Abstract
Children with perinatal stroke (PS) provide a unique opportunity to understand developing brain-behavior relations. Previous research has noted distinctive differences in behavioral sequelae between children with PS and adults with acquired stroke: children fare better, presumably due to the plasticity of the developing brain for adaptive reorganization. Whereas we are beginning to understand language development, we know little about another communicative domain, emotional expression. The current study investigates the use and integration of language and facial expression during an interview. As anticipated, the language performance of the five and six year old PS group is comparable to their typically developing (TD) peers, however, their affective profiles are distinctive: those with right hemisphere injury are less expressive with respect to affective language and affective facial expression than either those with left hemisphere injury or TD group. The two distinctive profiles for language and emotional expression in these children suggest gradients of neuroplasticity in the developing brain.
Keywords: Neuroplasticity, Focal lesions, Emotions, Expressivity, Narratives
1. Introduction
As early as the mid-eighteenth century it had been proposed that early brain injury would lead to more favorable outcomes than similar injury occurring later in life. Empirical studies later confirmed this hypothesis (Basser, 1962; Bates et al., 2001; Lenneberg, 1967; Reilly, Levine, Nass, & Stiles, 2008; Stiles, Reilly, Levine, Trauner, & Nass, 2012). Such positive results are generally attributed to neuroplasticity, the developing brain’s ability to flexibly adapt and reorganize (Cao, Vikingstad, Huttenlocher, Towle, & Levin, 1994; Chu, Huttenlocher, Levin, & Towle, 2000; Kirton & Deveber, 2006; Stiles et al., 2012). Evidence of brain plasticity stems from animal studies in a variety of areas: the motor cortex (Kennard, 1936, 1942), visual systems (Hubel & Wiesel, 1967; Hubel, Wiesel, & LeVay, 1977; Wiesel, 1982; Wiesel & Hubel, 1963, 1965), enhanced performance in enriched environments (Greenough & Chang, 1989; Hebb, 1947; Kempermann & Gage, 1998; Kempermann, Kuhn, & Gage, 1997; Rosenzweig & Bennett, 1972), the successful rewiring of cortical projections (Angelucci, Clasca, Bricolo, Cramer, & Sur, 1997; Neville, Schmidt, & Kutas, 1983; Sur, Garraghty, & Roe, 1988), and finally the development of transplanted fetal tissue (O’Leary and Stanfield, 1989).
While we have learned much about neuroplasticity from animal models, children with perinatal stroke (PS) offer an opportunity to better understand the nature and extent of neuroplasticity in humans. Looking across different cognitive domains in children with PS has yielded differing results (see Stiles et al., 2012 for a comprehensive review). For example, group studies of language in children with PS have shown initial delays in the onset of language regardless of lesion site (Bates et al., 2001; Rowe, Levine, Fisher, & Goldin-Meadow, 2009; Thal et al., 1991; Vicari et al., 2000). However, by middle childhood, spontaneous language is in the low-normal range (Bates et al., 2001; Reilly, Bates, & Marchman, 1998; Reilly, Losh, Bellugi, & Wulfeck, 2004). As such, the language profile of children with early stroke does not mirror that of adults with homologous lesions. Emotion is another communicative system. In contrast to the profile for early language, the few studies on emotion with infants and toddlers with PS (e.g., Nass & Koch, 1987; Reilly, Stiles, Larsen, & Trauner, 1995) have shown that the site of injury is associated with differential profiles of expressiveness and emotionality. Moreover, the infant profile is similar to that of adults with late onset strokes (Borod, Koff, Lorch, & Nicholas, 1985; Borod et al., 1998). To better understand the nature of neuroplasticity in the developing brain, the present study investigates language and emotional expression in young school age children with PS. Will their profile of affective expressivity follow that of language, such that by school age, the children with PS perform like their typically developing (TD) counterparts, regardless of lesion site? Or will the emotion profile continue to be characterized by site-specific deficits mirroring that of adults with acquired strokes? To address these questions, we investigate the production of emotional expression in both language and facial expression, as well as their integration during a semi-structured naturalistic biographical interview. Together these results will enhance our understanding of both the nature and extent of neuroplasticity in the developing brain.
1.1. Language development in children with perinatal stroke
Jules Cotard, a colleague of Paul Broca, suggested that unlike an adult with a comparable lesion, a child with an early left hemisphere stroke would not be aphasic (Cotard, 1868; Levin & Grafman, 2000). The reports of Lenneberg (1967) and Basser (1962) confirmed this observation leading to Lenneberg’s hypothesis of equipotentiality, that is, that either hemisphere of the brain could assume language functions. More recently, prospective studies of children with PS have provided an opportunity to understand the dynamics of early brain injury and their behavioral and cognitive sequelae (e.g. Ballantyne, Spilkin, Hesselink, & Trauner, 2008; Chilosi et al., 2005, 2008; Feldman, 2005; Reilly et al., 1998, 2004; Rowe et al., 2009; Stiles, Stern, Trauner, & Nass, 1996; Stiles et al., 2012; Yeatman & Feldman, 2013). Within the context of language development, studies have found distinctively different outcomes for children and adults with analogous lesions. In the adult language profile, the side, site, and the size of the lesion is associated with particular types of deficit. Studies from a broad range of languages have shown that adults with left hemisphere strokes are often aphasic (Broca, 1861; Goodglass, 1993; Goodglass & Hunter, 1970; Wernicke, 1874), whereas those with right hemisphere injury tend to be garrulous, and show deficits in discourse cohesion and in processing non-literal language (Abusamra, Côté, Joanette, & Ferreres, 2009; Joanette & Goulet, 1990, 1994; Joanette, Goulet, Ska, & Nespoulous, 1986; Lundgren, Brownell, & Keith, 2006). Imaging studies of healthy adults and neurological patients broadly confirm that for adults, a network subsuming left perisylvian regions is implicated in processing core aspects of language (Bookheimer, 2002; Brauer, Anwander, & Friederici, 2011; Price, 2010; Tyler & Marslen-Wilson, 2008; Tyler, Stamatakis, Post, Randall, & Marslen-Wilson, 2005). Importantly, young children with PS do not show the same pattern of linguistic deficits as adults with comparable lesions. Specifically, young children with either left or right hemisphere lesions present with delays in the major milestones of language acquisition: babbling, gestures, and both comprehension and production of first words (Bates et al., 1997; Marchman, Miller, & Bates, 1991; Reilly et al., 2008; Sauer, Levine, & Goldin-Meadow, 2010; Stiles, Bates, Thal, Trauner, & Reilly, 1998; Thal et al., 1991; Vicari et al., 2000). Within this profile of overall delay, several studies have found increased production deficits for those toddlers and preschoolers with left hemisphere injury (Bates et al., 1997; Chilosi, Cipriani, Bertuccelli, Pfanner, & Cioni, 2001; Vicari et al., 2000) whereas comprehension deficits were reported in toddlers with right posterior injury (Bates et al., 1997). Interestingly, comprehension deficits were not found in children with injuries to left temporal areas, as the adult profile would have predicted. Rather, those with left temporal injury showed delays in word production (Bates et al., 1997). Thus, during the early stages of language development, the children’s profiles do not map onto those of adults with homologous injuries. Moreover, the pattern of deficits strongly suggests that acquiring language, as opposed to maintaining a mature system, requires both the right and left cerebral hemispheres (Reilly et al., 2008). Imaging studies have lent support to this view: language processing in TD children is bilaterally mediated and left hemisphere specialization for language is a prolonged developmental process (Brown et al., 2005; Holland et al., 2001).
Remarkably, by the time children with PS enter school, their spontaneous language performance is broadly comparable to their TD peers. Bates et al. (2001) used speech samples from biographical interviews to examine spontaneous language production in brain-injured children and adults with comparable lesions. The adult stroke patients showed classic hemispheric differences with regards to language; none of these characteristics were found in children with early brain injury (ages 5–8). Overall the children with PS were in the normal range, that is, within one standard deviation of the normal mean for their ages, on all comparisons including frequency of morphological errors and use of complex syntax (Bates et al., 2001). In addition, performance of those children with left hemisphere injury (LHI) was comparable to that of children with right hemisphere injury (RHI). In sum, although there is an initial delay in reaching the early milestones of language acquisition for those with either LHI or RHI, children with PS eventually develop functional language and perform within the low-normal range in spontaneous speech by school age (Bates et al., 2001; Reilly et al., 1998, see Stiles et al., 2012 for a review).
This pattern of delay and development has also been demonstrated in oral picture story narratives. For example, Reilly and colleagues found that children with RHI or LHI were initially delayed on grammatical and narrative measures (Reilly et al., 1998, 2004). In the youngest group, ages 4–6, children with PS told shorter stories, made more morphological errors, produced fewer complex sentences, and included fewer story components in their narratives than did their TD peers. But by age 10, the PS group performed in the low-to-normal range on all morphosyntactic as well as narrative discourse measures, and there were no significant hemispheric differences.
These differential results in conversation and narratives point to the importance of discourse context in the linguistic performance of children with perinatal stroke. Some of the same children (ages 5–8) participated in both the conversational (Bates et al., 2001) and narrative studies (Reilly et al., 1998, 2004). In the conversation, their performance was in the normal range; in the picture story narrative, a cognitively more challenging task, the linguistic performance of these younger children with PS (both RHI and LHI) fell below their TD peers. In a complementary narrative study with 5–7 year olds with PS that used story stems, Demir, Levine, and Goldin-Meadow (2009) also found more impoverished narratives from the PS group. To investigate both later language development and how context influences performance, we asked children with PS (ages 7–16) to recount a personal narrative in response to the prompt, “Tell me about a time someone made you sad or mad,” (Reilly, Wasserman, & Appelbaum, 2012). In this case, not only is the child/adolescent asked to create her own story (as opposed to the pictures presenting the story), but the additional emotional component further increases the cognitive challenge. In this particular context we found that the language performance of the children with left hemisphere injury trailed that of the TD group in both morphosyntactic and discourse measures while the performance of the group with RHI was broadly comparable to the typically developing group.
In summary, after initial delay in the onset of language, children with PS tend to follow a steady developmental progression, acquiring language milestones in a similar sequence to their TD peers and performing within the low-to-normal range in spontaneous language production by middle childhood. Such profiles demonstrate the role of both hemispheres in acquiring language and the adaptive organization of the brain for language in the wake of an early insult. The distinctive patterns of results in varying contexts suggest that language in children with PS is more fragile than in the TD group, and finally, during adolescence, when language has lateralized to the left hemisphere in typically developing youngsters (e.g. Brown et al., 2005; Holland et al., 2009), we begin to see more site-specific deficits in the perinatal stroke group.
1.2. Emotional expression in children with perinatal stroke
In contrast to language, emotional expression in the PS group has garnered little attention. Although the literature on the perception of affect in both neurotypical and brain injured adults is large (e.g. Adolphs, Damasio, Tranel, & Damasio, 1996; Adolphs, Tranel, Damasio, & Damasio, 1994; Anderson & Phelps, 2000; DeKosky, Heilman, Bowers, & Valenstein, 1980; Fusar-Poli et al., 2009; Hamann, Stefanacci, Squire, Adolphs, & Tranel, 1996; Jansari, Tranel, & Adolphs, 2000; Kawasaki et al., 2001, 2005; Pessoa & Adolphs, 2010; Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009; Said, Haxby, & Todorov, 2011; Said, Moore, Engell, Todorov, & Haxby, 2010; Tsuchiya, Moradi, Felsen, Yamazaki, & Adolphs, 2009), few have investigated the spontaneous production of emotions in individuals with brain injury. The few studies examining production of emotions in the adult literature report a decrement in affective expression in those with RHI compared to those with LHI or neurotypical adults (Borod et al., 1998, 1985). Moreover, adults with RHI received ratings of less intense facial expressions when telling a monologue when compared to either LHI or TD groups (Kazandjian, Borod, & Brickman, 2007). Blonder and colleagues found that during interviews, adults with RHI showed reduced facial expressivity and smiled and laughed significantly less often than LHI and TD groups (Blonder, Burns, Bowers, Moore, & Heilman, 1993; Blonder et al., 2005).
Typically developing infants show a right hemisphere bias including temporo-occipital and frontal regions for processing facial expressions from as early as four months of age (e.g., Cassia, Kuefner, Westerlund, & Nelson, 2006; Grossmann et al., 2008) as well as activating core aspects of the adult face processing network as early as two months of age (Tzourio-Mazoyer et al., 2002). An early study by Davidson and Fox (1982) also demonstrated that by 10 months of age, the right and left frontal lobes were differentially activated in approach/avoidance situations. With respect to infants with PS, Reilly and colleagues investigated spontaneous facial expression in 10 infants with PS (5 LHI and 5 RHI: ages 6–24 months) compared to their TD peers in a mother–child free play situation (Reilly et al., 1995). They found that the infants with LHI clustered with the TD group and smiled often and easily. In contrast, the children with RHI showed depressed expression of positive affect while negative emotions were enhanced. These results mirror the adult stroke profile and are consonant with parental reports of toddlers with PS (Nass & Koch, 1987). As such, the infant profile for emotion expression mirrors that of adults; such findings suggest that early in the first year of life, the infant brain already reflects functional specification for affective expression.
1.3. The current study
The infant findings raise two important questions regarding development and the potential for reorganization, as well as the nature and extent of the observed deficit.
What is the nature of the deficit, if any, in emotional expression in children with right hemisphere injury? If so, does the deficit extend to language expression?
To what degree, if any, will the expressive deficit of the infants with right hemisphere injury change with age?
The children in the infant study (Reilly et al., 1995) study were pre-linguistic and totally dependent on non-verbal systems to communicate. As such, it may be that the decrement in positive facial expression in those infants with RHI reflects a broader emotional deficit. If this is the case, we might also see an atypical profile in the use of affective language. To address these questions, the current study investigates the production and use of emotional facial expression and affective language in spontaneous conversation in children with unilateral perinatal stroke. Specifically, to gauge overall expressivity while engaging in a dyadic interaction, we will first assess the structural proficiency of language, determining the rate of morphological errors committed and the use of complex syntax. Second, to assess the degree to which emotional language is implicated, we will calculate the percentage of affective clauses produced during the biographical interview and their valence. Third, we will investigate the production of facial expression during the entire biographical interview. Fourth, we will identify facial expressions during narrative segments with affective content; affective content will include positive and negative narrative segments. Fifth, we will examine the relationship between affective narrative and affective facial expression to determine the relations between emotional facial expression and the use of emotional language. For example, when a child tells a positive narrative, will they also produce a positive facial expression? Will negative facial expression be expressed when telling a negative toned story? And finally, if group differences exist within the PS group on these measures above, we will investigate whether lesion severity and gender play a role in the differences observed.
2. Methods
2.1. Participants
Perinatal stroke is defined as a cerebrovascular event that occurs in the last trimester of gestation, and up to the first month after birth. The estimated prevalence rate of PS is 1 in 4000 births (Lynch, Hirtz, DeVeber, & Nelson, 2002; Lynch & Nelson, 2001). A perinatal stroke often results in a significant lesion that can be diagnosed in utero, at birth, or months after birth when symptoms are noted. The participants in this study included 40 children between 5;0 to 6;8 years of age (see Table 1 for Demographic Information). Twenty children with unilateral PS (10 with LHI and 10 with RHI), and 20 TD children participated in this study. Archival data from a larger longitudinal study, the Project of Cognitive and Neural Development in San Diego, California were used for this study. The criteria for inclusion for all participants included: monolingual English background, normal hearing, and normal or corrected vision.
Table 1.
Demographic information.
| Group | TD | LHI | RHI |
|---|---|---|---|
| Number of subjects |
N = 20 | N = 10 | N = 10 |
| Gender | 10 Males, 10 Females |
4 Males, 6 Females |
6 Males, 4 Females |
| Mean age | 5.86 (0.52) | 5.79 (0.46) | 5.52 (0.61) |
Note: Standard deviations in parentheses.
The inclusion criteria for the PS group were a single, unilateral focal lesion in the absence of other more diffuse pathology (see Appendix A for lesion profiles). The insult must have occurred within the perinatal period, and was confirmed by a clinician and by Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) scan. Children in the TD group were recruited from the community, had no history of developmental delay and were neurologically intact, as confirmed by neurological assessments. In addition, all participants were checked for symmetrical facial expression, as hemiparesis (weakness on one side of the body) can influence the muscles of the face. A pre-screening of the video data was completed and no asymmetry was found on the face for any of the participants.
Appendix A.
Lesion group site(s) of lesion and severity.
| Child | Lesion side | Frontal | Temporal | Parietal | Occipital | Subcortical | Severity |
|---|---|---|---|---|---|---|---|
| 1 | LHI | + | + | + | + | + | 5 |
| 2 | LHI | + | + | − | − | + | 3 |
| 3 | LHI | + | − | − | − | + | 4 |
| 4 | LHI | + | + | + | − | + | 5 |
| 5 | RHI | + | + | + | + | + | 5 |
| 6 | LHI | + | + | + | + | + | 5 |
| 7 | RHI | − | + | + | − | + | 5 |
| 8 | RHI | + | + | + | + | + | 5 |
| 9 | LHI | − | − | − | − | + | 2 |
| 10 | RHI | − | − | + | − | + | 4 |
| 11 | RHI | + | − | − | − | − | 3 |
| 12 | RHI | + | + | + | + | + | 5 |
| 13 | LHI | + | − | − | − | + | 3 |
| 14 | LHI | + | + | + | + | + | 5 |
| 15 | RHI | + | + | − | + | + | N/A |
| 16 | RHI | + | + | + | + | − | N/A |
| 17 | LHI | + | − | − | − | + | 5 |
| 18 | RHI | + | + | + | − | N/A | 5 |
| 19 | RHI | + | − | − | − | + | 4 |
| 20 | LHI | + | − | + | − | + | N/A |
Note: +. Lesion is present in that region. −. No lesion present in that region.
Severity was rated on a 5-point scale with 1 being the smallest lesion and 5 being a large lesion involving multiple lobes.
A subset of these linguistic data (from 8 children with LHI and 8 with RHI) were reported in the Bates et al., 2001 study which investigated morphosyntax and compared the children’s grammatical production with that of adults with late onset lesions. Here we focus on the emotional aspects of their language and its integration with emotional facial expression.
2.2. Procedures
A semi-structured naturalistic biographical interview was conducted as subjects sat at a table across from the interviewer. The subjects were asked a set of open-ended questions (e.g., “what did you do over the weekend?”) and the interviewer subsequently followed up with questions and comments specific to the child’s responses. Sessions were videotaped and audio recorded. For video data, Video Home System (VHS) tapes were transformed into digital format to allow using the Eudico Linguistic Annotator (ELAN; Brugman & Russel, 2004; Lausberg & Sloetjes, 2009) for analyses. ELAN is a software platform used to create multi-tier annotations using audio and video data. ELAN permits real-time integration of behaviors while gathering information regarding duration, frequency, and latency.
Language data were recorded on audio cassettes and were transcribed using the Codes of Human Analysis of Transcripts (CHAT) system from the Child Language Data Exchange System database (CHILDES; McWhinney & Snow, 1985) transcribed texts. Coders evaluated the data for propositions, morphological errors, and instances of complex syntax. Narrative segments were then selected from the transcribed texts. Narrative segments were defined as two consecutive child-produced propositions on the same topic (Labov & Waletzky, 1967). Two independent raters then coded the narrative segments for emotional valence (positive, negative, neutral). Once narrative segments were selected from the texts, the corresponding video taped segments were isolated in ELAN for further analysis.
2.3. Facial expression coding conventions
All facial expressions produced during the biographical interview were isolated in ELAN and coded using the Facial Action Coding System (FACS; Ekman & Friesen, 1978; Ekman, Friesen, & Hager, 2002). FACS is a microanalytic coding system based on a taxonomy of facial behaviors identified by the individual muscle contractions that produce them. Facial muscle movements were coded from onset (beginning of muscle contraction) to apex (the highest intensity of the muscle movement). After facial expressions were FACS coded, they were then given a valence label (positive, negative, or other facial expression) according to FACS criteria (Ekman et al., 2002, p. 174).
2.4. Language coding conventions
The coding of language structures was modeled after Reilly et al.’s (1998, 2004) measures and is comparable to that of Bates et al., 2001 which include: language productivity, morphological proficiency, and use of complex syntax.
2.4.1. Language productivity: overall length
For language productivity, the total number of propositions produced by each subject was tallied. Propositions were defined as a verb and its arguments; from a semantic perspective a proposition roughly corresponds to a single event. The total number of child produced propositions was also used to control for varying lengths in the interviews as it serves as the denominator when examining morphological errors, use of complex syntax, production of affective language, and production of facial expression. A proportion is used to control for the variability in amounts of speech, as some children are more talkative than others.
2.4.2. Morphological proficiency: errors
All uncorrected morphosyntactic errors of commission or omission were tallied. Error types included: copula or auxiliary errors, agreement errors, errors in verb form, pronominal errors, and determiner errors. To control for varying speech lengths, the Rate of Morphological Errors was created by taking the total number of morphological errors and dividing by the total number of child produced propositions in the conversation.
2.4.3. Syntactic complexity
The number of complex sentences was counted to determine the frequency of complex syntax. Complex sentences are multiple propositions falling within a sentence intonation contour. The total number of complex sentences was tallied and included: all coordinate sentences, verb complements, relative clauses, passive sentences, and adverbial clauses. To control for length, the total number of complex sentences was divided by the total number of child-produced propositions to yield a Rate of Complex Syntax.
2.4.4. Affective propositions within narratives
Two independent raters identified the narrative segments within each biographical interview; then, narrative segments were coded for affective valence (positive, negative, or neutral). Affective propositions were obtained by tallying the propositions within the affective narrative segment. Examples of a positive, negative and neutral narrative segment follows:
Positive narrative segment:
CHI: I like to go to Hometown Buffet.
EXP: What kinds of things do you like to eat?
CHI: I like to eat grilled cheese sandwich…and I like macaroni and cheese…and noodles with brown sauce…and hot dogs…and cheeseburgers…
Negative narrative segment:
EXP: Oh are you like the head cheerleader?
CHI:No
CHI: I’m the uh mascot.
EXP: Oh!
CHI: But I…I don’t wanna wear it this season the um big thing the um the mascot and everything I don’t do that.
Neutral narrative segment:
EXP: Like what kind of art projects have you made?
CHI: The class, not everybody makes like bunnies, but I made a bunny.
CHI: I made butterflies with colored chalk that’s what everybody had to do.
(These three examples came from TD subjects between the ages of 6.41 and 6.50 years of age.)
2.5. Inter-rater reliability
For facial expression coding, a second independent coder coded 25% of the facial expression samples and agreement exceeded 85%. Both coders were FACS trained during a weeklong FACS workshop session hosted by Erika L. Rosenberg, Ph.D. on the campus of the University of California, Berkeley (Rosenberg, 2009, 2010). For the language samples, two independent coders coded 25% of the language samples for morphological proficiency and syntactic complexity; agreement exceeded 92%. For narrative segments, a second independent coder coded all of the narrative segments for valence and agreement exceeded 85%.
2.6. Lesion characteristics: location and severity of lesions
A clinical neuroradiologist provided documentation of lesion location and lesion severity (see Appendix A). Severity was rated qualitatively on a 5-point scale (adapted from Vargha-Khadem, O’Gorman, & Watters, 1985), with 1 being the smallest lesion and 5 being a lesion involving multiple lobes. Specifically, a rating of 1 indicated a focal ventricular dilation or atrophy seen on <3 cuts on CT or MRI. A rating of 2 showed a focal ventricular dilation or atrophy seen on >3 cuts on CT or MRI. A rating of 3 indicated a focal porencephaly involving one lobe only, <3 cuts on CT or MRI. A rating of 4 showed a focal porencephaly involving one lobe only, >3 cuts on CT or MRI. A rating of 5 indicated porencephaly or cortical atrophy involving multiple lobes. Scores were not available for three individuals with PS, 2 with RHI and 1 with LHI.
3. Results
To gauge the extent of expressivity within our groups, we will examine multiple channels of communication when children engage in a dyadic interaction. Language, facial expressions, and their interactions, will be investigated in children with PS to gauge the extent and limitations of neural plasticity in these domains after early brain injury. First, we will assess the formal aspects of language produced during affective narrative segments. Second, to assess the production of emotional content expressed in the children’s language, we will determine the percentage of affective propositions and their valence during the biographical interview. Third, we will investigate the production of facial expression during the entire biographical interview. Fourth, in order to investigate the production and use of emotional facial expression when telling an affective story, we will calculate the amount of facial expressions produced during affective narrative segments. Fifth, in order to assess the extent to which affective language and affective facial emotions co-occur we will examine the relationship between affective narrative and affective facial expression. Finally, if group differences exist between the PS group for the measures examined above, severity of lesion and gender will be examined to see whether these characteristics contributed to the group differences.
3.1. Measures of linguistic structure
The following set of analyses examines language performance during the biographical interview. Table 2 includes results for the linguistic variables, including mean values and standard errors for measures of length (narrative propositions), morphological error rates, and complex syntax rates for all three groups. As expected, and consonant with Bates et al., 2001, no significant differences were found between groups for measures of linguistic structure. However, a trend was found for the production of propositions (p = .063) during narrative segments: both the LHI and RHI groups produced fewer clauses than the TD group during the biographical interview.
Table 2.
Linguistic measures during narratives.
| Group number of subjects | TD n = 20 | LHI n = 10 | RHI n = 10 |
|---|---|---|---|
| Propositionsa | 56.4 (5.57) | 36.6 (9.34) | 32.2 (11.13) |
| Morphological error ratesb | 0.07 (0.01) | 0.04 (0.02) | 0.06 (0.02) |
| Complex syntax ratesc | 0.60 (0.02) | 0.55 (0.05) | 0.48 (0.09) |
Note: All parenthesized values represent standard error (SE).
Includes all propositions (positive, negative, & neutral) in narrative sequences.
Rate of morphological errors per proposition.
Rate of complex syntax per proposition.
3.2. Production of affective propositions during the biographical interview
In this series of analyses, we examined (1) whether children produce affective content during a dyadic interaction by calculating the proportion of affective propositions over all the propositions produced during the entire biographical interview; and (2) the valence (positive/negative) of these propositions. A proportion was calculated by dividing total affective propositions by the total propositions of the entire biographical interview. Results of a one-way analysis of variance (ANOVA) revealed a significant group difference, F(2, 37) = 6.844, p = .003. Post hoc analyses using Bonferroni adjusted alpha levels revealed a significant difference in the use of affective language between the RHI group and the LHI group (p = .010), and between the RHI group and the TD group (p = .005). The RHI group produced a smaller proportion of affective propositions during the biographical interview than either the LHI or TD groups (Fig. 1). Since group differences in the PS group were observed, we then investigated if the severity of the injury correlated with the production of affective propositions during the biographical interview. Rowe et al. (2009) found lesion size to play a significant role in the development of early language when examining mean length of utterances (MLU) in children with early brain injury. In their study, larger lesions resulted in lower MLU compared to TD children, while small and medium lesions did not differ from their TD peers (Rowe et al., 2009). With our data, we did not find a relation between the severity of the injury and the quantity of affective propositions produced for the RHI group, r = 0.16, n = 8, p = 0.691. Similarly, no differences were observed for the LHI group, r = 0.09, n = 9, p = 0.811. As for gender within the PS group, a t-test revealed no significant difference for males with PS (M = 0.36, SD = 0.20) and females with PS (M = 0.41, SD = 0.28); t(18) = 0.46, p = 0.64 in the production of affective clauses.
Fig. 1.
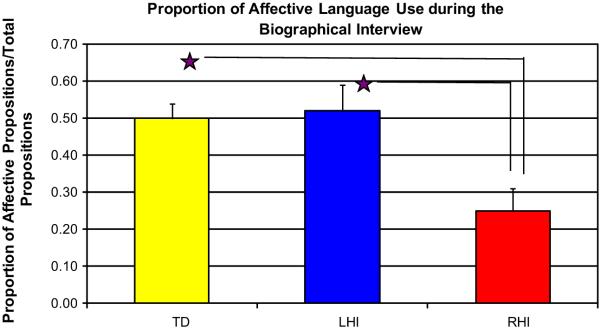
Proportion of affective language used during the biographical interview. The TD and LHI groups are producing twice as much affective content as the RHI group during the course of the interview.
Since all three groups are producing some affective content, a 3 × 2 (Group: TD, LHI, RHI × Affect Type: Positive, Negative) two-way ANOVA was used to examine the valence of these affective propositions (Fig. 2). An interaction was found for affect type between the TD and RHI groups F(2, 74) = 6.001, p = .004. The TD group on average produced more positive than negative content, while the RHI group produced more negative than positive content; the LHI group falls between the two groups.
Fig. 2.
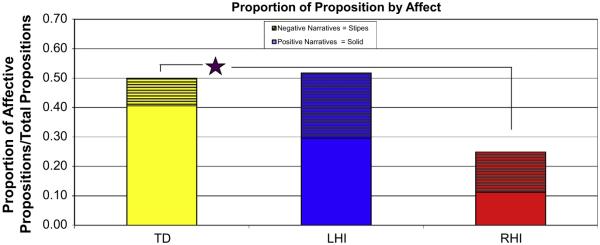
Proportion of proposition by affect for each group. The TD group is producing significantly more positive than negative affect in their narratives. While the RHI group show an almost even distribution of positive/negative narratives.
3.3. Production of facial expressions during the entire biographical interview
Our first question regarding facial expression concerns the production of all facial expressions during the biographical interview. Due to varying lengths of conversation, an average frequency of facial expression per minute was tabulated for each child. A one-way analysis of variance (ANOVA) revealed a significant group difference, F(2, 37) = 3.34, p = .046, in the production of facial expression during the entire interview (Fig. 3). The RHI group produced significantly fewer facial expressions per minute (M = 4.75) than either the TD (M = 6.70; p < .025) or LHI (M = 6.91; p < .03) groups. Since group differences in the PS group were observed, we then measured whether the severity of lesion correlated with the production of facial expression. For both the RHI, r = −0.26, n = 8, p = 0.530, and LHI, r = −0.43, n = 9, p = 0.237, no significant correlations were found. As for gender within the PS group, a t-test revealed no significant difference for males with PS (M = 5.32, SD = 1.75) and females with PS (M = 6.33, SD = 2.38); t(18) = 1.07, p = 0.29 in the production of facial expression.
Fig. 3.
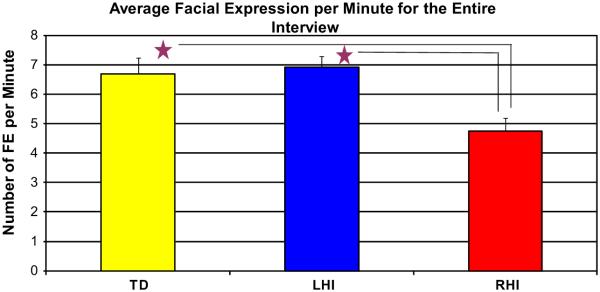
Frequency of facial expression per minute during the entire biographical interview. The TD and LHI groups are producing significantly more facial expression than the RHI group over the course of the biographical interview (Error bars reflect standard errors).
3.4. Production of facial expressions during affective narratives
This series of analyses focuses on how the children use facial expression as they relate an affective story, and the degree to which facial expression maps onto affective linguistic content. As noted previously, research in infants with PS found that the infant profile mirrored that of adults with late onset stroke: both infants and adults with RHI expressed less emotion overall, and more negative than positive affect. In the current study, a one-way ANOVA revealed no significant group differences in the frequency of facial expression when telling affective narratives (p = .308). Upon closer review, high levels of variability are seen in all three groups and although no statistical group differences were observed, the RHI group (M = 0.42, SE = 0.11) on average produced relatively fewer facial expressions than both the LHI (M = 0.64, SE = 0.10) and TD (M = 0.72, SE = 0.12) groups.
To examine the valence of the facial expressions, a 3 × 3 (Group: TD, LHI, RHI × FE Type: Positive, Negative, Other) two-way ANOVA was used. A main effect was found for FE Type, F(2, 111) = 19.968, p < .001, as positive facial expressions were produced more often than negative or other facial expressions.
3.5. Relationship between affective narratives and affective facial expressions
Our next analyses examined the relation between affective language in narratives and affective facial expression within each group. Overall, when telling positive narratives (Fig. 4a, left), all three groups produce semantically matching positive facial expression: for TD group t(38) = 4.285, p < .001, for LHI group t(12) = 3.08, p = .010, for RHI group t(8) = 2.892, p = .02. Very few negative facial expressions (mismatches) are produced when telling a positive narrative. When recounting negative narratives (Fig. 4b, right), positive facial expressions also predominate in all three groups. In other words, negative narratives are not accompanied by negative facial expressions; rather mismatches in affective valence are the rule, with positive expressions co-occurring with negative narrative segments. For negative narratives, within group comparisons yielded statistical significance for only the TD group: when telling negative narratives they were overwhelmingly producing more positive than negative facial expressions, t(22) = 2.654, p = .014.
Fig. 4.
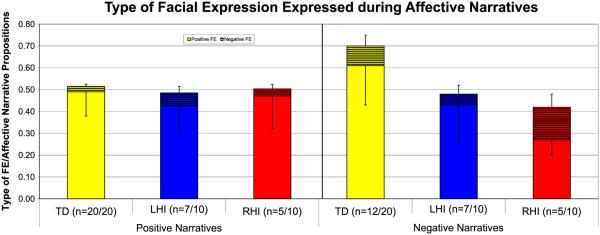
(a and b) Facial expression produced when telling an affective narrative. The n-values indicate the number of subjects producing affective facial expression when telling that particular type of affective narrative. (a) Positive Narratives: Overall, all groups use positive facial expressions when telling positive narratives. (b) Negative Narratives: all groups tend to use positive expressions when telling negative stories; however only the TD group reached statistical significance.
4. Discussion
This study investigated the nature and extent of neuroplasticity within the brain for emotional expression by examining the production of emotional narratives and the use of affective facial expression in children with perinatal stroke. A semi-structured naturalistic biographical interview was utilized to gather both spontaneous language and facial expression data in children at 5–6 years of age. First, when examining the structural aspects of language, no differences were found for the rates of morphological error or frequency of complex syntax across the three groups. Second, we examined whether children produced affective content during a social interaction by calculating the proportion of affective propositions and the accompanying valence of these propositions. Results showed the TD and LHI groups were producing twice as much affective content as the RHI group, in addition, the TD group on average produced more positive than negative content, while the RHI group produced more negative than positive content. Thirdly, we examined the entire biographical interview for the production of facial expression and found the RHI group produced significantly fewer facial expressions per minute than either the TD or LHI groups. Fourthly, facial expressions and their valence were calculated during affective linguistic content. Affective linguistic content included narrative segments that were judged to be either positive or negative in tone. Results revealed no group differences as large amounts of variability were observed in all three groups. With regards to valence, all three groups produced more positive than negative facial expressions. Finally, we examined the relation between affective language production and the co-occurrences of affective facial expression within each group. When recounting positive experiences, a matching positive facial expression is usually observed in all three groups. When telling negative experiences, positive facial expression (mismatches) generally is present for all three groups. Taken together, these results show evidence for persistent hemispheric differences in the expression of emotion: the children with RHI displayed a flatter affective profile for both the production of affective language and affective facial expressions than either the LHI or TD groups. With regards to valence, the RHI profile is characterized by a decrement in the expression of positive affect in their linguistic content, however this was not observed in their facial expression profile.
When examining language production, there was no statistical difference in terms of the quantity of talk between the three groups. From a structural or grammatical perspective, all three groups performed comparably as no differences were observed with respect to morphological errors and using complex syntax. As such these data are consistent with the findings from Bates et al., 2001. This provides further evidence that language production deficits seen before school age has resolved, at least in a dyadic conversational context. In addition, the results of this study contrast with the adult stroke model for language, as classic hemispheric differences were not present in children with PS at 5–6 years of age.
However, from a functional perspective, that is, how the children choose to use language, we see evidence for hemispheric differences: those children with RHI produce far fewer affective segments than either the LHI or TD groups. These findings suggest that emotion deficits, identified in the RHI group prelinguistically are still present at age 5 and 6; at this age, they are now also reflected in the linguistic expression of emotion. Considering the valence of the narratives, the TD group produced overwhelmingly more positive than negative propositions, followed by the LHI group, while the RHI group showed the reverse profile, producing more negative than positive content in their stories. The production of increased negative affect expressed through language in children with RHI again reflects the earlier identified profile, but in a newly acquired communicative system, language. Such a profile is also consonant with that of the adult stroke literature where negative affect is expressed more frequently by those with RHI as compared to LHI and TD groups (Blonder et al., 1993, 2005).
When examining the overall production of facial expression during the biographical interview, the TD group and those with LHI clustered together whereas the RHI group is less expressive overall than either the LHI or TD groups, again, mirroring both the infant and adult profiles. Even though the duration of the interviews were comparable across the three groups, the RHI group still produced fewer facial expressions compared to the LHI and TD groups when discussing their experiences. Gender and lesion severity were also examined in the PS group and no differences were observed for these two measures with respect to overall production of facial expression during the biographical interview.
When examining the production of facial expression during affective narratives, even with high variability within all three groups, the RHI group is once again less expressive compared to either the LHI or TD groups. With respect to the valence of the facial expressions produced, all three groups produced more positive than negative facial expressions while conversing during these narratives. Finally, when examining the relationship between the spoken affective narrative and its corresponding facial expression, different patterns were found depending on the valence of the story. When producing positive narratives, all three groups produce predominantly positive expressions, that is, semantic matches. For negative narratives, all three groups were also producing mostly positive facial expressions: semantic mismatches. The bias toward positive content and positive facial expression in the interviews is not too surprising considering the nature of the biographical interview: the majority of narrative topics discussed were neutral to positive. The tendency to produce positive facial expression during negative topics is more perplexing. These results may be attributed to several factors including: more self-control of their emotions, learning cultural and social display rules, and/or more experience with discourse level communication (Meza et al., 2010; Reilly & SalamancPlease update reference ‘Reily et al., in preparation; Salamanca, Littlewort, Bartlett, & Reilly, 2011). Reilly and colleagues have found similar results in their group of typically developing 7–8 year-olds, but interestingly, not in the 3–4 year-olds who are more likely to use both positive and negative face-story content matches. Given that different cultures have different social conventions for expressing emotions (so-called “display rules”), Reilly and colleagues suggest that as children age, they acquire culture-specific display rules, and the executive control to “mask” their emotions. In turn, masking implies increased cognitive control for emotion and the emergence of top-down processing of emotional expression (see Monk et al., 2008). An additional pertinent factor may be children’s increased ability to take multiple perspectives. Specifically, while telling a sad or distressing story, the older children, but not the younger, can to some extent attend both to their own story and their interlocutor.
Perhaps the most striking finding in our data is the apparently differing developmental profiles for these two communicative systems: emotional expression and language. The growing body of research on language development in the PS group points to initial delays with site specific deficits resolving by school age (see Stiles et al., 2012 for an overview; Reilly et al., 2012) and then reappearing under increased cognitive demands, whereas the few studies on emotional expression point to a right hemisphere profile of flatter affect as well as increased negative expression in the first year of life (Reilly et al., 1995). The results from the current study regarding flatter affect are broadly consistent with the infant and adult profile for emotional expression: five and six year-old children with RHI use less affective language and produce facial expression less frequently than either those with LHI or their TD peers. The flatter affect observed in children with RHI, but not in children with LHI, is consistent with past research on emotion processing and the role of the right hemisphere. In the late nineteenth century Hughlings Jackson (1874, 1880) observed the sparing of emotional words in aphasics, and Mills (1912) suggested a link between the right hemisphere and emotion processing as emotional expression was reduced when lesions were localized within the right hemisphere. More recently, studies from Borod et al. (1985, 1998) and Blonder et al. (1993, 2005) pinpoint the right hemisphere in emotional processing as individuals with RHI had flatter affect while this pattern was not observed in individuals with LHI.
Since the affective profile of deficits seen in the children with PS maps onto both that of infants with PS, as well as that of adult stroke patients, our results suggest more limited neuroplasticity for emotional expression than language in the developing brain. What might explain the differential degrees of plasticity for these two communicative systems, language and emotion? Several possible factors/explanations come to mind. One factor that may broadly contribute to differences observed in the degree of plasticity across these systems might be related to phylogeny. The affective system is evolutionarily older than language. Even animals as ancient as reptiles show emotional responses. For example, a tortoise whose territory has been invaded will bob his head to frighten a perceived enemy. Thus older brain regions, e.g., the limbic system, may be more predetermined and constrained than those of more recent systems, e.g., language, a cortically dominated system. Another, and complementary possibility is that for those systems that emerge early in development (implying early neural specification), there may be increased limitations on how the developing brain can reorganize after an early insult. Days old infants produce canonical facial expressions (Geangu, Benga, Stahl, & Striano, 2010; Oster, 1997) and studies have shown that by their first birthday, babies are competent emotional communicators, both interpreting (Stern, 2009) and producing emotional expressions meaningfully. For example, social smiles appear by six weeks of age (Spitz, 1965; Sroufe & Waters, 1976) and are used both to respond to others’ smiles and by three-four months to initiate an interaction. Stenberg, Campos, and Emde (1983) found that 7-month-olds express anger by furrowing their brows when cookies were withdrawn as well as when the babies were physically restrained. Infants at 7 months also show distress during the visual cliff experiment (Campos, Barret, Lamb, Goldsmith, & Stenberg, 1983). Such early developing behaviors reflect early neural specification: electrophysiological studies of infants show a right hemisphere bias for faces by three months of age (Cassia et al., 2006) and by six months, infants also show a right hemisphere bias for particular emotional expressions (Nelson & de Haan, 1997). As such, the developmental timing of emotional expression maturation may impose constraints on the development of alternative pathways.
In contrast, language development has a protracted time frame with mastery extending well into adolescence (Berman, 2004; Nippold, 1998). As a complex cognitive system, the protracted development of language may be associated with an increased potential to be supported by alternative networks. Lenneberg (1967) suggested that in the case of early insult, if the young brain has not yet committed its full complement of resources to its prespecified function, e.g., language, the brain has the capacity to reorganize, and uncommitted resources will support developing functions. In fact, he proposed that both hemispheres were ‘equipotential’ for acquiring language. Current functional imaging studies from typically developing children have demonstrated that for school age children, language is distributed, and that both the left and right hemispheres are recruited for language tasks (Brown et al., 2005; Holland et al., 2001). Moreover, it has been shown that the mature adult pattern of left hemisphere activation is progressive and does not emerge until well into adolescence (Holland et al., 2009; Szaflarski, Holland, Schmithorst, & Byars, 2006). These findings suggest that left lateralization for language is an extended developmental process. Studies using functional imaging (fMRI) with individuals with PS have confirmed that the right hemisphere plays a critical role for language in those with LHI (Fair, Brown, Petersen, & Schlaggar, 2006; Jacola et al., 2006; Staudt et al., 2002). However, those with LHI also recruit non-lesioned areas in the left hemisphere for language processing (Raja Beharelle et al., 2010; Saccuman et al., 2006). The bilateral distribution of language in typically developing children presents a potential opportunity for the children with PS to exploit this additional tissue. As such this typical bilateral profile may provide an explanation for the remarkable language development in the face of early insult, demonstrating that while not optimal, multiple regions in the developing brain can support core language functions.
5. Limitations and future directions
Limitations with this study include the lack of video data from the experimenter, the single context of investigation, its cross-sectional nature, and small sample size. Although the experimenters were following a script, their responses may influence the way the children responded to the question, but this is the nature of a conversation, each turn is adapted to their interlocutor’s previous utterance. Future studies would benefit from several discourse contexts. Because our study is cross-sectional, we cannot predict how these children will fare as they develop. Future research can address the developmental aspect of neuroplasticity in the PS group by conducting longitudinal follow-up studies to fully assess the extent and limitations of plasticity in children with early neural brain injuries. The sample in our study is small and participants in our lesion groups are fairly heterogeneous, future studies may select a more homogenous lesion profile, which may better capture potential brain-behavior relations.
6. Conclusion
Children with perinatal stroke provide an opportunity to explore neural plasticity within and across different communicative systems. Previous research has recognized that the behavioral and cognitive consequences of a stroke are greatly reduced in children relative to adults with comparable lesions. This is presumably due to the plastic nature of the developing brain in early childhood, and suggests adaptive reorganization through the development of alternate neural circuitry and/or utilizing existing neural circuitry to recruit and maintain functional processing. In the current study, two communicative systems, language and emotion expression, were investigated to gauge the extent and limitations of neural plasticity in five- and six-year-old children with PS. As anticipated, in this conversational context, the core aspects of language for the children with perinatal stroke group were comparable to their TD peers. However, hemispheric differences were found as the RHI group displayed a flatter profile both in the use and production of affective language and in the overall production of facial expressions when compared to the LHI and TD groups. The results are consistent with previous infant data as well as the adult stroke literature, suggesting that neural plasticity differs across communicative systems. Specifically, the two distinctive profiles for language and emotional expression in these children suggest graded plasticity in the developing brain.
Acknowledgments
The authors wish to thank the staff at PCND for helping with data collection and members of the Developmental Laboratory for Language & Cognition at San Diego State University for their assistance. We also thank our two reviewers with their suggestions and helpful comments. Mostly, we are grateful to the participants and their families. This research was supported by funding from Grant NIH-NINDS P50 NS22343 and from the Office of Graduate Studies and Center for Research in Language at UCSD.
References
- Abusamra V, Côté H, Joanette Y, Ferreres A. Communication impairments in patients with right hemisphere damage. Life Span and Disability/XII. 2009;1:67–82. [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. The Journal of Neuroscience. 1996;16(23):7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Perceiving emotion: There’s more than meets the eye. Current Biology. 2000;10(15):R551–R554. doi: 10.1016/s0960-9822(00)00612-6. [DOI] [PubMed] [Google Scholar]
- Angelucci A, Clasca F, Bricolo E, Cramer KS, Sur M. Experimentally induced retinal projections to the ferret auditory thalamus: Development of clustered eye-specific patterns in a novel target. The Journal of Neuroscience. 1997;17:2040–2055. doi: 10.1523/JNEUROSCI.17-06-02040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne A, Spilkin AM, Hesselink J, Trauner D. Plasticity in the developing brain: Intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008;131(11):2975. doi: 10.1093/brain/awn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser LS. Hempligia of early onset and the faculty of speech with special reference to the effects of hemispherectomy. Brain. 1962;85:427–460. doi: 10.1093/brain/85.3.427. [DOI] [PubMed] [Google Scholar]
- Bates E, Reilly J, Wulfeck B, Dronkers N, Opie M, Fenson J, et al. Differential effects of unilateral lesions on language production in children and adults. Brain and Language. 2001;79(2):223–265. doi: 10.1006/brln.2001.2482. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, Trauner D, Fenson J, Aram D, Eisele J, et al. From first words to grammar in children with focal brain injury. Developmental Neuropsychology. 1997;13:275–343. [Google Scholar]
- Berman RA. Language development across childhood and adolescence. Vol. 3. John Benjamins Publishing Company; 2004. [Google Scholar]
- Blonder LX, Burns AF, Bowers D, Moore RW, Heilman KM. Right hemisphere facial expressivity during natural conversation. Brain and Cognition. 1993;21(1):44–56. doi: 10.1006/brcg.1993.1003. [DOI] [PubMed] [Google Scholar]
- Blonder LX, Heilman KM, Ketterson T, Rosenbek J, Raymer A, Crosson B, et al. Affective facial and lexical expression in aprosodic versus aphasic stroke patients. Journal of the International Neuropsychological Society. 2005;11(06):677–685. doi: 10.1017/S1355617705050794. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Borod JC, Cicero BA, Obler LK, Welkowitz J, Erhan HM, Santschi C, et al. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology. 1998;12(3):446. doi: 10.1037//0894-4105.12.3.446. [DOI] [PubMed] [Google Scholar]
- Borod JC, Koff E, Lorch MP, Nicholas M. Channels of emotional expression in patients with unilateral brain damage. Archives of Neurology. 1985;42(4):345–348. doi: 10.1001/archneur.1985.04060040055011. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cerebral Cortex. 2011;21(2):459–466. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siège de la faculté du langage articule suivies d’une observation d’aphemie. Bulletin de la Société Anatomique. 1861;6:330–357. [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Brugman H, Russel A. Annotating multimedia/multi-modal resources with ELAN; Proceedings of LREC 2004, Fourth international conference on language resources and evaluation.2004. [Google Scholar]
- Campos J, Barret KC, Lamb ME, Goldsmith HH, Stenberg C. Socioemotional development. In: Mussen P, Haith M, Campos J, editors. Handbook of child psychology. II. Wiley; New York: 1983. Infancy and Development: Psychobiology. [Google Scholar]
- Cao Y, Vikingstad EM, Huttenlocher PR, Towle VL, Levin DN. Functional magnetic resonance studies of the reorganization of the human hand sensorimotor area after unilateral brain injury in the perinatal period. Proceedings of the National Academy of Sciences USA. 1994;91:9612–9616. doi: 10.1073/pnas.91.20.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassia VM, Kuefner D, Westerlund A, Nelson CA. A behavioural and ERP investigation of 3-month-olds’ face preferences. Neuropsychologia. 2006;44(11):2113. doi: 10.1016/j.neuropsychologia.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi AM, Cipriani P, Bertuccelli B, Pfanner L, Cioni G. Early cognitive and communication development in children with focal brain lesions. Journal of Child Neurology. 2001;16(5):309–316. doi: 10.1177/088307380101600502. [DOI] [PubMed] [Google Scholar]
- Chilosi AM, Cipriani P, Pecini C, Brizzolara D, Biagi L, Montanaro D, et al. Acquired focal brain lesions in childhood: Effects on development and reorganization of language. Brain and Language. 2008;106(3):211–225. doi: 10.1016/j.bandl.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Chilosi AM, Pecini C, Cipriani P, Brovedani P, Brizzolara D, Ferretti G, et al. Atypical language lateralization and early linguistic development in children with focal brain lesions. Developmental Medicine & Child Neurology. 2005;47(11):725–730. doi: 10.1017/S0012162205001532. [DOI] [PubMed] [Google Scholar]
- Chu D, Huttenlocher PR, Levin DN, Towle VL. Reorganization of the hand somatosensory cortex following perinatal unilateral brain injury. Neuropediatrics. 2000;31:63–69. doi: 10.1055/s-2000-7475. [DOI] [PubMed] [Google Scholar]
- Cotard J. Etude sur l’atrophie cerebrale. 1868. Doctoral dissertation.
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Heilman KM, Bowers D, Valenstein E. Recognition and discrimination of emotional faces and pictures. Brain and Language. 1980;9(2):206–214. doi: 10.1016/0093-934x(80)90141-8. [DOI] [PubMed] [Google Scholar]
- Demir OE, Levine SC, Goldin-Meadow S. Narrative skill in children with early unilateral brain injury: A possible limit to functional plasticity. Developmental Science. 2009;13:636–647. doi: 10.1111/j.1467-7687.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system. Consulting Psychologists Press; Palo Alto, CA: 1978. [Google Scholar]
- Ekman P, Friesen WV, Hager JC. Facial action coding system: An investigator’s guide. Research Nexus; Salt Lake City: 2002. [Google Scholar]
- Fair DA, Brown TT, Petersen SE, Schlaggar BL. A comparison of analysis of variance and correlation methods for investigating cognitive development with functional magnetic resonance imaging. Developmental Neuropsychology. 2006;30(1):531–546. doi: 10.1207/s15326942dn3001_2. [DOI] [PubMed] [Google Scholar]
- Feldman HM. Language learning with an injured brain. Language Learning and Development. 2005;1(3):265–288. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters. 2009;452(3):262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Geangu E, Benga O, Stahl D, Striano T. Contagious crying beyond the first days of life. Infant Behavior & Development. 2010;33(3):279–288. doi: 10.1016/j.infbeh.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Understanding aphasia. Academic Press; 1993. [Google Scholar]
- Goodglass H, Hunter M. A linguistic comparison of speech and writing in two types of aphasia. Journal of Communication Disorders. 1970;3:28–35. [Google Scholar]
- Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. Cerebral Cortex. 1989;7:391–440. [Google Scholar]
- Grossmann T, Johnson MH, Lloyd-Fox S, Blasi A, Deligianni F, Elwell C, et al. Early cortical specialization for face-to-face communication in human infants. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1653):2803–2811. doi: 10.1098/rspb.2008.0986. http://dx.doi.org/10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Stefanacci L, Squire LR, Adolphs R, Tranel D. Recognizing facial emotion. Nature. 1996;379(6565) doi: 10.1038/379497a0. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The effects of early experience on problem solving at maturity. American Psychologist. 1947;2:306–307. [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema J, Karunanayaka PR, et al. Functional MRI of language lateralization during development in children. International Journal of Audiology. 2009;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. Journal of Neurophysiology. 1967;30:1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Jackson JH. On the nature of the duality of the brain. The Medical Press & Circular. 1874;1:4–44. [Google Scholar]
- Jackson JH. On affections of speech from disease of the brain. Brain. 1880;2:203–222. [Google Scholar]
- Jacola LM, Schapiro MB, Schmithorst VJ, Byars AW, Strawsburg RH, Szaflarski JP, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37(1):46. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansari A, Tranel D, Adolphs R. A valence-specific lateral bias for discriminating emotional facial expressions in free field. Cognition and Emotion. 2000;14(3):341–353. [Google Scholar]
- Joanette Y, Goulet P. Right hemisphere and verbal communication: Conceptual, methodological and clinical issues. Clinical Aphasiology. 1994;22:1–23. [Google Scholar]
- Joanette Y, Goulet P. Narrative discourse in right-brain-damaged right-handers. In: Joanette Y, Brownell H, editors. Discourse ability and brain damage. Springer Series; New York: 1990. pp. 131–153. [Google Scholar]
- Joanette Y, Goulet P, Ska B, Nespoulous JL. Informative content of narrative discourse in right-brain-damaged right-handers. Brain and Language. 1986;29(1):81–105. doi: 10.1016/0093-934x(86)90035-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Adolphs R, Kaufman O, Damasio H, Damasio AR, Granner M, et al. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience. 2001;4(1):15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Adolphs R, Oya H, Kovach C, Damasio H, Kaufman O, et al. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(10):1509–1518. doi: 10.1162/089892905774597182. [DOI] [PubMed] [Google Scholar]
- Kazandjian S, Borod CJ, Brickman MA. Facial expression during emotional monologues in unilateral stroke: An analysis of monologue segments. Applied Neuropsychology. 2007;14(4):235–246. doi: 10.1080/09084280701719153. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Closer to neurogenesis in adult humans. Nature Medicine. 1998;4:555–556. doi: 10.1038/nm0598-555. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kennard MA. Age and other factors in motor recovery from precentral lesions in monkeys. American Journal of Physiology. 1936;115(138):146. [Google Scholar]
- Kennard MA. Cortical reorganization of motor function. Studies on series of monkeys of various ages from infancy to maturity. Archives of Neurology and Psychiatry. 1942;48:227–240. [Google Scholar]
- Kirton A, Deveber G. Perinatal ischemic stroke. Current Medical Literature: StrokeReview. 2006;10(2):38–47. [Google Scholar]
- Labov W, Waletzky J. Narrative analysis: Oral versions of personal experience. In: Helm J, editor. Essays on the verbal and visual arts. University of Washington Press; Seattle: 1967. [Google Scholar]
- Lausberg H, Sloetjes H. Coding gestural behavior with the NEUROGES-ELAN system. Behavior Research Methods, Instruments, & Computers. 2009;41(3):841–849. doi: 10.3758/BRM.41.3.841. [DOI] [PubMed] [Google Scholar]
- Lenneberg EH. Biological foundations of language. John Wiley & Sons; New York: 1967. [Google Scholar]
- Levin HS, Grafman J, editors. Cerebral reorganization of function after brain damage. Oxford University Press; Oxford: 2000. [Google Scholar]
- Lundgren K, Brownell H, Keith B. Encyclopedia of Language & Linguistics. Elsevier; Oxford: 2006. Narrative and discourse impairments; pp. 445–451. [Google Scholar]
- Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the national institute of neurological disorders and stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- Lynch JK, Nelson K. Epidemiology of perinatal stroke. Current Opinion in Pediatrics. 2001;13:499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Marchman VA, Miller R, Bates EA. Babble and first words in children with focal brain injury. Applied Psycholinguistics. 1991;12:1–22. [Google Scholar]
- McWhinney B, Snow CE. The child language data exchange system. Journal of Child Language. 1985;12:271–295. doi: 10.1017/s0305000900006449. [DOI] [PubMed] [Google Scholar]
- Meza R, Phan L, Felizardo J, Littlewort G, Bartlett, Movellan J, Reilly J. Why so many faces? Children’s spontaneous and voluntary facial expression production; Poster presented at the 3rd annual temporal dynamics of learning center all hands meeting; San Diego, California. Jan, 2010. [Google Scholar]
- Mills CK. The cerebral mechanisms of emotional expression. Transactions of the College of Physicians of Philadelphia. 1912;34:381–390. [Google Scholar]
- Monk C, Klein R, Telzer E, Schroth E, Mannuzza S, Moulton J, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Nass R, Koch D. Temperament differences in toddlers with early unilateral right- and left-brain damage. Developmental Neuropsychology. 1987;3(2):93–99. [Google Scholar]
- Nelson CA, de Haan M. A neurobehavioral approach to the recognition of facial expressions in infancy. The Psychology of Facial Expression. 1997:176–204. [Google Scholar]
- Neville HJ, Schmidt A, Kutas M. Altered visual-evoked potentials in congenitally deaf adults. Brain Research. 1983;266:127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- Nippold MA. Later language development: The school-age and adolescent years. Pro-Ed; Austin, TX: 1998. [Google Scholar]
- O’Leary DDM, Stanfield BB. Selective elimination of axons extended by developing cortical neurons is dependent on regional locale: Experiments utilizing fetal cortical transplants. Journal of Neuroscience. 1989;9:2230–2246. doi: 10.1523/JNEUROSCI.09-07-02230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H. Facial expression as a window on sensory experience and affect in newborn infants. In: Ekman P, Rosenberg E, editors. What the face reveals: Basic and applied studies of spontaneous expression using the Facial Action Coding System (FACS) Oxford University Press; New York, NY US: 1997. [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Science. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Raja Beharelle A, Dick AS, Josse G, Solodkin A, Huttenlocher PR, Levine SC, et al. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133(6):1707–1716. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JS, Bates EA, Marchman VA. Narrative discourse in children with early focal brain injury. Brain and Language. 1998;61(3):335–375. doi: 10.1006/brln.1997.1882. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Salamanca L, Meza R, Littlewort G, Bartlett M. in preparation.
- Reilly JS, Levine S, Nass R, Stiles J. Brain plasticity: Evidence from children with perinatal brain injury. In: Reed J, Warner-Rogers J, editors. Child neuropsychology: Concepts, theory and practice. Blackwell; Oxford: 2008. pp. 58–91. [Google Scholar]
- Reilly JS, Losh M, Bellugi U, Wulfeck B. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain and Language. 2004;88(2):229–247. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Reilly JS, Stiles J, Larsen J, Trauner D. Affective facial expression in infants with focal brain damage. Neuropsychologia. 1995;33:83–99. doi: 10.1016/0028-3932(94)00093-5. [DOI] [PubMed] [Google Scholar]
- Reilly J, Wasserman S, Appelbaum M. Later language development in narratives in children with perinatal stroke. Developmental Science. 2012:1–17. doi: 10.1111/j.1467-7687.2012.01192.x. DOI: 10.1111/j.1467-7687.2012.01192.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg E. FACS Workshop May 2009. University of California, Berkeley; Slottman Hall, Berkeley, CA: May 4–8, 2009. [Google Scholar]
- Rosenberg E. FACS Workshop December 2010. University of California, Berkeley; Slottman Hall, Berkeley, CA: Dec 13–17, 2010. [Google Scholar]
- Rosenzweig MR, Bennett EL. Cerebral changes in rats exposed individually to an enriched environment. Journal of Comparative and Physiological Psychology. 1972;80:304–313. doi: 10.1037/h0032978. [DOI] [PubMed] [Google Scholar]
- Rowe ML, Levine SC, Fisher J, Goldin-Meadow S. The joint effects of biology and input on the language development of brain-injured children. Developmental Psychology. 2009;45:90–102. doi: 10.1037/a0012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccuman MC, Cappa SF, Bates EA, Arevalo A, Della Rosa P, Danna M, et al. The impact of semantic reference on word class: An fMRI study of action and object naming. NeuroImage. 2006;32(4):1865. doi: 10.1016/j.neuroimage.2006.04.179. [DOI] [PubMed] [Google Scholar]
- Said CP, Haxby JV, Todorov A. Brain systems for assessing the affective value of faces. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1571):1660–1670. doi: 10.1098/rstb.2010.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Moore CD, Engell AD, Todorov A, Haxby JV. Distributed representations of dynamic facial expressions in the superior temporal sulcus. Journal of Vision. 2010;10(5) doi: 10.1167/10.5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanca L, Littlewort G, Bartlett M, Reilly J. Children’s production of facial expressions during problem solving contexts; Poster presented at the 4th annual Inter-science of learning student and post-doc conference; Washington, DC. 2011. [Google Scholar]
- Sauer E, Levine SC, Goldin-Meadow S. Early gesture predicts language delay in children with pre- or perinatal brain lesions. Child Development. 2010;81:528–539. doi: 10.1111/j.1467-8624.2009.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz RA. The first year of life. University Press; Internat: 1965. [Google Scholar]
- Sroufe LA, Waters E. The ontogenesis of smiling and laughter: A perspective on the organization of development in infancy. Psychological Review. 1976;83(3):173. [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis A TMS and fMRI study. Brain. 2002;125(10):2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Stenberg CR, Campos JJ, Emde RN. The facial expression of anger in seven-month-old infants. Child Development. 1983:178–184. doi: 10.1111/j.1467-8624.1983.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Stern D. Pre-reflexive experience and its passage to reflexive experience: A developmental view. Journal of Consciousness Studies. 2009;16(10–12):307–331. [Google Scholar]
- Stiles J, Bates EA, Thal D, Trauner D, Reilly J. Linguistic, cognitive, and affective development in children with pre- and perinatal focal brain injury: A ten-year overview from the San Diego Longitudinal Project. In: Rovee-Collier C, Lipsitt LP, Hayne H, editors. Advances in infancy research. Ablex; Stamford, CT: 1998. pp. 131–163. [Google Scholar]
- Stiles J, Reilly JS, Levine SC, Trauner DA, Nass R. Neural plasticity and cognitive development: Insights from children with perinatal brain injury. Oxford University Press; New York, NY: 2012. [Google Scholar]
- Stiles J, Stern C, Trauner D, Nass R. Developmental change in spatial grouping activity among children with early focal brain injury: Evidence from a modeling task. Brain and Cognition. 1996;31(1):46–62. doi: 10.1006/brcg.1996.0024. [DOI] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science. 1988;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. FMRI study of language lateralization in children and adults. Human Brain Mapping. 2006;27(3):202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DJ, Marchman V, Stiles J, Aram D, Trauner D, Nass R, et al. Early lexical development in children with focal brain injury. Brain and Language. 1991;40(4):491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nature Neuroscience. 2009;12(10):1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1493):1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Post B, Randall B, Marslen-Wilson W. Temporal and frontal systems in speech comprehension: An fMRI study of past tense processing. Neuropsychologia. 2005;43(13):1963–1974. doi: 10.1016/j.neuropsychologia.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, De Schonen S, Crivello F, Reutter B, Aujard Y, Mazoyer B. Neural correlates of woman face processing by 2-month-old infants. Neuroimage. 2002;15(2):454–461. doi: 10.1006/nimg.2001.0979. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, O’Gorman AM, Watters GV. Aphasia and handedness in relation to hemispheric side, age at injury and severity of cerebral lesion during childhood. Brain and Language. 1985;108:677–696. doi: 10.1093/brain/108.3.677. [DOI] [PubMed] [Google Scholar]
- Vicari S, Albertoni A, Chilosi AM, Cipriani P, Cioni G, Bates E. Plasticity and reorganization during language development in children with early brain injury. Cortex. 2000;36:31–46. doi: 10.1016/s0010-9452(08)70834-7. [DOI] [PubMed] [Google Scholar]
- Wernicke C. In: Der aphasische Symptomenkomplex. Cohn, Weigert, editors. Breslau: 1874. [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Receptive fields of cells in striate cortex of very young, visually inexperienced kittens. Journal of Neurophysiology. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Feldman HM. Neural plasticity after pre-linguistic injury to the arcuate and superior longitudinal fasciculi. Cortex. 2013;49(1):301–311. doi: 10.1016/j.cortex.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


