Abstract
The efficacy of seven-day clarithromycin-based standard triple therapy (STT) for Helicobacter pylori has decreased in Korea over the past decade. The aim of this meta-analysis was to clarify the efficacy of first-line and second-line therapies in Korea. This systematic review will provide an overview of H. pylori eradication and present new therapeutic strategies used in Korea. An extensive search of the literature concerning STT, sequential therapy (SET), concomitant therapy (CT), bismuth-containing quadruple therapy (BCQT) and various other therapies used in Korea was performed. All selected studies were randomized controlled trials (RCTs). Eighteen RCTs were eligible for systematic review. The alternative regimens comparing seven-day STT as a first-line therapy include SET, CT, levofloxacin-based therapy (LBT), BCQT, and STT with prolonged duration. The results of the meta-analysis suggest that SET is superior to seven-day STT. The overall eradication rate by intention to treat (ITT) analysis was 69.8% for STT and 79.7% for SET. The overall eradication rate by per-protocol (PP) analysis was 77.0% for STT and 85.0% for SET. The odds ratios for the ITT and PP eradication rate were 0.57 (95% confidence interval [CI], 0.43 to 0.74) and 0.52 (95% CI, 0.35 to 0.76), respectively. In the subgroup analysis, however, there were no significant differences between SET and STT with prolonged durations. Alternative regimens to seven-day BCQT as second-line therapy include LBT, moxifloxacin-based therapy and 14-day BCQT. The eradication rates of these alternative regimens were not superior to that of the conventional treatment. SET is superior to seven-day STT but not to STT with prolonged duration.
Graphical Abstract
Keywords: Helicobacter pylori, Treatment, Disease Eradication, Review, Systematic
INTRODUCTION
Seven-day standard triple therapy (STT) has been the most recommended and used first-line therapy for the eradication of H. pylori in many countries, including Korea. However, the efficacy of STT has decreased over the past decade (1,2). Primary treatment failure of H. pylori-related disease is a large problem in Korea. Several previous Korean meta-analyses have reported SET to be superior to STT for eradication of H. pylori (3,4,5,6). However, no study has performed subgroup analysis of duration of STT. There thus remains controversy with regard to the use of 10-day STT or 14-day STT and second-line therapy such as bismuth-containing quadruple therapy (BCQT). According to this systematic review and meta-analysis, SET is superior only when STT is used for seven days. Patients who fail H. pylori eradication must be treated by 'rescue' therapy. According to the Maastricht IV Consensus Report, BCQT remains the best second-line therapy option, if available (1). However, this regimen requires the complex administration of four drugs. Therefore, there is concern that BCQT with poor compliance will result in lower efficacy. A previous study compared the eradication rate of quinolone-based therapy with that of BCQT as a second-line therapy (7). However, there was no benefit of quinolone-based therapy with regard to eradication rate in Korea. The reason for this result may be the rapidly increasing resistance to fluoroquinolones in Korea (8). In addition, the present systematic review proposes new therapeutic strategies for use in Korea.
MATERIALS AND METHODS
Study sources and search strategy
An extensive search of the literature was performed for this review. The Cochrane Library, PubMed/Medline, EMBASE and Korean medical databases (KoreaMed, KMbase, KISS, and RISS) were searched. International digestive congresses such as Digestive Disease Week, United European Gastroenterology and European Helicobacter Study Group were also searched. Researchers independently retrieved information including year of publication. These searches included data published up to March 2013. Searches of the literature used combinations of the following terms: Helicobacter pylori and Korea or Korean and regimen, therapy, treatment, concomitant, quadruple, dual, sequential, triple, standard, clarithromycin, moxifloxacin, levofloxacin, tetracycline, amoxicillin, metronidazole or rifabutin.
Selection criteria
Abstracts and full articles of relevant studies were independently reviewed by two investigators (S.W.L., J.G.K.), and those meeting the inclusion criteria were considered for further evaluation. An expert on meta-analysis study (H.J.K.) was involved in this systematic review and meta-analysis. The abstracts or full manuscripts of all studies identified by literature searches were reviewed and selected according to the following criteria for inclusion: 1) Randomized controlled trials with at least two parallel groups; 2) H. pylori infection demonstrated by at least one high-accuracy diagnostic test (histologic evaluation, biopsy urease test, fecal antigen test, or urea breath test); 3) H. pylori eradication evaluated by any of these tests a minimum of four weeks after treatment; 4) Subjects older than 18 yr; 5) Reported Korean data. Experimental articles were excluded, as were case reports, letters, editorials, commentaries, reviews, and abstracts with insufficient details to meet the inclusion criteria. If multiple publications of the same trial were retrieved or if there was a case mix between publications, only one publication was included.
Data extraction and quality assessment
Two investigators (S.W.L., J.G.K.) independently extracted data. Disagreement was resolved by discussion and consensus by the two researchers. The quality of each study was assessed using Cochrane risk of bias criteria.
Statistics
The pre-defined outcome measure was the proportion of patients with successful eradication after treatment. The odds ratio (OR) of eradicating H. pylori infection was the primary endpoint. ORs of adverse events of treatment were the secondary endpoints. The inconsistency index (I2) statistic was used to assess statistical heterogeneity among the reported treatment effects. Low, moderate and high degrees of heterogeneity were considered for I2 values of 25%, 50%, and 75%, respectively. However, all cases were analyzed using the random-effect model as clinical heterogeneity existed even if statistical heterogeneity did not. The 95% confidence interval (CI) was used to gauge the clinical importance of the relative benefit of the alternative therapy group compared with the control group in terms of percentage reduction in failure to eradicate H. pylori. We also determined pooled ORs for adverse events of the treatments. Forest plots were constructed for a visual display of individual studies and pooled results. All of the calculations were performed with Cochrane Collaboration's software, Review Manager 5.
RESULTS
Search results
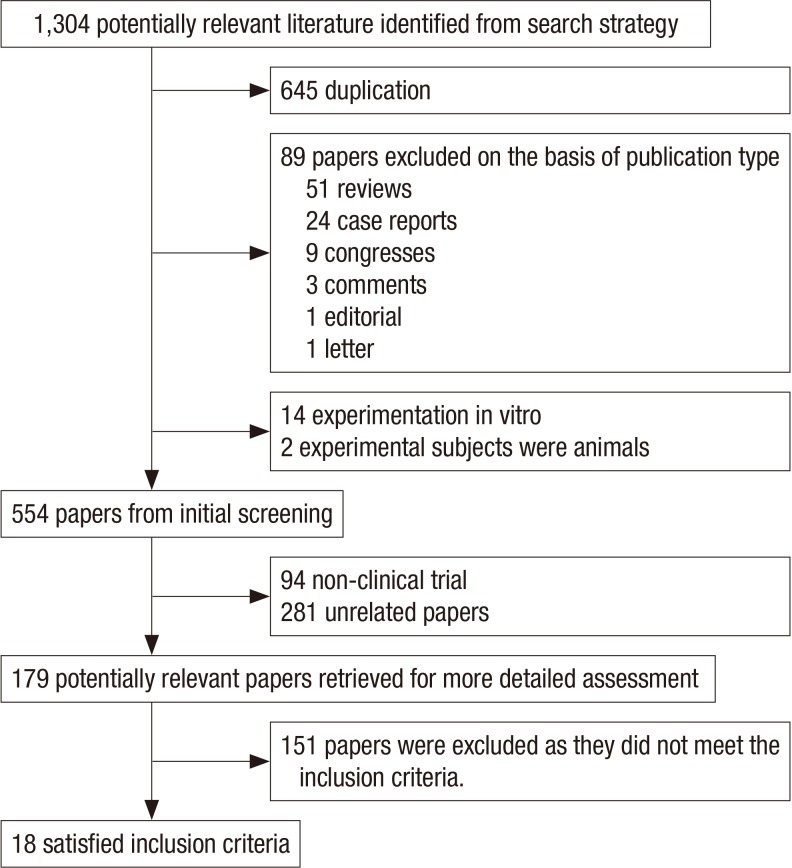
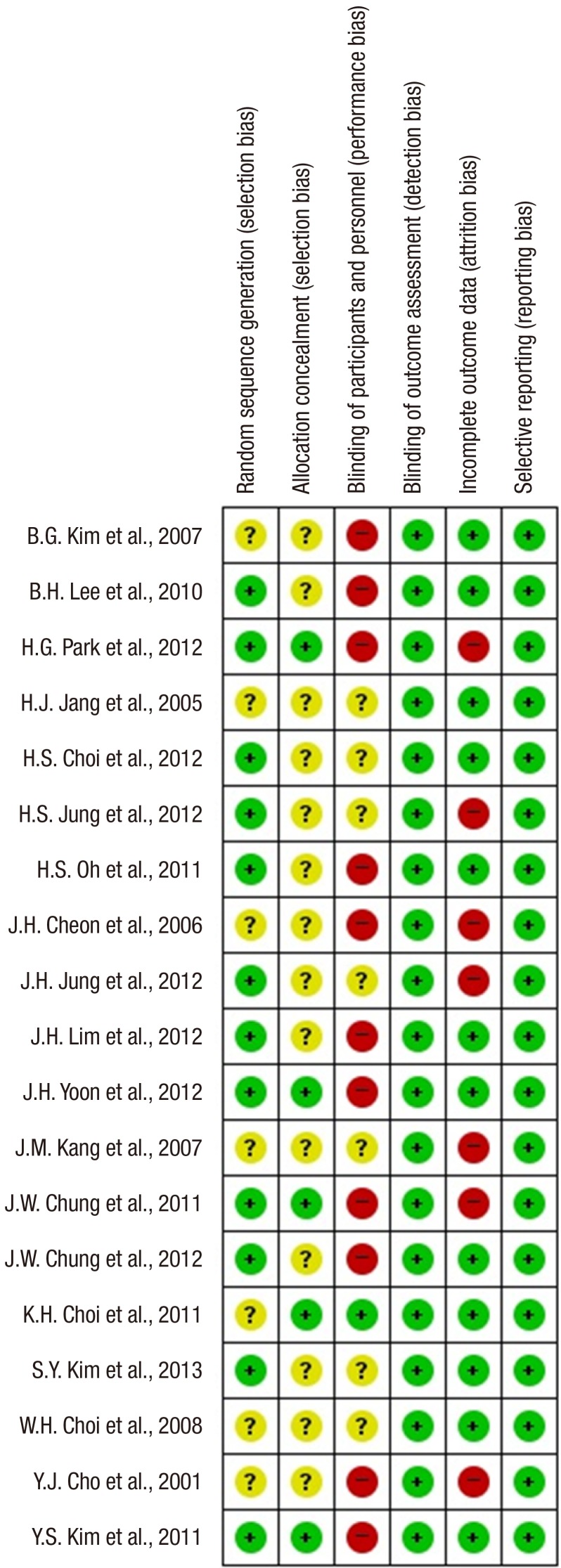
Our initial search yielded 1,304 citations. After applying the inclusion and exclusion criteria, 18 articles were eligible for systematic review. A flow diagram of the selected trials is shown in Fig. 1. Characteristics of the studies included are shown in Table 1. Risk of bias according to the Cochrane Collaboration was applied to the included studies (Fig. 2).
Fig. 1. Flow diagram of selected trials. The initial search yielded 1,304 citations. Ultimately, 18 articles were eligible for systematic review.
Table 1. Main characteristics of included studies.
| Author | Publication year | Drop-out rate (%) | No. of patients | Control group regimen | Experimental group regimen |
|---|---|---|---|---|---|
| J. W. Chung et al. (9) | 2012 | 15.0 | 159 | 10-day STT | 10-day SET |
| H. G. Park et al. (11) | 2012 | 23.7 | 326 | 7-day STT | 10-day SET |
| H. S. Choi et al. (10) | 2012 | 8.6 | 460 | 7,10 and 14-days STT | 10-day SET |
| Y. S. Kim et al. (12) | 2011 | 11.7 | 410 | 14-day STT | 10-day SET |
| H. S. Oh et al. (13) | 2011 | 4.3 | 246 | 7-day STT | 10-day SET |
| W. H. Choi et al. (14) | 2008 | 9.0 | 158 | 7-day STT | 10-day SET |
| S. Y. Kim et al. (15) | 2013 | 14.8 | 270 | 7-day STT | 5-day CT |
| J. H. Lim et al. (16) | 2013 | 3.8 | 164 | 14-day STT | 14-day CT |
| J. H. Jang et al. (17) | 2005 | 16.2 | 149 | 7-day STT | 7-day BCQT |
| K. H. Choi et al. (18) | 2011 | 11.2 | 197 | 7-day STT | 7-day LBT |
| B. G. Kim et al. (20) | 2007 | 15.1 | 598 | 7-day STT | 14-day STT |
| Y. J. Cho et al. (19) | 2001 | NR | 255 | 7-day STT | 14-day STT |
| H. S. Jung et al. (26) | 2012 | 22.2 | 76 | 7-day BCQT | 7-day LBT |
| J. M. Kang et al. (22) | 2007 | 25 | 192 | 14-day BCQT | 10-day MBT |
| J. H. Cheon et al. (21) | 2006 | 20.7 | 85 | 7-day BCQT | 7-day MBT |
| B.H. Lee et al. (23) | 2010 | 18.2 | 227 | 7-day BCQT | 14-day BCQT |
| J. H. Yoon et al. (24) | 2012 | 4.7 | 169 | 7-day BCQT | 14-day BCQT |
| J. W. Chung et al. (25) | 2011 | 20.8 | 199 | 7-days BCQT | 14-day BCQT |
BCQTSTT, standard triple therapy; SET, sequential therapy; CT, concomitant therapy; BCQT, bismuth-containing quadruple therapy; LBT, levofloxacin-based therapy; MBT, moxifloxacin-based therapy; NR, not reported.
Fig. 2. The risk of bias summary. Risk of bias according to the Cochrane Collaboration was used to assess risk of bias in the included studies. Many items were judged as unclear (reported in yellow) because the study did not report enough information for a proper evaluation. The figures show that the overall risk of bias is low (reported in green), but performance bias is high (reported in red).
Regimens
Regimens used in included studies were as follows: 1) Standard triple therapy (STT) consisting of twice a day amoxicillin (1,000 mg), clarithromycin (500 mg) and standard dose proton pump inhibitor (PPI); 2) Sequential therapy (SET) consisting of standard dose PPI and 1,000 mg amoxicillin for five days; followed by standard dose PPI, 500 mg clarithromycin, and 500 mg metronidazole for five days; 3) Concomitant therapy (CT) consisting of twice a day amoxicillin (1,000 mg), clarithromycin (500 mg), metronidazole (500 mg) and standard dose PPI; 4) Bismuth-containing quadruple therapy (BCQT) consisting of tripotassium dicitrate bismuthate (300 mg q.i.d.), metronidazole (500 mg t.i.d.), and tetracycline (500 mg q.i.d.) and standard dose PPI (b.i.d.); 5) Levofloxacin-based therapy (LBT) consisting of twice a day amoxicillin (1,000 mg), levofloxacin (200 mg), and PPI; 6) Moxifloxacin-based therapy (MBT) consisting of moxifloxacin (400 mg q.i.d.), amoxicillin (1,000 mg b.i.d.), and standard dose PPI (b.i.d.); 7) Rifabutin-based therapy consisting of twice a day amoxicillin (1,000 mg), rifabutin (150 mg) and standard dose PPI; 8) Rifaximin plus levofloxacin-based rescue regime consisting of rifaximin (200 mg t.i.d.), levofloxacin (500 mg q.i.d.), PPI (b.i.d.); 9) Dual therapy consisting of twice a day amoxicillin (1,000 mg) and standard dose PPI.
First-line therapies
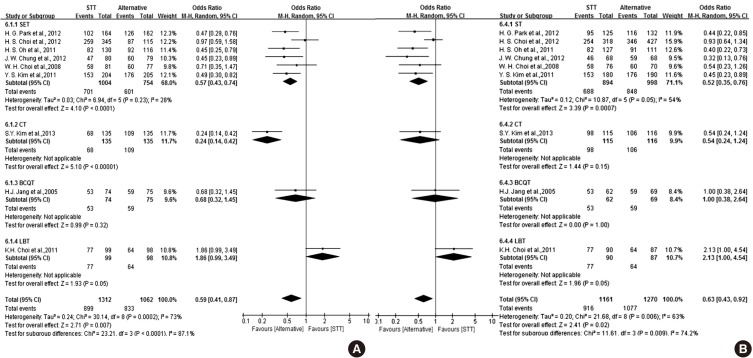
The results of the meta-analysis concerning STT compared to other therapies as first-line therapy are shown in Fig. 3. A total of nine RCTs comparing STT with other therapies for H. pylori eradication met the inclusion criteria. The effect of STT was inferior to other therapies with regard to eradication rate. The ITT analysis of all of the selected studies comparing STT and alternative regimens yielded an OR of 0.59 (95% CI, 0.41 to 0.87). The overall eradication rate by ITT analysis was 68.5% for STT. The PP analysis yielded an OR of 0.63 (95% CI, 0.43 to 0.92). The overall eradication rate by PP analysis was 78.9% for STT.
Fig. 3. Forest plot of the nine studies comparing STT with alternative regimens of first-line therapy including SET, CT, BCQT and LBT according to ITT analysis (A) and PP analysis (B). The effect of STT was inferior to other therapies with regard to eradication rate. STT, standard triple therapy; SET, sequential therapy; CT, concomitant therapy; BCQT, bismuth-containing quadruple therapy; LBT, levofloxacin-based therapy; CI, confidence interval. The studies concerning SET are as follows: 1) H.G. Park et al., 2012; 2) H.S Choi et al., 2012; 3) H.S Oh et al., 2011; 4) J.W. Chung et al., 2012; 5) W.H. Choi et al., 2008; 6) Y.S. Kim et al., 2011; The study concerning on CT is 7) S.Y. Kim et al., 2013. The study on bismuth-containing quadruple therapy is 8) J.H. Jang et al.,2005. The study concerning levofloxacin-based therapy is 9) K.H. Choi et al., 2011.
In our systematic review, six RCTs comparing SET with STT for H. pylori eradication met the inclusion criteria (9,10,11,12,13,14). The forest plot of the six studies is shown in Fig. 4. The effect of SET was superior to STT with regard to eradication rate. The ITT analysis of all of the selected studies comparing STT and SET yielded an OR of 0.57 (95% CI, 0.43 to 0.74). The overall eradication rates by ITT analysis were 69.8% for STT and 79.7% for SET. The PP analysis yielded an OR of 0.52 (95% CI, 0.35 to 0.76). The overall eradication rates by PP analysis were 77.0% for STT and 85.0% for SET. The subgroup analysis showed an inconsistent result, with SET being superior to seven-day STT. The ITT analysis of selected studies comparing seven-day STT and SET yielded an OR of 0.56 (95% CI, 0.42 to 0.74). The overall eradication rates by ITT analysis were 65.9% for seven-day STT and 77.7% for SET. The PP analysis yielded an OR of 0.49 (95% CI, 0.35 to 0.69). The overall eradication rates by PP analysis were 72.6% for seven-day STT and 84.5% for SET. A total of two studies comparing 10-day STT and SET provided data on 389 adult patients. The ITT analysis of selected studies comparing 10-day STT and SET yielded an OR of 0.67 (95% CI, 0.32 to 1.39). The PP analysis yielded an OR of 0.58 (95% CI, 0.19 to 1.75). The overall eradication rates by ITT and PP analysis were 68.2% and 76.3%, respectively, for the 10-day STT group. A total of two studies comparing 14-day STT and SET provided data on 639 adult patients. The ITT analysis of selected studies comparing 14-day STT and SET yielded an OR of 0.78 (95% CI, 0.31 to 2.00). The PP analysis yielded an OR of 0.72 (95% CI, 0.28 to 1.86). The overall eradication rates by ITT and PP analysis were 76.8% and 84.8%, respectively, for the 14-day STT group. Adverse event rates were 23.3% for SET and 18.0% for STT. In the subgroup analysis, however, there was no significant difference between SET and STT with regard to prolonged treatment duration. The pooled OR of adverse events was 0.83 (95% CI, 0.65 to 1.06). The incidences of frequent adverse events like diarrhea, nausea, abdominal pain and taste disturbance were similar in the two groups.
Fig. 4. Forest plot of the six studies comparing STT with SET as first-line therapy and subgroup analysis by duration according to ITT analysis (A) and PP analysis (B). SET was superior to STT with regard to eradication rate. In the subgroup analysis, however, there was no significant difference between SET and STT with regard to prolonged treatment duration. STT, standard triple therapy; SET, sequential therapy; CI, confidence interval. The studies concerning seven-day STT are as follows: 1) H. G. Park et al., 2012; 2) H. S. Choi et al., 2012; 3) H. S. Oh et al., 2011; 4) W. H. Choi et al., 2008. The studies concerning 10-day STT are as follows: 1) H. S. Choi et al., 2012; 2) J. W. Chung et al., 2012. The studies concerning 14-day STT are as follows: 1) H. S. Choi et al., 2012; 2) Y. S. Kim et al., 2011.
There was one RCT comparing seven-day STT with CT for first-line H. pylori eradication (15). There were no significant differences in eradication rates between the two groups (ITT, P=0.150 and PP, P=0.157). In the ITT analysis, the eradication rate was 72.6% in the STT group and 80.7% in the CT group. As for the PP analysis, the eradication rate was 85.2% in the STT group and 91.4% in the CT group. There were no significant differences in the proportion of patients with adverse events between the two groups.
In another RCT comparing CT with SET for first-line H. pylori eradication, there were no significant between-group differences with regard to eradication rate (16). In the ITT and PP analyses, eradication rates were 75.6% and 76.8% in the SET group, respectively, and 80.8% and 81.3% in the CT group. There were no significant differences between the two groups with regard to adverse events.
There was only one RCT conducted to compare the efficacy of STT and BCQT as a first-line treatment (17). The effect of BCQT showed no significant differences to STT in eradication rate. Eradication rates in the STT and BCQT groups were 78.7% and 71.6% by ITT analysis, respectively (P=0.424). By PP analysis, eradication rates of the STT and BCQT group were 85.5% and 85.5%, respectively (P=1.012). The incidence of common adverse events was similar between the STT group and BCQT group.
Only one RCT was found comparing STT with LBT (18). The eradication rate achieved with STT was higher than with LBT on the ITT (77.7%, 65.3%, P=0.05) and PP analysis (P=0.04). LBT was not effective as a first-line therapy in Korea.
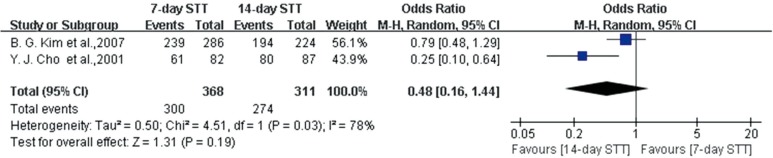
We performed a systematic literature review and meta-analysis for two RCTs concerning treatment duration of STT (19,20). One study did not describe the ITT analysis and so could not be analyzed on an ITT basis (19). In the meta-analysis of RCTs comparing different durations of STT, there were no significant differences between the effects of seven-day and 14-day STT on eradication rate by PP analysis (Fig. 5). The analysis of selected studies comparing seven-day STT and 14-day STT yielded an OR of 0.48 (95% CI, 0.16 to 1.44). Overall, the eradication rate was 81.5% for seven-day STT and 88.1% for 14-day STT. The pooled OR of adverse events was 1.19 (95% CI, 0.69 to 2.06).
Fig. 5. Forest plot of the two studies on treatment duration of STT according to PP analysis. There was no significant difference between the effect of seven-day and 14-day STT on eradication rate by PP analysis. STT, standard triple therapy; CI, confidence interval.
Second-line therapies
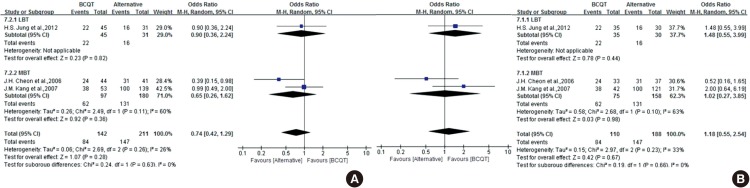
The most commonly prescribed second-line therapy was BCQT. The results of the meta-analysis concerning alternative second-line therapies are shown in Fig. 6. There was only one RCT comparing BCQT with LBT (7). There was no significant difference in H. pylori eradication rate between the two groups (ITT, P= 0.815 and PP, P=0.437). The eradication rates according to ITT and PP analyses were 51.6% and 53.3% in the LBT group and 48.9% and 62.9% in the BCQT group, respectively. LBT was better tolerated than BCQT, with a lower incidence of adverse events (10.0% vs. 31.4%, P=0.03).
Fig. 6. Forest plot of the three studies comparing BCQT with alternative regimens of second-line therapy including LBT and MBT on ITT analysis (A) and PP analysis (B). There were no significant differences between BCQT and other therapies. BCQT, bismuth-containing quadruple therapy; LBT, levofloxacin-based therapy; MBT. moxifloxacin-based therapy; CI, confidence interval. The study concerning LBT is 1) H. S. Jung et al., 2012. The studies concerning MBT are as follows: 1) J. H. Cheon et al., 2006; 2) J. M. Kang et al., 2007.
There were two RCTs concerning MBT as second-line therapy (21,22). The effect of MBT as second-line therapy did not show a significant difference from BCQT with regard to eradication rate. However, data were heterogeneous, and the confidence interval was relatively wide. Therefore, the results were inconclusive. The ITT analysis of all of the selected studies comparing MBT and BCQT yielded an OR of 0.65 (95% CI, 0.26 to 1.62). Overall, the eradication rate by ITT analysis was 63.9% for BCQT and 72.8% for MBT. The PP analysis yielded an OR of 1.02 (95% CI, 0.27 to 3.85). Overall, the eradication rate by PP analysis was 82.7% for BCQT and 82.9% for MBT. There was a significantly lower rate of overall total adverse events with MBT. The pooled OR of adverse events was 0.23 (95% CI, 0.12 to 0.44).
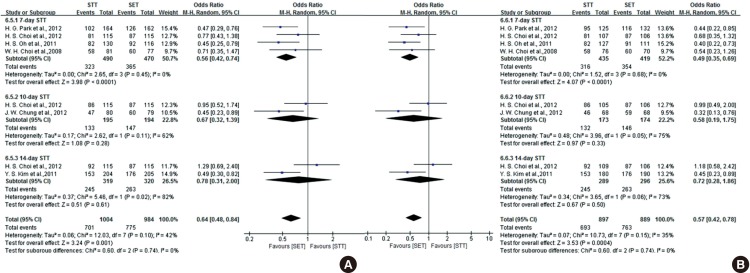
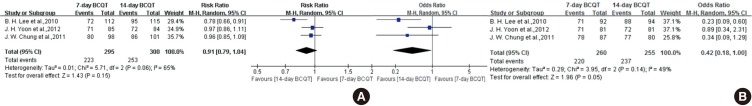
In our systematic review, three RCTs comparing 14-day with seven-day BCQT for H. pylori eradication met the inclusion criteria (23,24,25). The forest plot of the three studies concerning treatment duration of BCQT is shown in Fig. 7. There was no significant difference between the effect of seven-day and 14-day BCQT on eradication rate by ITT analysis. The ITT analysis of all of the selected studies comparing seven-day therapy and 14-day therapy yielded an OR of 0.91 (95% CI, 0.79 to 1.04). Overall, the eradication rate by ITT analysis was 75.6% for seven-day BCQT and 84.3% for 14-day BCQT. In comparison, the PP analysis yielded an OR of 0.42 (95% CI, 0.18 to 1.00). Overall, the eradication rate by PP analysis was 84.6% for seven-day BCQT and 92.9% for 14-day BCQT. There were no significant differences between the two groups with regard to adverse events.
Fig. 7. Forest plot of the three studies on treatment duration of BCQT on ITT analysis (A) and PP analysis (B). There was no significant difference between the effect of seven-day and 14-day BCQT on eradication rate by ITT analysis. However, there was borderline statistical significance between the effect of seven-day and 14-day BCQT on eradication rate by PP analysis. BCQT, bismuth-containing quadruple therapy; CI, confidence interval.
Third-line therapies
In Korea, there was no RCT for third-line therapies. Only four non-randomized studies were available (26,27,28,29). As a result, the eradication rates of alternative regimens as third-line therapies have not reached acceptable levels.
DISCUSSION
The reasonable targets of H. pylori eradication are as follows (30): The cure rate by ITT analysis should be greater than 80%, and the cure rate by PP analysis should be greater than 90%. In our systematic review, however, the eradication rates of seven-day STT did not reach the acceptable level by either ITT or PP analysis. The overall eradication rate of STT was 68.5% by ITT analysis and 78.9% by PP analysis (Fig. 3). In attempts to overcome eradication failure, recent interest has focused on SET, which was introduced in 2000. With regard to the results, SET appears to be superior to STT as a first-line therapy in the eradication of H. pylori. In the subgroup analysis, however, there were no significant differences between SET and STT with prolonged treatment durations (10-day STT or 14-day STT). This result suggests that lengthening the duration of STT may increase the success of eradication of H. pylori in Korea. A recent published meta-analysis showed similar results (31). According to the meta-analysis of 46 RCTs concerning SET, SET is superior to seven-day STT but not superior to 14-day STT. Another meta-analysis concerning optimum duration of treatment showed that 14-day STT is superior to seven-day STT (32). These results suggest that lengthening the duration of STT may increase the success of eradication of H. pylori. According to our meta-analysis, however, this strategy will not achieve a sufficient eradication rate. It seems clear that SET is superior to seven-day STT. STT is usually used for seven days, and the most common regimen of the studies in the meta-analysis is the seven-day STT. There is, however, still controversy with regard to the use of 10-day STT or 14-day STT. Therefore further high-quality RCTs are needed to compare SET and STT with prolonged treatment durations.
There have been few studies concerning CT in Korea. In our systematic review, two studies were identified. One RCT compared the efficacy and safety of CT to STT (15). Another RCT compared the efficacy and safety of CT to SET in Korea (16). According to Kim et al., the effect of CT was not significantly different from that of STT (15). According to Lim et al., the effect of CT was not significantly different from that of SET in the ITT and PP analyses (16). We were not able to perform a meta-analysis with Korean data. Five-day CT showed a trend toward a higher eradication rate than STT, although the difference did not reach statistical significance. CT may be an alternative to STT as a first-line therapy of H. pylori eradication in regions where clarithromycin resistance is rapidly increasing, such as Korea. A recent published meta-analysis showed that CT was more effective than STT (33). There were no significant differences between hybrid therapy, CT and SET according to Wang B et al. (34). In the future, well designed RCTs using CT with prolonged treatment durations are needed. BCQT administered for 7-10 days has been proposed as a solution for the declining eradication rates seen with STT. According to Jang et al., seven-day BCQT as first-line therapy did not show any advantage over STT (17). However, the quality of their study was not high and included relatively old data that did not reflect real, current clinical practice. Only one RCT concerning LBT as a first-line therapy was identified (18); in that study, the LBT was inferior to STT. The unacceptable eradication rate of LBT is due to the higher prevalence of primary levofloxacin resistance, which has increased to 21.5% in Korea (35). However, in second-line therapy, there was demonstrated no significant difference in H. pylori eradication rate between the LBT group and BCQT group (7). This discrepancy may result from two limitations of the original studies. One is that the statistical power was low due to the small sample size. The other is that the heterogeneity of results was high. There a previously published meta-analysis paper concerning LBT in other country. A systematic review and meta-analysis concerning LBT revealed that STT is superior to LBT in Asian countries (36). In European areas, however, LBT may be considered as an alternative therapy according to a subgroup analysis of studies in different geographic areas.
Several strategies have been proposed to improve eradication rates of existing therapies. It has been suggested that lengthening the duration of STT may increase success. However, there is still no consensus on the optimal duration of treatment (7, 10, or 14 days). We performed a systematic literature review and meta-analysis to compare the efficacies of seven-day and 14-day STT. In this meta-analysis of RCTs comparing different durations of STT, there was no significant difference between the effects of seven-day and 14-day STT on eradication rate by PP analysis. However, this study was not able to perform an analysis on an ITT basis, and the results were based on relatively old data that did not reflect real, current clinical practice. On the other hand, lengthening the duration of STT in the subgroup analysis of SET appeared to increase the eradication of H. pylori in our meta-analysis (Fig. 4).
The Korean College of Helicobacter and Upper Gastrointestinal Research recommended either one- or two-week BCQT as a second-line therapy in cases with initial treatment failure (30). However, there is still debate on the ideal duration of BCQT. According to the present meta-analysis, there was no significant difference between the effect of seven-day and 14-day BCQT on eradication rate by ITT analysis. On the other hand, there was borderline statistical significance between the effect of seven-day and 14-day BCQT on eradication rate by PP analysis. Well-designed multicenter RCTs are needed to determine the ideal duration of BCQT.
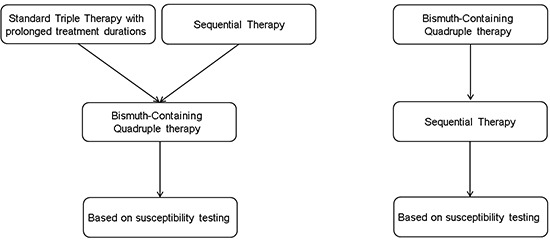
Based on current evidence, we propose a new therapeutic strategy for use in Korea. STT with prolonged treatment duration and SET are promising alternatives to seven-day STT as a first-line therapy in Korea. After failure of first-line therapy, BCQT can be recommended. As an alternative, BCQT can be recommended for first-line therapy. After failure of BCQT, SET is recommended. There was no RCT for a third-line therapy in Korea. Empirical third-line therapies like LBT, quinolone-based therapy, rifabutin-based triple therapy and dual therapy could be prescribed.
There are limitations to our systematic review. First, there was a relatively small number of RCTs. Second, the quality of the reviewed RCTs was not high. Third, most of the studies were conducted in a single center. Finally, many studies were based on relatively old data.
In conclusion, seven-day STT is not effective in Korea as a first-line therapy, with SET being superior to seven-day STT. The effect of CT is inconclusive. Quinolone-based therapy may not be effective in Korea. An effective empirical third-line therapy is not conclusive. Well-designed multicenter RCTs are needed to determine the optimal first-line therapy in Korea (SET, CT, BCQT and STT with prolonged treatment duration).
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Concept & design: Kim JG. Data collection: Kim HJ, Lee SW. Writing manuscript: Lee SW. Revision of manuscript: Kim HJ. Final approval of submission: all authors.
References
- 1.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection: the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 2.Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim DH, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011;58:246–250. [PubMed] [Google Scholar]
- 3.Kim JS, Ji JS, Choi H, Kim JH. Sequential therapy or triple therapy for Helicobacter pylori infection in Asians: systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:118–125. doi: 10.1016/j.clinre.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoon H, Lee DH, Kim N, Park YS, Shin CM, Kang KK, Oh DH, Jang DK, Chung JW. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801–1809. doi: 10.1111/jgh.12397. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Kim BW, Ham JH, Park HW, Kim YK, Lee MY, Ji JS, Lee BI, Choi H. Sequential therapy for Helicobacter pylori infection in Korea: systematic review and meta-analysis. Gut Liver. 2013;7:546–551. doi: 10.5009/gnl.2013.7.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JW, Ha M, Yun SC, Kim JH, Lee JJ, Kim YJ, Kim KO, Kwon KA, Park DK, Lee DH. Meta-analysis: sequential therapy is superior to conventional therapy for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2013;62:267–271. doi: 10.4166/kjg.2013.62.5.267. [DOI] [PubMed] [Google Scholar]
- 7.Jung HS, Shim KN, Baik SJ, Na YJ, Kang MJ, Jung JM, Ha CY, Jung SA, Yoo K. Efficacy of levofloxacin-based triple therapy as second-line Helicobacter pylori eradication. Korean J Gastroenterol. 2008;51:285–290. [PubMed] [Google Scholar]
- 8.Yoon H, Kim N, Lee BH, Hwang TJ, Lee DH, Park YS, Nam RH, Jung HC, Song IS. Moxifloxacin-containing triple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate. Helicobacter. 2009;14:77–85. doi: 10.1111/j.1523-5378.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, Lee SM, Hahm KB, Lee SM, Jeong JY, et al. Ten-day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open-label, randomized trial. J Gastroenterol Hepatol. 2012;27:1675–1680. doi: 10.1111/j.1440-1746.2012.07249.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi HS, Chun HJ, Park SH, Keum B, Seo YS, Kim YS, Jeen YT, Um SH, Lee HS, Kim CD, et al. Comparison of sequential and 7-, 10-, 14-d triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18:2377–2382. doi: 10.3748/wjg.v18.i19.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, Lee JH, Yang CH, Kim ES, Cho KB, et al. Randomised clinical trial: a comparative study of 10-day sequential therapy with 7-day standard triple therapy for Helicobacter pylori infection in naive patients. Aliment Pharmacol Ther. 2012;35:56–65. doi: 10.1111/j.1365-2036.2011.04902.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, Kim DY, Min HJ, Park SH, Shin WG, et al. Randomised clinical trial: the efficacy of a 10-day sequential therapy vs. a 14-day standard proton pump inhibitor-based triple therapy for Helicobacter pylori in Korea. Aliment Pharmacol Ther. 2011;34:1098–1105. doi: 10.1111/j.1365-2036.2011.04843.x. [DOI] [PubMed] [Google Scholar]
- 13.Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Ten-day sequential therapy is more effective than proton pump inhibitor-based therapy in Korea: a prospective, randomized study. J Gastroenterol Hepatol. 2012;27:504–509. doi: 10.1111/j.1440-1746.2011.06922.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, Song MJ, Park SK, Park JH, Kim HJ, et al. Effectiveness of 10 day-sequential therapy for Helicobacter pylori eradication in Korea. Korean J Gastroenterol. 2008;51:280–284. [PubMed] [Google Scholar]
- 15.Kim SY, Lee SW, Hyun JJ, Jung SW, Koo JS, Yim HJ, Park JJ, Chun HJ, Choi JH. Comparative study of Helicobacter pylori eradication rates with 5-day quadruple "concomitant" therapy and 7-day standard triple therapy. J Clin Gastroenterol. 2013;47:21–24. doi: 10.1097/MCG.0b013e3182548ad4. [DOI] [PubMed] [Google Scholar]
- 16.Lim JH, Lee DH, Choi C, Lee ST, Kim N, Park YS, Shin CM, Cho HJ. Two weeks sequential and concomitant therapy for Helicobacter pylori infection and factors associated with eradication: a prospective randomized trial. Journal of Gastroenterology and Hepatology; 111 River ST, Hoboken 07030-5774. NJ USA: Wiley-Balckwell; 2012. p. 60. [Google Scholar]
- 17.Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, Eun CS, Kim JB, Kae SH, Kim DJ, et al. Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication. Korean J Gastroenterol. 2005;46:368–372. [PubMed] [Google Scholar]
- 18.Choi KH, Chung WC, Lee KM, Paik CN, Kim EJ, Kang BK, Oak JH, Jung SH. Efficacy of levofloxacin and rifaximin based quadruple therapy in Helicobacter pylori associated gastroduodenal disease: a double-blind, randomized controlled trial. J Korean Med Sci. 2011;26:785–790. doi: 10.3346/jkms.2011.26.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YJ, Chun HJ, Kim ST, Koh DW, Park JH, Park DK, Park CH, Lee SJ, Jeen YT, Lee HS, et al. Analysis of eradication rate of Helicobacter pylori according to treatment duration by using 13C-urea breath test comparison of OAC 7, 10 or 14 days regimen. Korean J Gastrointest Endosc. 2001;23:207–212. [Google Scholar]
- 20.Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, Kim SW, Kim SK, Kim JJ, Kim HY, et al. Comparison of 7-day and 14-day proton pump inhibitor-containing triple therapy for Helicobacter pylori eradication: Neither treatment duration provides acceptable eradication rate in Korea. Helicobacter. 2007;12:31–35. doi: 10.1111/j.1523-5378.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheon JH, Kim N, Lee DH, Kim JM, Kim JS, Jung HC, Song IS. Efficacy of moxifloxacin-based triple therapy as second-line treatment for Helicobacter pylori infection. Helicobacter. 2006;11:46–51. doi: 10.1111/j.0083-8703.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang JM, Kim N, Lee DH, Park YS, Kim YR, Kim JS, Jung HC, Song IS. Second-line treatment for Helicobacter pylori infection: 10-Day moxifloxacin-based triple therapy versus 2-week quadruple therapy. Helicobacter. 2007;12:623–628. doi: 10.1111/j.1523-5378.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC, et al. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: Effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010;15:38–45. doi: 10.1111/j.1523-5378.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoon JH, Baik GH, Kim YS, Suk KT, Shin WG, Kim KH, Kim KO, Park CH, Baik IH, Jang HJ, et al. Comparison of the eradication rate between 1- and 2-week bismuth-containing quadruple rescue therapies for Helicobacter pylori eradication. Gut Liver. 2012;6:434–439. doi: 10.5009/gnl.2012.6.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung JW, Lee JH, Jung HY, Yun SC, Oh TH, Choi KD, Song HJ, Lee GH, Kim JH. Second-line Helicobacter pylori eradication: a randomized comparison of 1-week or 2-week bismuth-containing quadruple therapy. Helicobacter. 2011;16:289–294. doi: 10.1111/j.1523-5378.2011.00844.x. [DOI] [PubMed] [Google Scholar]
- 26.Jeong MH, Chung JW, Lee SJ, Ha M, Jeong SH, Na S, Na BS, Park SK, Kim YJ, Kwon KA, et al. Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori. Korean J Gastroenterol. 2012;59:401–406. doi: 10.4166/kjg.2012.59.6.401. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Lee SW, Park JY, Kwon BS, Kim SY, Hyun JJ, Kim JH, Jung SW, Koo JS, Yim HJ, et al. Effectiveness and safety of repeated quadruple therapy in Helicobacter pylori infection after failure of second-line quadruple therapy: repeated quadruple therapy as a third-line therapy. Helicobacter. 2011;16:410–414. doi: 10.1111/j.1523-5378.2011.00870.x. [DOI] [PubMed] [Google Scholar]
- 28.Park HK, Lee DH, Suh S, Seo PJ, Kim N, Jeong SH, Kim JW, Hwang JH, Park YS, Lee SH, et al. Dual therapy trial using esomeprazole and amoxicillin as third-line rescue therapy for Helicobacter pylori infection. Clin Endosc. 2011;44:33–37. doi: 10.5946/ce.2011.44.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun SP, Seon HG, Ok CS, Yoo KH, Kang MK, Kim WH, Kwon CI, Ko KH, Hwang SG, Park PW, et al. Rifaximin plus levofloxacin-based rescue regimen for the eradication of Helicobacter pylori. Gut Liver. 2012;6:452–456. doi: 10.5009/gnl.2012.6.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS Korean College of Helicobacter and Upper Gastrointestinal Research; Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 31.Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. doi: 10.1136/bmj.f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan Y, Ford AC, Khan KJ, Gisbert JP, Forman D, Leontiadis GI, Tse F, Calvet X, Fallone C, Fischbach L, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. doi: 10.1002/14651858.CD008337.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23–34. doi: 10.2147/CEG.S25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Wang YH, Lv ZF, Xiong HF, Wang H, Yang Y, Xie Y. Review: efficacy and safety of hybrid therapy for Helicobacter pylori infection: a systematic review and meta-analysis. Helicobacter. 2015;20:79–88. doi: 10.1111/hel.12180. [DOI] [PubMed] [Google Scholar]
- 35.Kim JM, Kim JS, Kim N, Kim SG, Jung HC, Song IS. Comparison of primary and secondary antimicrobial minimum inhibitory concentrations for Helicobacter pylori isolated from Korean patients. Int J Antimicrob Agents. 2006;28:6–13. doi: 10.1016/j.ijantimicag.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Xiao SP, Gu M, Zhang GX. Is levofloxacin-based triple therapy an alternative for first-line eradication of Helicobacter pylori? A systematic review and meta-analysis. Scand J Gastroenterol. 2014;49:528–538. doi: 10.3109/00365521.2014.887765. [DOI] [PubMed] [Google Scholar]