Abstract
The kidney collecting duct is an important renal tubular segment for the regulation of body water and salt homeostasis. Water reabsorption in the collecting duct cells is regulated by arginine vasopressin (AVP) via the vasopressin V2-receptor (V2R). AVP increases the osmotic water permeability of the collecting duct cells through aquaporin-2 (AQP2) and aquaporin-3 (AQP3). AVP induces the apical targeting of AQP2 and transcription of AQP2 gene in the kidney collecting duct principal cells. The signaling transduction pathways resulting in the AQP2 trafficking to the apical plasma membrane of the collecting duct principal cells, include AQP2 phosphorylation, RhoA phosphorylation, actin depolymerization and calcium mobilization, and the changes of AQP2 protein abundance in water balance disorders have been extensively studied. These studies elucidate the underlying cellular and molecular mechanisms of body water homeostasis and provide the basis for the treatment of body water balance disorders.
Keywords: Arginine vasopressin, Aquaporins, Body water balance
Body Water Balance and Water Channel Proteins in the Kidneys
The Kidneys regulate body water and sodium balance1). Absorption of water in the kidney tubule depends on the driving force for water reabsorption and osmotic equilibration of water across the tubular epithelium. The majority of glomerular filtrate is constitutively reabsorbed by the proximal tubules and descending thin limbs of Henle's loop. The ascending thin limbs, thick ascending limbs, and distal convoluted tubules are relatively impermeable to water and they deliver the tubular fluid into the connecting tubules and collecting ducts. The collecting ducts are importantly involved in the regulation of body water balance, since vasopressin-regulated water reabsorption occurs in this tubular segment2,3).
Body water balance is achieved and finely regulated by a number of cellular and molecular processes, including kidney tubular reabsorption/secretion of water and sodium through water channels (aquaporins: AQPs) and sodium transporters 1,2,3,4,5). Since water can slowly diffuse through lipid bilayers, all biological membranes exhibit some degree of water permeability6). Nevertheless, renal tubular epithelial cells have to have the plasma membranes which have distinctively high water permeability for water transport and urinary concentration. Consistent with this, an identification of aquaporin membrane water channel provided a novel insight into the physiology of water balance and the pathophysiology of water balance disorders7,8).
Thirteen mammalian aquaporins have been identified. Three major subtypes of aquaporins are known: (1) the classical aquaporins (AQP1, -2, -4, and -5), which are water-selective channels transporting water molecules; (2) aquaglyceroporins (AQP3, -7, -9, and -10), which are permeated by small uncharged molecules in addition to water; and (3) unorthodox aquaporins (AQP6, -8, -11, and -12), whose function is currently being studied. Of the known aquaporins, eight aquaporins (AQP1, -2, -3, -4, -6, -7, -8, and -11) are expressed in mammalian kidney1,2,3,5). These renal aquaporins are expressed in (1) the proximal tubules, (2) the descending thin limbs and vasa recta, and (3) the collecting ducts.
In the proximal tubule which has an extraordinary high water permeability9,10), AQP1 is abundantly expressed in the apical and basolateral plasma membranes11). The AQP1 expression contributes to the near-isosmotic fluid reabsorption that is driven by an active sodium transport via Na, K-ATPase. In the descending thin limbs and descending vasa recta, AQP1 expression is involved in the countercurrent exchange process of the renal medulla12). The high water permeability of the descending vasa recta lowers blood flow to the inner medulla by shunting water to ascending vasa recta and hence it allows perfusion of the renal medulla without washing out the salt and urea gradients necessary for urine concentration12).
In the collecting ducts cells, vasopressin-regulated water channel AQP2 is highly abundant in the apical plasma membrane and subapical vesicles13,14). Water reabsorption in the collecting duct is regulated by both short-term regulation and long-term adaptational mechanisms, both of which are mainly dependent on AQP2 expression1,2,3,5). Because the collecting duct is the final site for regulation of renal water excretion, the changes in both AQP2 trafficking and protein abundance, and hence the changes in the water permeability of the collecting duct could be involved in a variety of disease conditions demonstrating altered capacity to concentrate urine1,2,3,5). In contrast to the apical water transport through AQP2, water transport across the basolateral plasma membrane of the collecting duct principal cells are mediated by AQP3 and AQP415,16). Rats with lithium-induced nephrogenic diabetes insipidus have dramatically reduced apical AQP2 and basolateral AQP3 expression levels, along with a marked polyuria and urinary concentrating defect17,18). Moreover, transgenic mice lacking AQP3 are severely polyuric19) and the inner medullary collecting ducts from AQP4 deficient mice have a significant reduction in vasopressin-stimulated water permeability20). Therefore, these findings demonstrate that basolateral membrane water transport can also play a critical role in water reabsorption.
Regulation of Body Water Homeostasis by Vasopressin
Vasopressin is a peptide hormone that controls plasma osmolality through the regulation of renal water excretion/reabsorption21). The feedback mechanism involving the hypothalamus, the posterior pituitary gland, and the kidneys play a key role in the whole body osmotic regulation1). The Verney receptor in the hypothalamus senses the changes of plasma osmolality and peptide hormone vasopressin is released when plasma osmolality rises to a level above a physiological threshold (290-295mOsm/KgH2O for most individuals). The main action site of vasopressin is the kidney collecting duct, where it regulates water, urea, and sodium transport1,2,3,5,21). In the kidney collecting duct principal cells, vasopressin binds to the basolateral G-protein coupled vasopressin V2 receptors that, through a complex regulatory mechanism, results in increased osmotic water transport across the epithelium of the collecting duct, returning filtered water back to the blood. The ability of vasopressin to decrease water excretion occurs largely through actions on the renal collecting duct cells that result in the regulation of two molecular water channels, AQP2 and AQP3.
Regulation of Renal Aquaporin-2 by Vasopressin
The signaling transduction pathways involved in the apical trafficking and endocytosis of AQP2, and the changes of AQP2 protein abundance, have been extensively studied (Table 1). AQP2 plays a key role in both short-term regulation and long-term adaptation of collecting duct water permeability1,2,3,5,21). Short-term regulation is the process by which vasopressin rapidly increases water permeability of collecting duct principal cells by stimulating vasopressin V2-receptor (V2R) in the basolateral plasma membrane and translocation of AQP2 from intracellular vesicles to the apical plasma membrane. This response was seen within 5 to 30 minutes after increasing the peritubular vasopressin concentration. Long-term adaptation of collecting duct water permeability is seen when circulating vasopressin levels are increased over a period of hours to days, resulting in an increase of the AQP2 abundance per cell in the collecting ducts. This process allows urine concentration and is essential for water balance homeostasis.
Table 1. Intracellular signaling pathways for AQP2 trafficking or endocytosis.
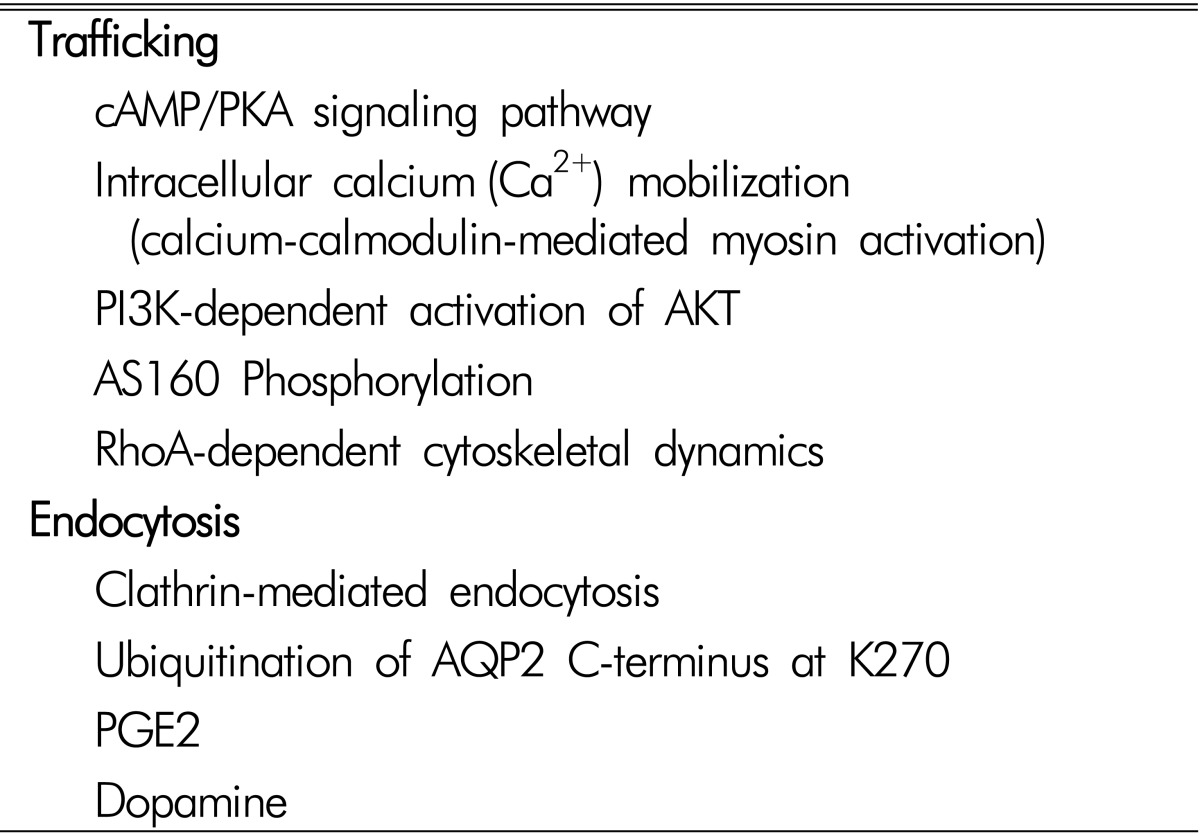
Cellular and Molecular Mechanisms for the Regulation of AQP2
Vasopressin V2 receptor is expressed not only in the principal cells of the collecting duct, but also in the cells of the thick ascending limb and the cells of the distal convoluted tubule22). In the thick ascending limb, vasopressin regulates active sodium chloride reabsorption, thereby playing a role in both countercurrent multiplication and luminal dilution. Vasopressin also accelerates active sodium chloride reabsorption in the distal convoluted tubule, the site of action of the thiazide diuretics. Despite these actions on sodium chloride transport, the most important action from the perspective of body water balance is on the collecting duct via the regulation of two water channels, AQP2 and AQP3.
The short-term regulation of AQP2 occurs as a result of membrane trafficking14,23). In the absence of vasopressin stimulation, AQP2 water channels were found predominantly in the recycling endosomes2,14). This was demonstrated by the findings of colocalization of AQP2 and Rab11 protein, a marker of apical recycling endosomes24). In contrast, when vasopressin was added to isolated collecting ducts, AQP2 water channels were seen predominantly in the apical plasma membrane. These actions of vasopressin are associated with changes in the phosphorylation of the AQP2 protein at four serine sites (S256, S261, S264, and S269) near the C-terminus25,26,27). Exocytosis of AQP2 is associated with phosphorylation at S25628). Vasopressin also markedly increases phosphorylation at S26926). This phosphorylation event, which is increased by vasopressin, inhibits AQP2 endocytosis29,30). Vasopressin decreases phosphorylation of S261 by reducing the activity of one or more MAP kinases31,32). Phosphorylation at this site has been found to be associated with decreased stability of the AQP2 protein31). Moreover, phosphorylation of AQP2 could influence an interaction between AQP2-containing vesicles and the cell cytoskeleton, microtubules, or accessory cross-linking proteins. A recent study demonstrated that AQP2 phosphorylation could be affected by extracellular pH changes33).
The long-term regulation of AQP2 occurs as a result of a vasopressin-induced increase in the total abundance of the AQP2 protein in collecting duct cells. These long-term actions are thought to be associated with regulatory processes at the transcriptional or post-transcriptional level. The half-life of the AQP2 protein could be increased by vasopressin. In cultured mpkCCD cells, the half-life increased from 9 to 14 hours34). Vasopressin enhances AQP2 protein abundance by altering its proteasomal degradation through a PKA- and p38-MAP kinase dependent pathway31). AQP2 is degraded in the proteasome and lysosome35,36). The process of endocytosis and subsequent targeting to the proteasome and lysosome is thought to be regulated or ubiquitylation of the C-terminal tail of the AQP2 protein at lysine 27035). Identification of E3 ubiquitin-protein ligases specific to AQP2 degradation is currently under investigation36). The relationship between S269 phosphorylation induced by vasopressin and ubiquitylation at lysine 270 is a matter of current investigation. Moreover, transcription of the AQP2 gene is markedly increased by vasopressin, resulting in increased cellular mRNA levels and increased AQP2 translation37). Transcriptional regulation of AQP2 is thought to be a result of vasop ressin-induced increase in intracellular cAMP levels with concomitant increases in activation of protein kinase A.
In addition to the transcriptional regulation of AQP2, microRNA(miRNA) appears to play a role in post-transcriptional regulation through inhibition of translation of target mRNA via translational regression of the RNA-induced silencing complex (RISC)38). A recent study specifically predicted miRNAs targeting AQP2 expression by in silico analysis, and two predicted AQP2-targeting miRNAs (miR-32 and miR-137) were further investigated to understand a novel molecular mechanism of AQP2 protein regulation39). This study provides a novel insight on the regulation of AQP2 protein expression, at least in part, via RNA interference, i.e., AQP2-targting miRNAs39). Other signal transduction pathways including prostaglandins, angiotensin II, aldosterone, PI3K/Akt pathways, cytoskeleton, intracellular Ca2+concentration, and vesicle-targeting receptors have been described in previous studies and reviews2,3,5,23,33,40,41,42,43).
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Korea (2010-0019393, 2013R1A1A2007266, 2014R1A5A2009242)
Footnotes
Conflicts of Interest: Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372:1349–1358. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 3.Kwon TH, Frokiaer J, Nielsen S. Regulation of aquaporin-2 in the kidney: A molecular mechanism of bodywater homeostasis. Kidney Res Clin Pract. 2013;32:96–102. doi: 10.1016/j.krcp.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knepper MA. Systems biology in physiology: the vasopressin signaling network in kidney. Am J Physiol Cell Physiol. 2012;303:C1115–C1124. doi: 10.1152/ajpcell.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon TH, Nielsen J, Moller HB, Fenton RA, Nielsen S, Frokiaer J. Aquaporins in the kidney. Handb Exp Pharmacol. 2009:95–132. doi: 10.1007/978-3-540-79885-9_5. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein A. Water and nonelectrolyte permeability of lipid bilayer membranes. J Gen Physiol. 1976;68:127–135. doi: 10.1085/jgp.68.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263:15634–15642. [PubMed] [Google Scholar]
- 8.Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991;266:6407–6415. [PubMed] [Google Scholar]
- 9.Andreoli TE, Schafer JA, Troutman SL. Perfusion rate-dependence of transepithelial osmosis in isolated proximal convoluted tubules: estimation of the hydraulic conductance. Kidney Int. 1978;14:263–269. doi: 10.1038/ki.1978.118. [DOI] [PubMed] [Google Scholar]
- 10.Schafer JA, Patlak CS, Troutman SL, Andreoli TE. Volume absorption in the pars recta. II. Hydraulic conductivity coefficient. Am J Physiol. 1978;234:F340–F348. doi: 10.1152/ajprenal.1978.234.4.F340. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallone TL, Kishore BK, Nielsen S, Agre P, Knepper MA. Evidence that aquaporin-1 mediates NaCl-induced water flux across descending vasa recta. Am J Physiol. 1997;272:F587–F596. doi: 10.1152/ajprenal.1997.272.5.F587. [DOI] [PubMed] [Google Scholar]
- 13.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol. 1995;269:F775–F785. doi: 10.1152/ajprenal.1995.269.6.F775. [DOI] [PubMed] [Google Scholar]
- 16.Ecelbarger CA, Terris J, Frindt G, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 17.Kwon TH, Laursen UH, Marples D, et al. Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2000;279:F552–F564. doi: 10.1152/ajprenal.2000.279.3.F552. [DOI] [PubMed] [Google Scholar]
- 18.Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S. Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest. 1995;95:1838–1845. doi: 10.1172/JCI117863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma T, Song Y, Yang B, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou CL, Ma T, Yang B, Knepper MA, Verkman AS. Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am J Physiol. 1998;274:C549–C554. doi: 10.1152/ajpcell.1998.274.2.C549. [DOI] [PubMed] [Google Scholar]
- 21.Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol. 1994;14:302–321. [PubMed] [Google Scholar]
- 22.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moeller HB, Fenton RA. Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch. 2012;464:133–144. doi: 10.1007/s00424-012-1129-4. [DOI] [PubMed] [Google Scholar]
- 24.Barile M, Pisitkun T, Yu MJ, et al. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics. 2005;4:1095–1106. doi: 10.1074/mcp.M500049-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffert JD, Fenton RA, Moeller HB, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283:24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffert JD, Pisitkun T, Knepper MA. Phosphoproteomics of vasopressin signaling in the kidney. Expert Rev Proteomics. 2011;8:157–163. doi: 10.1586/epr.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 29.Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int. 2009;75:295–303. doi: 10.1038/ki.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci U S A. 2010;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedvetsky PI, Tabor V, Tamma G, et al. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol. 2010;21:1645–1656. doi: 10.1681/ASN.2009111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinschen MM, Yu MJ, Wang G, et al. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci U S A. 2010;107:3882–3887. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HJ, Jung HJ, Kwon TH. Extracellular pH affects phosphorylation and intracellular trafficking of AQP2 in inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2015;308:F737–F748. doi: 10.1152/ajprenal.00376.2014. [DOI] [PubMed] [Google Scholar]
- 34.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol. 2013;24:1793–1805. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamsteeg EJ, Hendriks G, Boone M, et al. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci U S A. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YJ, Lee JE, Choi HJ, et al. E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol. 2011;301:F883–F896. doi: 10.1152/ajprenal.00117.2011. [DOI] [PubMed] [Google Scholar]
- 37.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol. 1997;8:861–867. doi: 10.1681/ASN.V86861. [DOI] [PubMed] [Google Scholar]
- 38.Lee RC, Feinbaum RL, Ambros V. The C. elegansheterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 39.Kim JE, Jung HJ, Lee YJ, Kwon TH. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am J Physiol Renal Physiol. 2015;308:F749–F764. doi: 10.1152/ajprenal.00334.2014. [DOI] [PubMed] [Google Scholar]
- 40.Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003;285:F152–F165. doi: 10.1152/ajprenal.00307.2002. [DOI] [PubMed] [Google Scholar]
- 41.Lee YJ, Song IK, Jang KJ, Nielsen J, Frokiaer J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 42.Jung HJ, Kim SY, Choi HJ, et al. Tankyrase-mediated beta-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol. 2015;308:F473–F486. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 43.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology. 2005;146:5063–5070. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]


