trends were found for the endpoints of stroke or death and for the more robust endpoint of death alone as well as when excluding the main sources of heterogeneity in the registry data.
It is important to discuss the limitations of this study. First, source data for individual studies were presented over a range of years and breakdown by year was not possible due to the age of some of the studies. This was accounted for by assigning the mean 30-day incidence to the midpoint of the study period and excluding studies that spanned a decade or more. Second, a minority of patients had more than one procedure, which may have introduced a small degree of clustering. Third, it is well-established that a volume-outcome relationship exists with carotid surgery46 and it is probable that poor performers were less likely to submit data and therefore registry results may represent high volume, safer centers. Risks outside of these institutions cannot be extrapolated from this study. Fourth, it is acknowledged that registry data are less robust than trial data. It was important to incorporate registry data in this analysis for several reasons: registries capture real-world practice, have greater power to detect temporal trends in rare outcomes due to larger numbers, report the results of modern-day procedures performed up to 5 years after randomized trials close, and report results outside of the United Kingdom and United States. This problem was addressed by comparing the results of registries with the results of trials to look for consistency, as well as considering the endpoint of death alone, which did not rely on the adjudication of an independent neurologist. Fifth, results for stenosis subgroups were not examined, as subgroup data were not extractable. However, it has been demonstrated that stenosis is a weak predictor of future stroke47 and did not predict benefit from endarterectomy in ACAS3 or ACST.4 It is therefore largely irrelevant in considering operative safety. Sixth, due to the small number of research trials, no quantitative analysis could be performed, as the results would be sensitive to outliers. Finally, it could be suggested that the results from the Veterans Affairs trial are an outlier. Nevertheless, US case series from the same decade show a perioperative stroke incidence of 7.7%48 and 4.2%,49 respectively, with mortality quoted in the former of 3.1%. These corroborate the results of Veterans Affairs during this time period.
In an earlier study of symptomatic patients undergoing endarterectomy, no significant change in perioperative risks was found between 1985 and 2008.9 During this period, symptomatic patients were being operated on sooner after the index event, at more advanced ages with an anticipated increase in perioperative risks.50 Elective asymptomatic carotid surgery is different as time is available for prior optimization of medical therapy and for stringent patient and operator selection, which should improve outcomes.
The results of this study can be interpreted as follows: over the last 30 years, there has been on average a decrease by almost two-thirds in perioperative stroke or death and death risk during CEA. The reasons for this are multifactorial, but we suggest the following: more technical experience, increasing preoperative use of statin51 and dual antiplatelet therapy,52 postoperative blood pressure control,53 improved patient selection, centralization of carotid surgery,46 and patch closure.54 For example, in the early Veterans Affairs trial, medical therapy consisted of aspirin alone, whereas by the end of ACST-1 in 2007, more than 80% of patients were receiving antihypertensive and statin therapy.4 Similarly, before the early results of ACST-1, surgeons were routinely operating on asymptomatic patients older than 75 years, whereas European guidelines changed after this trial, recommending an age limit of <75 years in men and lower in women.55 The publication of better high-volume center outcomes in the United Kingdom has led to its Vascular Society recommending fewer, higher-volume vascular units.56 Finally, the introduction of patch arterial closure was demonstrated by Cochrane reviews to lower the perioperative risk of stroke or death.54 To address the variability in these factors, national audit of consecutive cases is recommended to allow centers to compare outcomes. In the United Kingdom, this has now been extended to individual surgeon outcomes. The results of this study provide a benchmark against which to compare outcomes internationally for surgeons, trialists, and health care funders.
It is argued by some that CEA for asymptomatic atherosclerosis has no place in modern medicine. However, despite improvements in primary prevention, the EUROASPIRE studies demonstrated that a large proportion of the European population are still failing to achieve control of cardiovascular risk factors, notably smoking, hypertension, diabetes, and obesity.57 Among those who fail to attain best medical therapy targets, there may be candidates for preventative carotid surgery, in whom the background stroke risk may be much higher.
The implications of this study for clinicians and patients are that the risks quoted from the early landmark ACST-1 and ACAS trials are out of date. New benchmarks for high-quality endarterectomy are a 30-day stroke or death rate of 1.2% and a 30-day mortality of 0.4%. The results of trials are today closely replicated in centers submitting registry data. Whether long-term efficacy for endarterectomy over medical therapy still exists for a specific population requires reexamination based on this evidence.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the following persons for supplying data: Prof. T.G. Brott (CREST), Dr. S. Lewis (GALA trial), Dr. T. Curran and Prof. M. Schermerhorn (NSQIP data), Dr. H. Söllner and Prof. H.-H. Eckstein (the QA registry of the BQS and the AQUA institute), Dr. N. Duschek (Austrian data), Dr. M.C. Stoner (NSQIP data), and S. Waton (National Vascular Registry). National Vascular Registry data are collected by or on behalf of the Healthcare Quality Improvement Partnership, who have no responsibility or liability for the accuracy, currency, reliability, or correctness of this publication.
GLOSSARY
- ACAS
Asymptomatic Carotid Atherosclerosis Study
- ACST-1
Asymptomatic Carotid Surgery Trial 1
- CaRESS
Carotid Revascularization Using Endarterectomy or Stenting Systems
- CEA
carotid endarterectomy
- CREST
Carotid Revascularization Endarterectomy versus Stenting Trial
- CrI
credible interval
- GALA
General Anaesthesia Versus Local Anaesthesia for Carotid Surgery
- OR
odds ratio
- PrI
prediction interval
Footnotes
Editorial, page 302
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
A.B.M.: participated in drafting and revising the manuscript for intellectual content, designing the study and interpreting the data, as well as coordinating the study and contributing to the acquisition of data. A.J.F.: participated in drafting the manuscript for intellectual content and interpreting the data, as well as performing the formal statistical analyses. M.I.Q.: participated in revising the manuscript for intellectual content, as well as contributing to the acquisition of data. A.T.: participated in drafting the manuscript for intellectual content, the conception of the study and analyzing the data, as well as supervising the study and contributing to the acquisition of data. A.H.D.: participated in revising the manuscript for intellectual content and the conception of the study, as well as supervising and coordinating the study.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Munster and A. Franchini report no disclosures relevant to the manuscript. M. Qureshi receives funding from the Circulation Foundation, Joint Royal College of Surgeons/Dunhill Medical Trust Fellowship, the Graham-Dixon Charitable Trust, and the Rosetrees Trust. A. Thapar and A. Davies report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Javid H, Ostermiller WE, Hengesh JW, et al. Carotid endarterectomy for asymptomatic patients. Arch Surg 1971;102:389–391. [DOI] [PubMed] [Google Scholar]
- 2.Halm EA, Tuhrim S, Wang JJ, Rojas M, Hannan EL, Chassin MR. Has evidence changed practice? Appropriateness of carotid endarterectomy after the clinical trials. Neurology 2007;68:187–194. [DOI] [PubMed] [Google Scholar]
- 3.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995;273:1421–1428. [PubMed] [Google Scholar]
- 4.Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 2010;376:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–e583. [DOI] [PubMed] [Google Scholar]
- 6.den Hartog AG, Achterberg S, Moll FL, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke 2013;44:1002–1007. [DOI] [PubMed] [Google Scholar]
- 7.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010;41:e11–e17. [DOI] [PubMed] [Google Scholar]
- 8.Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010;67:180–186. [DOI] [PubMed] [Google Scholar]
- 9.Rerkasem K, Rothwell PM. Temporal trends in the risks of stroke and death due to endarterectomy for symptomatic carotid stenosis: an updated systematic review. Eur J Vasc Endovasc Surg 2009;37:504–511. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology 2011;22:128. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke 1996;27:266–269. [DOI] [PubMed] [Google Scholar]
- 12.Mattos MA, Modi JR, Mansour A, et al. Evolution of carotid endarterectomy in two community hospitals: Springfield revisited: seventeen years and 2243 operations later. J Vasc Surg 1995;21:719–728. [DOI] [PubMed] [Google Scholar]
- 13.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698–702. [Google Scholar]
- 14.Kakisis JD, Avgerinos ED, Antonopoulos CN, Giannakopoulos TG, Moulakakis K, Liapis CD. The European Society for Vascular Surgery guidelines for carotid intervention: an updated independent assessment and literature review. Eur J Vasc Endovasc Surg 2012;44:238–243. [DOI] [PubMed] [Google Scholar]
- 15.Ricotta JJ, AbuRahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg 2011;54:e1–e31. [DOI] [PubMed] [Google Scholar]
- 16.White HD, Thygesen K, Alpert JS, Jaffe AS. Clinical implications of the Third Universal Definition of Myocardial Infarction. Heart 2014;100:424–432. [DOI] [PubMed] [Google Scholar]
- 17.Plummer M. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. Presented at the 3rd International Workshop on Distributed Statistical Computing; March 2003; Vienna.
- 18.Reiff T, Stingele R, Eckstein HH, et al. Stent-protected angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy: SPACE2: a three-arm randomised-controlled clinical trial. Int J Stroke 2009;4:294–299. [DOI] [PubMed] [Google Scholar]
- 19.Rudarakanchana N, Dialynas M, Halliday A. Asymptomatic Carotid Surgery Trial-2 (ACST-2): rationale for a randomised clinical trial comparing carotid endarterectomy with carotid artery stenting in patients with asymptomatic carotid artery stenosis. Eur J Vasc Endovasc Surg 2009;38:239–242. [DOI] [PubMed] [Google Scholar]
- 20.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004;351:1493–1501. [DOI] [PubMed] [Google Scholar]
- 21.Brown MM, Rogers J, Bland JM. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 2001;357:1729–1737. [PubMed] [Google Scholar]
- 22.Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004;54:318–324. [DOI] [PubMed] [Google Scholar]
- 23.Liu CW, Liu B, Ye W, et al. Carotid endarterectomy versus carotid stenting: a prospective randomized trial [in Chinese]. Zhonghua Wai Ke Za Zhi 2009;47:267–270. [PubMed] [Google Scholar]
- 24.Ling F, Jiao L-Q. Preliminary report of trial of endarterectomy versus stenting for the treatment of carotid atherosclerotic stenosis in China (TESCAS-C). Chin J Cerebrovasc Dis 2006;3:4–8. [Google Scholar]
- 25.Hobson RW, II, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis: The Veterans Affairs Cooperative Study Group. N Engl J Med 1993;328:221–227. [DOI] [PubMed] [Google Scholar]
- 26.GALA Trial Collaborative Group, Lewis SC, Warlow CP, et al. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet 2008;372:2132–2142. [DOI] [PubMed] [Google Scholar]
- 27.Zarins CK, White RA, Diethrich EB, Shackelton RJ, Siami FS. Carotid revascularization using endarterectomy or stenting systems (CaRESS): 4-year outcomes. J Endovasc Ther 2009;16:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brott TG, Hobson RW, II, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yates GN, Bergamini TM, George SM, Jr, Hamman JL, Hyde GL, Richardson JD. Carotid endarterectomy results from a state vascular society: Kentucky Vascular Surgery Society Study Group. Am J Surg 1997;173:342–344. [DOI] [PubMed] [Google Scholar]
- 30.Cebul RD, Snow RJ, Pine R, Hertzer NR, Norris DG. Indications, outcomes, and provider volumes for carotid endarterectomy. JAMA 1998;279:1282–1287. [DOI] [PubMed] [Google Scholar]
- 31.Kresowik TF, Bratzler D, Karp HR, et al. Multistate utilization, processes, and outcomes of carotid endarterectomy. J Vasc Surg 2001;33:227–234. [DOI] [PubMed] [Google Scholar]
- 32.Horner RD, Oddone EZ, Stechuchak KM, et al. Racial variations in postoperative outcomes of carotid endarterectomy: evidence from the Veterans Affairs National Surgical Quality Improvement Program. Med Care 2002;40:I35–I43. [PubMed] [Google Scholar]
- 33.Findlay JM, Nykolyn L, Lubkey TB, Wong JH, Mouradian M, Senthilselvan A. Auditing carotid endarterectomy: a regional experience. Can J Neurol Sci 2002;29:326–332. [DOI] [PubMed] [Google Scholar]
- 34.Middleton S, Donnelly N. Outcomes of carotid endarterectomy: how does the Australian state of New South Wales compare with international benchmarks? J Vasc Surg 2002;36:62–69. [DOI] [PubMed] [Google Scholar]
- 35.Kragsterman B, Parsson H, Lindback J, Bergqvist D, Bjorck M; Swedish Vascular Registry (Swedvasc). Outcomes of carotid endarterectomy for asymptomatic stenosis in Sweden are improving: results from a population-based registry. J Vasc Surg 2006;44:79–85. [DOI] [PubMed] [Google Scholar]
- 36.Stoner MC, Abbott WM, Wong DR, et al. Defining the high-risk patient for carotid endarterectomy: an analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg 2006;43:285–295. [DOI] [PubMed] [Google Scholar]
- 37.Feasby TE, Kennedy J, Quan H, Girard L, Ghali WA. Real-world replication of randomized controlled trial results for carotid endarterectomy. Arch Neurol 2007;64:1496–1500. [DOI] [PubMed] [Google Scholar]
- 38.Nault P, Elkouri S, Daniel V, Blanchard ME, Okrainec K; Québec Vascular Outcome Research Team with Electronic eXpertise (Vortex). Modification of outcomes by lowering ischemic events after reconstruction of extracranial vessels (MOLIERE): an internet-based prospective study to evaluate and improve the effectiveness of carotid endarterectomy. J Vasc Surg 2008;47:530–536. [DOI] [PubMed] [Google Scholar]
- 39.Calvillo-King L, Xuan L, Zhang S, Tuhrim S, Halm EA. Predicting risk of perioperative death and stroke after carotid endarterectomy in asymptomatic patients: derivation and validation of a clinical risk score. Stroke 2010;41:2786–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vikatmaa P, Mitchell D, Jensen LP, et al. Variation in clinical practice in carotid surgery in nine countries 2005-2010: lessons from VASCUNET and recommendations for the future of national clinical audit. Eur J Vasc Endovasc Surg 2012;44:11–17. [DOI] [PubMed] [Google Scholar]
- 41.Duschek N, Ghai S, Sejkic F, et al. Homocysteine improves risk stratification in patients undergoing endarterectomy for asymptomatic internal carotid artery stenosis. Stroke 2013;44:2311–2314. [DOI] [PubMed] [Google Scholar]
- 42.Deutsch L, Haller B, Söllner H, Storck M, Eckstein HH. Trends and results of carotid artery surgery in Germany 2003–2011: part 1: clinical stages, perioperative morbidity and mortality and assessment of quality indicators [in German]. Gefässchirurgie 2013;18:558–567. [Google Scholar]
- 43.Gupta PK, Ramanan B, Mactaggart JN, et al. Risk index for predicting perioperative stroke, myocardial infarction, or death risk in asymptomatic patients undergoing carotid endarterectomy. J Vasc Surg 2013;57:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran T, Lo RC, Fokkema M, et al. Predictors of 30-day readmission and postdischarge mortality following carotid endarterectomy in the ACS-NSQIP. J Vasc Surg 2013;57:93S–94S. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waton S, Johal A, Groene O, Cromwell D, Mitchell D, Loftus I. UK Carotid Endarterectomy Audit. Round 5. London: The Royal College of Surgeons of England; 2013. [Google Scholar]
- 46.Holt PJ, Poloniecki JD, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between hospital volume and outcome following carotid endarterectomy. Eur J Vasc Endovasc Surg 2007;33:645–651. [DOI] [PubMed] [Google Scholar]
- 47.Nicolaides AN, Kakkos SK, Kyriacou E, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486–1496. [DOI] [PubMed] [Google Scholar]
- 48.Brott T, Thalinger K. The practice of carotid endarterectomy in a large metropolitan area. Stroke 1984;15:950–955. [DOI] [PubMed] [Google Scholar]
- 49.Hertzer NR, Flanagan RA, Jr, Beven EG, O'Hara PJ. Surgical versus nonoperative treatment of asymptomatic carotid stenosis: 290 patients documented by intravenous angiography. Ann Surg 1986;204:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naylor AR. Time is brain! Surgeon 2007;5:23–30. [DOI] [PubMed] [Google Scholar]
- 51.Heyer EJ, Mergeche JL, Bruce SS, et al. Statins reduce neurologic injury in asymptomatic carotid endarterectomy patients. Stroke 2013;44:1150–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payne DA, Jones CI, Hayes PD, et al. Beneficial effects of clopidogrel combined with aspirin in reducing cerebral emboli in patients undergoing carotid endarterectomy. Circulation 2004;109:1476–1481. [DOI] [PubMed] [Google Scholar]
- 53.Bouri S, Thapar A, Shalhoub J, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg 2011;41:229–237. [DOI] [PubMed] [Google Scholar]
- 54.Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev 2009;4:CD000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liapis CD, Bell PR, Mikhailidis D, et al. ESVS guidelines: invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg 2009;37(suppl 4):1–19. [DOI] [PubMed] [Google Scholar]
- 56.NHS England. 2013/2014 NHS Standard Contract for specialised vascular services [online]. Available at: www.vascularsociety.org.uk/wp-content/uploads/2013/06/Service-Specification.pdf. Accessed November 25, 2014.
- 57.Kotseva K, Wood D, De Backer G, et al. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet 2009;373:929–940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.