Abstract
Purpose
This phase I dose-escalation trial was performed to determine the maximum-tolerated dose, dose-limiting toxicities, and pharmacokinetics of CPX-351.
Patients and Methods
CPX-351 induction was administered on days 1, 3, and 5 by 90-minute infusion to 48 relapsed or refractory patients with acute myeloid leukemia (AML) or high-risk myelodysplasia. Doses started at 3 units/m2 with dose doublings in single-patient cohorts until a pharmacodynamic effect (treatment-related adverse events or reduction in bone marrow cellularity or blast count) was observed, followed by 33% escalations in three patient cohorts until dose-limiting toxicity (DLT) occurred.
Results
The maximum-tolerated dose was 101 units/m2. DLTs consisted of hypertensive crisis, congestive heart failure, and prolonged cytopenias. Adverse events were consistent with cytarabine and daunorubicin treatment. Response occurred at doses as low as 32 units/m2. Of 43 patients with AML, nine had complete response (CR) and one had CR with incomplete platelet recovery; of patients with acute lymphoblastic leukemia, one of three had CR. Eight CRs were achieved among the 31 patients with prior cytarabine and daunorubicin treatment. CR in AML occurred in five of 26 patients age ≥ 60 years and in five of 17 patients younger than age 60 years. Median half-life was 31.1 hours (cytarabine) and 21.9 hours (daunorubicin), with both drugs and their metabolites detectable > 7 days after the last dose. The targeted 5:1 molar ratio was maintained at all dose levels for up to 24 hours.
Conclusion
The recommended dose of CPX-351 for phase II study is 101 units/m2. Further exploration of efficacy and safety is ongoing in phase II trials in newly diagnosed and first-relapse patients with AML.
INTRODUCTION
Daily bolus daunorubicin (days 1 to 3) with continuous infusion cytarabine (days 1 to 7; 7 + 3), has been the standard induction treatment for acute myeloid leukemia (AML) for more than 30 years, despite limited potential for cure. A farrago of clinical trials examining alternative doses, schedules, and combinations with new cytotoxic and targeted therapies have led to few improvements.1 Although recent studies with dose-escalated daunorubicin2 suggest increased efficacy, the underlying schedule and method of drug administration remains the same. Cytotoxic combinations based on the principles of non–cross resistance3 and avoidance of overlapping toxicities4 usually ignore how drug-drug interactions affect efficacy. Active single agents can be synergistic, additive, or even antagonistic when combined with other agents in vitro, depending on the molar ratio of the two agents. Differences in pharmacokinetics and pharmacodynamics between cytotoxic drugs lead to constantly changing drug ratios in vivo and prevent the maintenance of any particular drug ratio. Specifically designed drug delivery vehicles enable a specific drug ratio to be achieved.
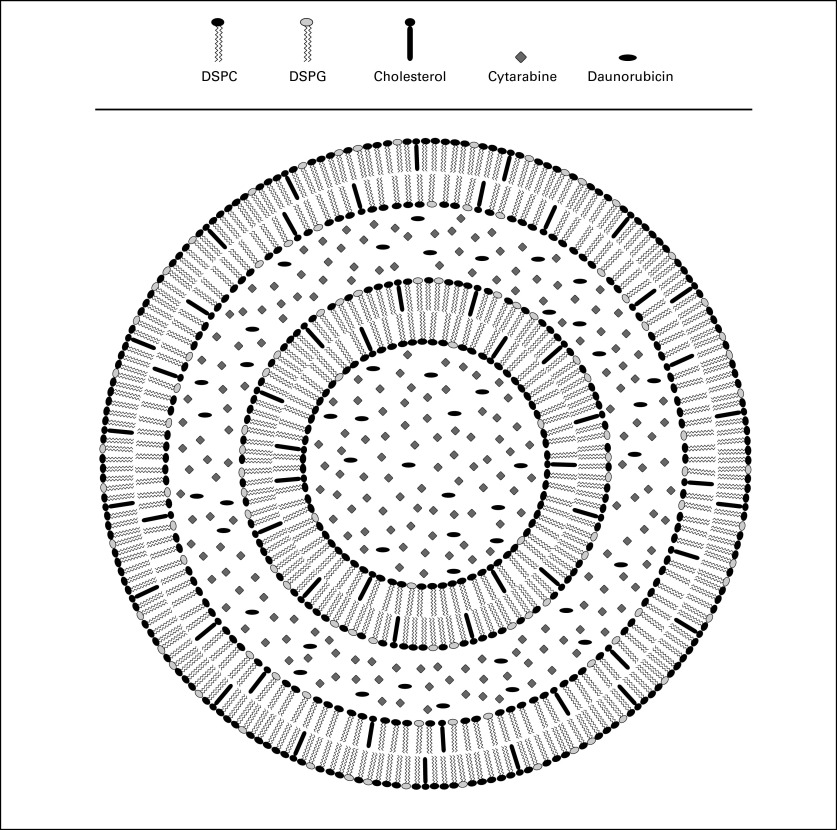
Nano-scale liposomal drug delivery vehicles prolong and maintain particular drug ratios in vivo resulting in enhanced efficacy in multiple murine tumor models.5 CPX-351 fixes a 5:1 molar ratio of cytarabine to daunorubicin within a liposomal carrier. The structure of CPX-351 is shown schematically in Figure 1. The liposomal membrane consists of a 7:2:1 ratio of distearylphosphatidylcholine, distearylphosphatidylglycerol, and cholesterol. Each unit of CPX-351 contains 1 mg cytarabine and 0.44 mg daunorubicin. Evaluations across multiple leukemic and solid-tumor cell lines in vitro demonstrated avoidance of antagonism and retention of synergy.6 The 5:1 molar ratio was maintained in the plasma and bone marrow in vivo for more than 24 hours and increased survival of leukemia-bearing mice compared with conventional cytarabine and daunorubicin.6 A phase I study was designed to identify the maximum-tolerated dose (MTD), evaluate the persistence of the 5:1 ratio of cytarabine to daunorubicin in plasma, assess the safety profile, and determine whether complete remissions (CRs) could be achieved in patients with advanced hematologic malignancies.
Fig 1.
Schematic representation of CPX-351 (cytarabine:daunorubicin) liposome injection. The liposomes are bilamellar with a diameter of 100 nm for the outer vesicle. The membrane is composed of desaturated phosphatidylcholine (DSPC): distearylphosphatidylglycerol (DSPG):cholesterol in a 7:2:1 molar ratio. The active agents, cytarabine and daunorubicin, are encapsulated in the aqueous space of both vesicles at a 5:1 molar ratio. The liposomes are suspended in phosphate-buffered sucrose, pH 7.4. While inside the liposome, daunorubicin is complexed with copper as copper gluconate, giving CPX-351 its characteristic purple color. The strength of CPX-351 is 5 units/mL, where 1 unit = 1.0 mg cytarabine plus 0.44 mg daunorubicin (base).
PATIENTS AND METHODS
Patient Selection
Adults with advanced AML or acute lymphoblastic leukemia (ALL) based on WHO classification or high-risk myelodysplasia (International Prognostic Scoring System [IPSS] intermediate-2 or higher) were eligible. Patients had Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, creatinine < 1.5 mg/dL, bilirubin < 1.5 mg/dL, AST/ALT < 150 U/L, and left ventricular ejection fraction (LVEF) by echocardiogram or multiple gated acquisition > 50%. Patients with CNS leukemia and active infections (fungal, HIV, or hepatitis C) were excluded. Prior chemotherapy was permitted up to 2 weeks before study start. Hydroxyurea was allowed up to 24 hours before CPX-351. Bone marrow karyotype was generally available but was not required for study entry. Written informed consent was obtained from all patients.
Treatment
CPX-351 was supplied by Celator Pharmaceuticals (Princeton, NJ). Induction treatment was infused via central venous catheter over 90 minutes on days 1, 3, and 5. Treatment began at 3 units/m2 for single-patient cohorts. Doses were escalated by doublings until a pharmacodynamic effect (chemotherapy-related adverse event [AE] of any grade or reduction in bone marrow [BM] blast count or cellularity on day 14) was observed, which led to cohorts of three patients and dose escalations of 33%. Patients were added to cohorts to evaluate AEs and in situations where patients became nonevaluable for safety because of early death.
Evaluations
The phase II study dose (MTD) was defined as the dose level at which more than one dose-limiting toxicity (DLT) was observed among six or fewer patients during the first induction course. AEs were assessed using Common Terminology Criteria of Adverse Events (CTCAE) v3.7 Hematologic DLT was defined as persistent (> 56 days) grade 4 cytopenia (absolute neutrophil count < 500/μL), and/or platelet count < 10,000/μL, or platelet transfusion dependency in the absence of leukemia. Nonhematologic DLT was defined as any AE of grade ≥ 3 severity due to study drug except for nausea or vomiting that responded to antiemetic therapy; grade 3 dysphagia, esophagitis, mucositis, and/or odynophagia persisting < 7 days; grade 3 febrile neutropenia or infection controlled with antibiotics; or grade 3 diarrhea controlled by antidiarrheal agents.
BM aplasia was assessed on day 14 and required reduction of cellularity to < 20% with < 5% blast count. A second induction course (CPX-351 on days 1 and 3) was allowed on the basis of individual investigator assessment of the BM at day 14 showing reduced cellularity and percentage blast count. Patients with minimal or no antileukemic effect were removed from study. Patients achieving aplasia had repeat marrow examination 2 to 4 weeks later, when rising peripheral blood neutrophil and platelet counts indicated marrow recovery. CR was defined by International Working Group Criteria.8 CR with incomplete platelet recovery (CRp) fulfilled the same criteria except for platelets < 100,000/μL without platelet transfusion dependency. Patients achieving CR could receive an additional course of CPX-351 (day 1 and 3) for consolidation.
Pharmacokinetic Studies
Cytarabine, daunorubicin, uracil arabinoside, and daunorubicinol levels were assessed for overall exposure, dose proportionality, and drug accumulation before and after each dose of the first induction course. Plasma samples were obtained on day 1: predose, 45 minutes, mid-infusion, 90 minutes, and 2, 4, 6, 8, 12, and 24 hours; day 3: predose, 45 and 90 minutes; and on day 5: predose, 45 and 90 minutes, and 2, 4, 6, 8, 12, 24, 48, 72, 96, and 168 hours. Validated analytic methods employed liquid chromatography–tandem mass spectrometry for quantification. The uracil arabinoside assay used a gemcitabine internal standard. The cytarabine and daunorubicin assays included a step for dissolving the liposome membrane to liberate the encapsulated drugs. Accordingly, these methods measured total (encapsulated and nonencapsulated) plasma cytarabine and daunorubicin.
Maximum concentration (Cmax) and time to Cmax (tmax) were taken directly from the observed data. The area under the serum concentration-time curve [AUC(0-last)] from time 0 to the time of the last observable concentration (Clast) was calculated using the linear trapezoidal rule. For day 1, the AUC from time zero to time infinity [AUC(0-inf)] was calculated as AUC(0-inf) + Clast/λZ. For day 5, the AUC from time 0 to the time τ [AUC(0-τ)] (end of the dosing interval) was calculated using the linear trapezoidal rule. Noncompartmental pharmacokinetic analysis was performed on individual plasma CPX-351 concentration-time data using WinNonlin (Scientific Consultant, Apex, NC) Professional Version 5.2 Noncompartmental Analysis Program. Nominal administration times and sample collection times were used for the analysis.
Extension Phase
After completion of dose escalation, patients with AML in first relapse were entered at the MTD (expansion phase) to obtain additional safety and preliminary efficacy data.
RESULTS
Patient Demographics
Forty-eight patients were enrolled between October 2, 2006, and October 8, 2008. Patient characteristics are listed in Table 1. There were 31 males and 17 females, the median age was 62 years (range, 23 to 81 years), and 90% had ECOG performance status of 0 or 1. Diagnoses included AML (43), ALL (three), and myelodysplastic syndrome (two). The median number of prior treatment regimens was two. CPX-351 was administered as first salvage treatment to 23 patients with AML (five refractory to initial treatment and 18 in first relapse), as second salvage to 10 patients, and as third or greater salvage to 10 patients.
Table 1.
Patient Characteristics
| Characteristic | Cohorts 1-4 at Dose Level 3-24 units/m2 (n = 8) |
Cohorts 5-8 at Dose Level 32-76 units/m2 (n = 14) |
Cohorts 9-10 at Dose Level 101-134 units/m2 (n = 26) |
All Cohorts at Dose Level 3-134 units/m2 (N = 48) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Male | 6 | 75.0 | 9 | 69.6 | 16 | 61.5 | 31 | 64.6 |
| Female | 2 | 25.0 | 5 | 35.7 | 10 | 38.5 | 17 | 35.4 |
| Age, years | ||||||||
| Median | 60.5 | 64 | 60.5 | 62 | ||||
| Minimum–maximum | 25-78 | 24-77 | 23-81 | 23-81 | ||||
| < 60 | 4 | 4 | 13 | 21 | ||||
| ≥ 60 | 4 | 10 | 13 | 27 | ||||
| AML | 4 | 50.0 | 13 | 92.9 | 26 | 100.0 | 43 | 89.6 |
| Primary refractory | 1 | 12.5 | 1 | 7.1 | 3 | 11.5 | 5 | 10.4 |
| 1st salvage | 2 | 25.0 | 2 | 14.3 | 14 | 58.3 | 18 | 37.5 |
| 2nd salvage | 0 | 0.0 | 5 | 35.7 | 5 | 19.2 | 10 | 20.8 |
| > 2nd salvage | 1 | 12.5 | 5 | 35.7 | 4 | 15.4 | 10 | 20.8 |
| ALL | 2 | 25.0 | 1 | 7.1 | 0 | 0.0 | 3 | 6.3 |
| MDS | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 2 | 4.2 |
| ECOG performance status | ||||||||
| 0 | 3 | 37.5 | 3 | 21.4 | 12 | 46.2 | 18 | 37.5 |
| 1 | 4 | 50.0 | 8 | 57.1 | 13 | 50.0 | 25 | 52.1 |
| 2 | 1 | 12.5 | 3 | 21.4 | 1 | 3.8 | 5 | 10.4 |
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndromes; ECOG, Eastern Cooperative Oncology Group.
Dose Escalation
The dose levels studied were 3, 6, 12, 24, 32, 43, 57, 76, 101, and 134 units/m2. Cohorts 1 to 4 used dose doublings and entered single patients into the first two cohorts, two patients in the third cohort (to evaluate a grade 2 skin rash), and four patients in the fourth cohort (one patient was nonevaluable and the next patient had evidence of > 50% reduction in BM cellularity, leading to a decision to study three patients in this cohort). Later cohorts used 33% increments and entered three or more patients. Cohorts 5 and 6 had four patients after replacement of nonevaluable patients. Cohorts 7, 8, and 9 treated three patients in each cohort. The 10th cohort (134 units/m2) observed DLTs in three of six patients consisting of congestive heart failure (CHF; one patient), hypertensive crisis (one patient), and persistent cytopenias beyond 56 days (one patient). A patient with 369 mg/m2 of prior daunorubicin exposure (556 mg/m2 following CPX-351) suffered symptomatic shortness of breath and reduced LVEF (52% to 29%) during a period of sepsis on study day 25. The sepsis resolved, and a repeat LVEF 3 weeks later demonstrated substantial recovery (29% to 47%), suggesting that CHF may have been largely due to the episode of sepsis. This event was considered a DLT because the final assessment was that study drug may have contributed to this event. A second patient had an episode of hypertensive crisis (blood pressure of 210/110 mmHg) complicated by seizures and persistent mental status changes. A third patient was switched to CPX-351 28 days after showing minimal reduction in leukemia to the 7 + 3 regimen, achieved aplasia following CPX-351, but was still markedly cytopenic and remained dependent on platelet transfusions at day 56, meeting protocol-defined criteria for hematologic DLT. BM examination at time of partial recovery confirmed clearance of leukemia and disappearance of a 5q deletion. Full recovery from cytopenias occurred on day 112 at which time criteria for CR were met. On the basis of these events, three additional patients were studied in cohort 9 (101 units/m2) without occurrence of DLT. The 101 units/m2 dose level was declared to be the MTD and was recommended for phase II study. Altogether, 34 patients were studied in the dose-escalation phase of the study. An additional 14 patients, most in first relapse, were subsequently added to the 101 units/m2 cohort to confirm safety and to evaluate potential efficacy in earlier stage patients.
AEs
Every patient had at least one AE. Thirty-five patients died during the treatment or follow-up phase of the study. Progressive leukemia was the cause of death in 26 patients. Six deaths (12.5%) occurred during the first 30 days. Only four patients discontinued treatment prematurely (< day 21) after completing one induction course due to AEs that were not related to the study drug, which included brainstem hemorrhage (43 units/m2), progressive leukemia, pericardial effusion, and pneumonia/staphylococcal bacteremia (101 units/m2). AE frequency and severity were dose related, with the highest dose levels associated with the highest proportion of treatment discontinuations, serious AEs, and grade 3 and 4 AEs. Neutropenia and thrombocytopenia were universal and were associated with episodes of fever, infectious complications, and bleeding episodes. Other nonhematologic toxicities were relatively mild and qualitatively similar to those seen with conventional cytarabine and daunorubicin (Table 2). Nausea and vomiting were well controlled with antiemetics. A distinct maculopapular rash, starting within 1 week of treatment, was seen in 71% of patients (grade 3 in three patients; 6%). The rashes resolved following topical corticosteroids, and intravenous corticosteroid prophylaxis was effective. Mild pruritus was reported in five patients. Surprisingly, alopecia was reported in only six patients (12.5%) during the entire study.
Table 2.
Nonhematologic Toxicity
| Select Adverse Events | Cohorts 1-4 at Dose Level 3-24 units/m2 (n = 8) |
Cohorts 5-8 at Dose Level 32-76 units/m2 (n = 14) |
Cohorts 9-10 at Dose Level 101-134 units/m2 (n = 26) |
All Cohorts at Dose Level 3-134 units/m2 (N = 48) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Mucositis | — | — | — | — | 1 | 3.8 | — | 1 | 2.1 | — | ||||||
| Diarrhea | — | — | — | — | — | — | — | — | ||||||||
| Nausea | — | — | — | — | — | — | — | — | ||||||||
| Vomiting | — | — | — | — | — | — | 1 | 2.1 | — | |||||||
| Skin rash | — | — | 1 | 7.1 | — | 2 | 7.7 | — | 3 | 11.5 | — | |||||
| Alopecia | — | — | — | — | — | — | — | — | ||||||||
| Cardiac (LVEF) | — | — | — | — | 2 | 7.7 | — | 2 | 4.2 | — | ||||||
| Creatinine | — | — | — | — | — | — | — | — | ||||||||
| Bilirubin | — | — | — | — | 1 | 3.8 | — | 1 | 2.1 | — | ||||||
| AST/ALT | — | — | — | — | 2 | 7.7 | — | 2 | 4.2 | — | ||||||
Abbreviation: LVEF, left ventricular ejection fraction.
Baseline LVEF was assessed in all patients, and post-treatment LVEF was available in 23 patients. Information about prior anthracycline exposure was obtained from all patients but had to be estimated from the number of cycles reported in nine patients. Eight patients had no prior anthracycline exposure, 20 received < 200 mg/m2, seven received between 201 and 300 mg/m2, nine received between 301 and 400 mg/m2, two received between 401 and 500 mg/m2, and single patients received 501 to 600 mg/m2 and 601 to 700 mg/m2, respectively. Among the 23 patients evaluable for change in LVEF, 12 had cumulative daunorubicin exposure (including daunorubicin derived from CPX-351) of > 400 mg/m2, while 11 received ≤ 400 mg/m2. Clinical CHF occurred in two patients who, following treatment with CPX-351, had cumulative daunorubicin exposure of 546 and 966 mg/m2, respectively. Three other patients, following CPX-351 therapy, had LVEF decreases of ≥ 10% but maintained LVEF > 50% and were without evidence of clinical cardiac toxicity; these patients had cumulative daunorubicin exposure following CPX-351 of 439, 357, and 95 mg/m2. Higher life-time anthracycline exposure may be associated with higher risk for CHF. As a result, the phase I protocol was amended to exclude patients with cumulative daunorubicin exposure > 500 mg/m2.
No grade 3 or 4 serum creatinine values were observed at any time during the study in any cohort. Liver function worsened by one grade or more in 29% for bilirubin, 25% for AST, and 23% for ALT. No patient in any cohort had grade 4 hepatic dysfunction during the study. Grade 3 abnormalities were observed in single patients (2%) for bilirubin, AST, and ALT.
Efficacy
In patients with AML, nine CRs and one CRp were observed (10 [23%] of 43 patients) and in patients with ALL, one CR in three patients). Two patients with high-risk myelodysplastic syndrome were treated in early cohorts without remission. One patient with multiply relapsed AML achieved CRp after two inductions at 32 units/m2. CRs following single inductions occurred at dose levels as low as 43 units/m2. Three patients achieved a longer CR duration following CPX-351 salvage than following initial induction therapy. For patients with AML, CR or CRp was achieved in the first salvage/primary refractory setting (seven of 23), second salvage setting (one of 10), and third or greater salvage setting (two of 10). Thirty-one (72%) of the 43 patients with AML had received prior cytarabine + anthracycline therapy, and eight of these patients achieved CR following CPX-351. Twelve other patients with AML had no history of prior cytarabine + anthracycline treatment and two achieved CR with CPX-351. CR was achieved in five of 26 patients with AML who were age ≥ 60 years and in five of 17 patients younger than age 60 years (Table 3).
Table 3.
CRs by Dose Level
| Dose Level (units/m2)/Cohort | No. of CRs | Aplasia | Response to CPX-351 | Age (years) | CPX-351 as Salvage | Duration of First CR (months) | Duration of CPX-351 Remission (months) |
|---|---|---|---|---|---|---|---|
| 32 | 3 | 2 | CRp | 55 | > 2nd | 2 | 5.4 |
| 43 | 3 | 2 | CR (ALL) | 44 | 1st | 8 | 0.5 |
| CR | 74 | > 2nd | 7 | 6.0 | |||
| 76 | 3 | 1 | CR | 60 | 1st | 3 | 1.6 |
| 101 | 20 | 13 | CR | 36 | 1st | 12 | < 12 |
| CR | 59 | 1st | 12 | 16+ | |||
| CR | 56 | 1st | 20 | 15+ T | |||
| CR | 72 | 1st | 8 | 9 | |||
| CR | 37 | 2nd | 24 | 18+ T | |||
| 134 | 6 | 4 | CR | 62 | 1st | 0 | 6.7 |
| CR | 67 | 1st | 56 | 22+ |
Abbreviations: CR, complete remission; CRp, CR with incomplete platelet recovery; ALL, acute lymphoblastic leukemia; T, consolidated with stem- cell transplantation.
One of five patients with primary induction failure achieved CR. Of patients with AML who were in first relapse and who were age 18 to 65 years, four (50.0%) of eight achieved CR compared with only two (20.0%) of 10 older than age 65 years. The median duration of remission for patients with AML was 6.9 months, with three patients remaining in remission 1 year after study completion. A single course of post-remission therapy (CPX-351 days 1 and 3) was allowed for responding patients. Among patients with AML, two underwent allogeneic stem-cell transplantation while in CR, one had markedly delayed hematopoietic recovery preventing administration of additional therapy, and seven received CPX-351 consolidation therapy. One patient with ALL relapsed before post-remission therapy could be started.
Pharmacokinetics
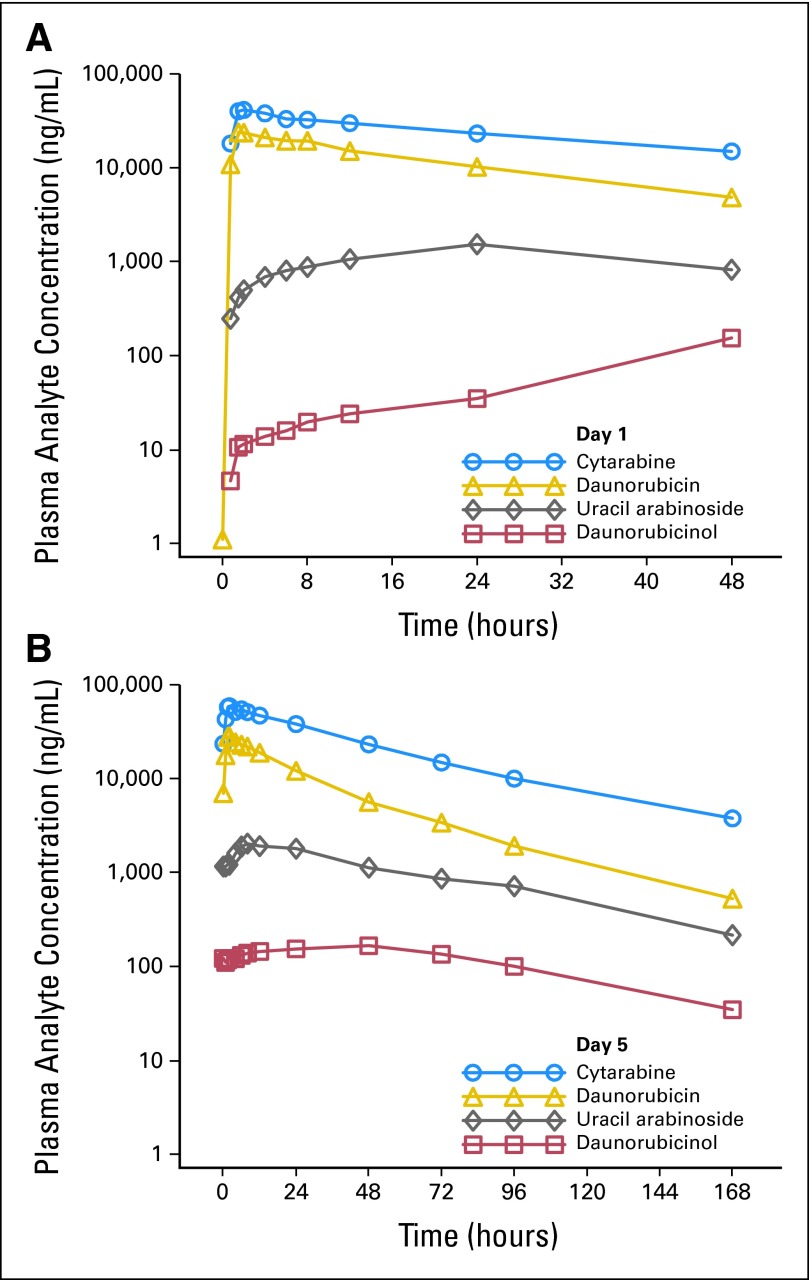
Cytarabine, daunorubicin, uracil arabinoside, and daunorubicinol exhibited mono-exponential, first-order elimination with minimal early-phase distribution (Fig 2). Plasma cytarabine and daunorubicin showed linear single-dose (day 1; Fig 2A) and multiple-dose (day 5; Fig 2B) pharmacokinetic characterist*ics (Cmax and AUC(0-τ)). The metabolites demonstrated generally linear pharmacokinetic characteristics on day 1. On day 5, uracil arabinoside showed generally linear characteristics, while Cmax and AUC(0-τ) for daunorubicinol increased in a greater than proportional manner with increasing CPX-351 dose. The 5:1 molar ratio of cytarabine to daunorubicin was maintained for up to 24 hours on days 1 and 5 at all dose levels.
Fig 2.
(A) Day 1 mean plasma cytarabine, daunorubicin, uracil arabinoside, and daunorubicinol concentrations, time 0 to 48 hours, 101 units/m2, 90-minute infusion. (B) Day 5 mean plasma cytarabine, daunorubicin, uracil arabinoside, and daunorubicinol concentrations, time 0 to 168 hours, 101 units/m2, 90-minute infusion.
The AUC and half-lives for total plasma cytarabine and daunorubicin are substantially greater than what would be expected after infusion of the conventional drugs (Table 4). The mean elimination half-life estimates for cytarabine ranged from 38 to 64 hours for doses between 24 and 134 units/m2. In contrast, conventional cytarabine infusions have a terminal half-life of about 3 hours, suggesting that most of the circulating cytarabine after CPX-351 administration remains within the liposome. Cytarabine and daunorubicin clearance for CPX-351 doses > 24 units/m2 were consistently < 0.5 L/h/m2 (Table 4) and were much less than those reported for conventional daunorubicin (38.6 L/h/m2)9 or cytarabine (134 L/h/m2).10 The AUC of nonencapsulated drug is estimated to be < 1% of the total AUC following CPX-351. Thus, the measured plasma drug concentrations following CPX-351 overwhelmingly come from encapsulated drug.
Table 4.
Pharmacokinetics of Cytarabine and Daunorubicin Following CPX-351 (101 units/m2, day 5)
| Variable | Cmax(ng/mL) | tmax(hours) | AUC(0-τ)(ng·hr/mL) | t1/2(hours) | CL (mL/h/m2) |
|---|---|---|---|---|---|
| Cytarabine | |||||
| No. of patients | 13 | 13 | 13 | 13 | 13 |
| Median | 55,800 | 2 | 1,487,638 | 31.1 | 67.9 |
| Mean | 64,608 | 3.02 | 1,851,089 | 36.9 | 67.3 |
| SD | 23,230 | 2.25 | 934,523 | 24.5 | 30.6 |
| Daunorubicin | |||||
| No. of patients | 13 | 13 | 13 | 13 | 13 |
| Median | 29,200 | 2 | 633,579 | 21.9 | 70.1 |
| Mean | 30,185 | 1.87 | 666,640 | 25.2 | 72.9 |
| SD | 6,198 | 0.74 | 209,198 | 11.6 | 23 |
Abbreviations: Cmax, maximum serum concentration; tmax, time to maximum serum concentration; AUC(0-τ), area under the serum concentration-time curve from time 0 to time τ (end of the dosing interval); t1/2, terminal half-life; CL, clearance; SD, standard deviation.
DISCUSSION
CPX-351 appears to be well-tolerated and capable of inducing CRs in patients with relapsed or refractory AML. The recommended dose and schedule for phase II study (MTD) is 101 units/m2 administered on days 1, 3, and 5 of each induction course. DLTs consisted of hypertensive crisis, CHF, and prolonged cytopenias.
The targeted 5:1 molar ratio of cytarabine to daunorubicin was maintained for up to 24 hours at all dose levels. The cytarabine and daunorubicin half-lives were markedly prolonged with detectable encapsulated drug present > 7 days after the last dose. Cytarabine and daunorubicin release within the vascular space appears to be minimal, and elimination via intact liposomes is likely. Mice exposed to CPX-351 demonstrated significant accumulation of daunorubicin and cytarabine in the BM, and in vitro incubation experiments showed leukemic cells actively transporting CPX-351 liposomes into the cytoplasm followed by drug release from the liposomal carrier within the cell.11 Encapsulation of cytarabine and daunorubicin within liposomes increases the magnitude and duration of drug exposure, preserves the 5:1 molar ratio until delivery to the target leukemia cell, and potentially increases the specificity of drug delivery to the leukemia cells. These differences are profound and suggest an explanation for the prolonged cytopenias observed in one patient treated at the highest dose level.
CHF with documented decreases in LVEF below 50% was observed in two patients with cumulative daunorubicin exposure (including daunorubicin from CPX-351) of 546 and 966 mg/m2. Encapsulation of daunorubicin with the CPX-351 liposome has not abrogated the risk of CHF; however, there is insufficient evidence at this time to determine the cardiotoxic potential of encapsulated daunorubicin. Future studies will have a limit placed on prior anthracycline exposure until sufficient evidence is available to estimate this risk. Assessment of the relative risk for CHF will require a randomized study comparing CPX-351 against conventional 7 + 3 chemotherapy.
An ongoing randomized phase II study (NCT00788892; Trial of CPX-351 in Newly Diagnosed Elderly AML Patients) directly compares the efficacy and safety of CPX-351 against a 7 + 3 control arm in newly diagnosed patients with AML (age 60 to 75 years) and is intended to assess whether control of drug ratios will result in therapeutic improvement. A second randomized phase II study for patients with AML in first relapse is also underway (NCT00822094; Trial of CPX-351 in Adult Patients With First Relapse Acute Myeloid Leukemia [AML]).
To the best of our knowledge, this phase I study is the first clinical trial of cytarabine and daunorubicin administered according to ratiometric dosing principles, which predict that maintenance of synergistic, nonantagonistic molar ratios may lead to increased efficacy without unacceptable toxicity. In this study, we determined a recommended dose of 101 units/m2 for phase II studies, found evidence that the 5:1 molar ratio is maintained in plasma for > 24 hours, and observed strong signs of clinical activity in the form of complete remissions in patients with advanced, previously treated, hematologic malignancies.
Footnotes
Supported by Celator Pharmaceuticals.
Presented in part in abstract form at the 50th Annual Meeting of the American Society of Hematology, December 6-9, 2008, San Francisco, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00389428.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Ekatherine Asatiani, Merck Serono (C); Lawrence D. Mayer, Celator Pharmaceuticals (C); Arthur C. Louie, Celator Pharmaceuticals (C) Consultant or Advisory Role: Christine Swenson, Celator Pharmaceuticals (C) Stock Ownership: Lawrence D. Mayer, Celator Pharmaceuticals; Christine Swenson, Celator Pharmaceuticals; Arthur C. Louie, Celator Pharmaceuticals Honoraria: None Research Funding: Eric J. Feldman, Celator Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eric J. Feldman, Arthur C. Louie
Financial support: Arthur C. Louie
Provision of study materials or patients: Eric J. Feldman, Jeffrey E. Lancet, Jonathan E. Kolitz, Ellen K. Ritchie, Gail J. Roboz, Alan F. List, Steven L. Allen, Ekatherine Asatiani, Arthur C. Louie
Collection and assembly of data: Arthur C. Louie
Data analysis and interpretation: Eric J. Feldman, Jeffrey E. Lancet, Lawrence D. Mayer, Christine Swenson, Arthur C. Louie
Manuscript writing: Eric J. Feldman, Jeffrey E. Lancet, Jonathan E. Kolitz, Ellen K. Ritchie, Gail J. Roboz, Alan F. List, Steven L. Allen, Ekatherine Asatiani, Lawrence D. Mayer, Christine Swenson, Arthur C. Louie
Final approval of manuscript: Eric J. Feldman, Jeffrey E. Lancet, Jonathan E. Kolitz, Ellen K. Ritchie, Gail J. Roboz, Alan F. List, Steven L. Allen, Ekatherine Asatiani, Lawrence D. Mayer, Christine Swenson, Arthur C. Louie
REFERENCES
- 1.Schiffer CA, Stone RM. Acute myeloid leukemia in adults. In: Kufe DW, Bast RC, Hait WN, et al., editors. Cancer Medicine 7. Hamilton, Ontario, Canada: BC Decker; 2006. pp. 1739–1760. [Google Scholar]
- 2.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 3.Goldie JH, Coldman AJ. Application of theoretical models to chemotherapy protocol design. Cancer Treat Rep. 1986;70:127–131. [PubMed] [Google Scholar]
- 4.Frei E, Eder JP. Principles of dose, schedule, and combination therapy. In: Kufe DW, Bast RC, Hait WN, et al., editors. Cancer Medicine 7. Hamilton, Ontario, Canada: BC Decker; 2006. pp. 590–599. [Google Scholar]
- 5.Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 5:1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 6.Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0. August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 8.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Robert J, Rigal-Huguet F, Hurteloup P. Comparative pharmacokinetic study of idarubicin and daunorubicin in leukemia patients. Hematol Oncol. 1992;10:111–116. doi: 10.1002/hon.2900100207. [DOI] [PubMed] [Google Scholar]
- 10.Fleming RA, Capizzi RL, Rosner GL, et al. Clinical pharmacology of cytarabine in patients with acute myeloid leukemia: A cancer and leukemia group B study. Cancer Chemother Pharmacol. 1995;36:425–430. doi: 10.1007/BF00686192. [DOI] [PubMed] [Google Scholar]
- 11.Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]