Abstract
In order to increase inhaled corticosteroid (ICS) use and to reduce hospitalization, emergency department visits and ultimately the economic burden of asthma, "Korean Asthma Management Guideline for Adults 2007" was developed. To assess the guideline effects on physician's ICS prescription for asthma, we conducted segmented regression and multilevel logistic regression using National Health Insurance claims database of outpatient visits from 2003 to 2010. We set each quarter of a year as a time unit and compared ICS prescription between before and after guideline dissemination. A total of 624,309 quarterly visits for asthma was observed. The ICS prescription rate before and after guideline dissemination was 13.3% and 16.4% respectively (P < 0.001). In the segmented regression, there was no significant guideline effect on overall ICS prescription rate. In multilevel logistic regression analyses, the effect of guideline on overall ICS prescription was not significant (odds ratio, 1.03; 95% CI, 1.00-1.06). In subgroup analysis, ICS prescription increased in secondary care hospitals (odds ratio, 1.15; 95% CI, 1.02-1.30) and in general hospitals (odds ratio, 1.10; 95% CI, 1.04-1.16). However, in primary clinics, which covered 81.7% of asthma cases, there was no significant change (odds ratio, 0.98; 95% CI, 0.94-1.02). From the in-depth interview, we could identify that the reimbursement criteria of the Health Insurance Review and Assessment Service and patient's preference for oral drug were barriers for the ICS prescription. The domestic asthma clinical guideline have no significant effect on ICS prescription, especially in primary clinics.
Graphical Abstract
Keywords: Asthma, Guideline, Corticosteroid
INTRODUCTION
There is growing concern about the increasing prevalence, chronic morbidity, and mortality of asthma, an inflammatory disease of the lower airway system (1). Asthma also substantially impacts national economics for both industrialized and developing countries (2). Asthma-related costs represent 0.1%-0.3% of the gross domestic product (GDP) for some countries (3). In Korea in 2004, the economic burden to patients was USD 4.11 billion, which was equivalent to 0.44% of the national GDP (4). When considering the increasing trend of asthma prevalence in Korea, the socioeconomic burden for asthma may become extremely high within a few decades (5).
The drug types used to control asthma can be divided into two categories: anti-inflammatory medications and bronchodilators. Among these two drug categories, anti-inflammatory medications, especially inhaled corticosteroids (ICS), are considered first-line treatments for maintenance therapy (6).
In the United States, the current overall rate of anti-inflammatory medication use for asthma is 20.1%, and ICS represents a major portion of that at 72.5%. (7). In Europe, about 43% of the population has used ICS for asthma (8). However, the ICS prescription rate in Korea is much lower. In a survey conducted in 2000, which evaluated asthma control in the Asia-Pacific region, the reported ICS use was the lowest as 1.2% in Korea (9).
In order to increase ICS use and reduce hospitalization, emergency department visits, and ultimately the economic burden of asthma costs, the Korean Academy of Asthma, Allergy and Clinical Immunology; the Korean Academy of Tuberculosis and Respiratory Diseases; and the Korean Academy of Medical Science cooperated to develop a clinical guideline for asthma, which was funded by the Korea Centers for Disease Control and Prevention (KCDC). This guideline was published in November 2007 and revised in March 2011 (10).
Recently, a study was published that used the National Health Insurance (NHI) claims database to assess drug prescription patterns for asthma (11). The researchers observed a slightly increasing trend of ICS prescription, but the effects of the asthma guideline dissemination on this trend was not evaluated.
Our aim was to evaluate the effects of the "Korean Asthma Management Guideline for Adults 2007" on physicians' ICS prescription rate.
MATERIALS AND METHODS
Data source and study population
Our study used claims data from the National Health Insurance Corporation (NHIC). Korea has a compulsory National Health Insurance (NHI) system with universal coverage. Except for a small subpopulation (3%-4%) covered by an alternative health care program for the very poor (the Medical Aid Program, MAP), the NHI system covers the insurance needs of the majority of the Korean population. The NHI claims database includes information about patients' social security numbers, hospital visit dates, the grade of hospitals visited, diagnostic codes according to the 10th International Statistical Classification of Diseases and Related Health Problems (ICD-10), specialty of physicians, prescribed drug codes, etc. (12).
We randomly selected 3% of the database population, resulting in 1,162,354 adult participants (age 20 yr or more on December 31, 2002) insured by the NHIC. For this sample, the claims data from January 1, 2003 to December 31, 2010 were collected.
Definitions
From these data, we defined the cases of asthma based on the ICD-10 codes, J45.X. Cases were defined as having asthma if there were codes for asthma listed as either the principal or first additional diagnosis. Principal diagnosis refers to a disease for which the patient primarily visits a clinic, and the first additional diagnosis indicates a disease that the patient is already being treated for or is diagnosed with for the first time during the same visit for the principal diagnosis. Although J46 (status asthmaticus) is an ICD-10 code for acute severe asthma cases, we excluded it from the analysis because treatment plans must differ in those cases. Likewise, we excluded inpatient stays for the same reason (13). ICS was defined according to the Anatomical Therapeutic Chemical (ATC) classification (R03), and we included beta-agonists along with steroid inhalers.
We classified each patient visit into two categories: 1) were not prescribed any ICS, and 2) were prescribed at least one kind of ICS. Because ICS is a maintenance medication and can be used for a certain period after one prescription, we divided each year into quarters and designated a quarter as the relevant time unit to divide patients into the aforementioned groups. The first group (ICS not prescribed) would include patients who visited a clinic for asthma but were not prescribed ICS during a quarter of a year; in contrast, if ICS was prescribed at least once in a quarter, the patient would be included in the second group (ICS prescribed). Specifically, if a patient visited a clinic for asthma every month from January to March 2003 and was prescribed ICS in February 2003, this would classify the patient in the latter group; if the patient was not prescribed any ICS from January to March, this patient would be included in the former group. Physicians' specialties were subdivided into four categories: 1) internal medicine, 2) otolaryngology, 3) family medicine, and 4) others (specialties with total ICS prescription cases of fewer than 1,000). We subdivided the grades of hospitals into three categories: 1) primary clinics, 2) secondary care hospitals (hospitals with 30 or more inpatient beds), and 3) general hospitals (more than 7 specialties and 100 beds, including tertiary hospitals) (14).
Statistical analyses
We conducted a retrospective, population-based study to estimate the effects of the "Korean Asthma Management Guideline for Adults 2007" on the ICS prescription rate. As the guideline was published in November 2007, we considered the first quarter of 2008 as the starting point for the period after guideline dissemination, which means that December 2007 was included in the pre-dissemination period of the guideline. To compare the trends of ICS prescription before and after guideline dissemination, we performed segmented regression analysis. Segmented regression is a powerful method of evaluating interventions to improve the quality of medication use, such as policies and clinical practice guidelines. It depicts the trends before and after an intervention. We then can evaluate the intervention by comparing the slopes of the two trends and the intercepts at the point of intervention (15).
To assess the guideline effects on ICS prescription and the level of variations among different regions, multilevel logistic regression was performed with a binomial dependent variable and a random intercept model across the 2 levels (each visit nested in regions). Regional level data were collected from local address codes of 251 municipal districts. In the main analysis, we assumed that there were fixed effects of age, sex, year of visit, grade of hospital, and specialty of physician. After the main analysis, we found that there was a significant interaction between the guideline effects and the grade of hospitals; therefore, we performed subgroup analyses stratified by grade of hospitals. We calculated the intra-class coefficients (ICCs) before and after guideline dissemination to estimate regional variation changes.
Since there are some limitations using claims data such as the definition of asthma case, we performed sensitivity analyses using the asthma cases which had asthma as the principal diagnoses.
Qualitative interview
To determine the barriers to ICS prescription as recommended in the guideline, we conducted in-depth interviews with one general hospital physician who participated in the development of the "Korean Asthma Management Guideline for Adults 2007," as well as 8 primary physicians who were not involved in the development of the guideline. The interviews were performed by showing the physicians the main results of our analyses. The questions used in the interviews were as follows: "Have you ever heard about the Korean Asthma Management Guideline for Adults 2007," "Do you agree with the ICS prescription recommendation in the guideline," "Is the observed rate of ICS prescription in primary clinic enough," and "What were the barriers to prescribing ICS following the guideline recommendations?"
Ethics statement
This study was approved by the institutional review board of Seoul National University Hospital (IRB No. 1206-055-414). Informed consent was exempted by the board.
RESULTS
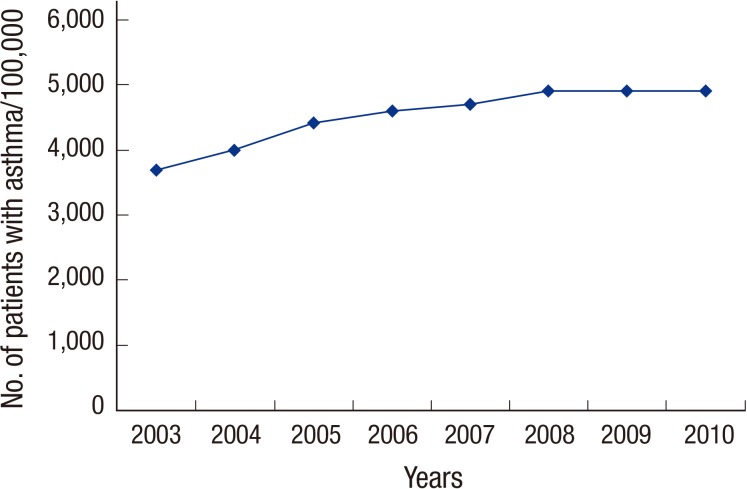
From pre-defined cohorts, 235,755 participants (20.3%) had visited clinics for asthma during our study period. Annual prevalence of asthma during the study period ranged from 3,708 to 4,925 per 100,000 adults (Fig. 1).
Fig. 1. Annual prevalence of asthma in Korea.
There were a total of 624,309 quarterly visits for asthma during the study period. ICS prescription rates before and after the guideline dissemination were 13.3% and 16.4% respectively (P< 0.001). The most commonly visited hospital grade was primary clinic (81.7%), and the most commonly visited specialty was internal medicine (80.8%). Females visited more frequently for asthma (59.9%) than males did. ICS prescription rates before and after the guideline dissemination were, respectively, 7.8% and 10.6% in primary clinics (P<0.001); 19.3% and 21.0% in secondary care hospitals (P<0.001); and 43.1% and 48.9% in general hospitals (P<0.001) (Table 1).
Table 1. Characteristics of quarterly visits for asthma (No, %).
| Parameters | Before guideline dissemination (n=368,193) | After guideline dissemination (n=256,116) | Total (n=624,309) | |||
|---|---|---|---|---|---|---|
| with ICS* | without ICS | with ICS | without ICS | with ICS | without ICS | |
| No. of visits for asthma | 48,808 (13.3) | 319,385 (86.7) | 41,935 (16.4) | 214,181 (83.6) | 90,743 (14.5) | 533,566 (85.5) |
| Mean age (SD), yr | 55.0 (14.9) | 53.4 (16.5) | 57.3 (14.7) | 55.2 (16.0) | 56.1 (14.9) | 54.1 (16.3) |
| Aged 65 or more | 15,302 (31.4) | 96,672 (30.3) | 15,503 (37.0) | 72,617 (33.9) | 30,805 (33.9) | 169,289 (31.7) |
| Female | 24,563 (50.3) | 196,902 (61.7) | 21,445 (51.1) | 131,036 (61.2) | 46,008 (50.7) | 327,938 (61.5) |
| Grade of hospital | ||||||
| Primary clinic | 23,585 (48.3) | 277,570 (86.9) | 22,158 (52.8) | 186,787 (87.2) | 45,716 (50.4) | 464,357 (87.0) |
| Secondary care hospital | 2,953 (6.1) | 12,338 (3.9) | 2,480 (5.9) | 9,308 (4.3) | 5,433 (6.0) | 21,646 (4.1) |
| General hospital | 22,297 (45.7) | 29,477 (9.2) | 17,297 (41.2) | 18,086 (8.4) | 39,594 (43.6) | 47,563 (8.9) |
| Specialty of physician | ||||||
| Internal medicine | 45,754 (93.7) | 254,366 (79.6) | 38,960 (92.9) | 165,452 (77.2) | 84,714 (93.4) | 419,818 (78.7) |
| Otolaryngology | 736 (1.5) | 24,535 (7.7) | 820 (2.0) | 21,999 (10.3) | 1,556 (1.7) | 46,534 (8.7) |
| Family medicine | 895 (1.8) | 12,358 (3.9) | 785 (1.9) | 8,658 (4.0) | 1,680 (1.9) | 21,016 (3.9) |
| Others† | 1,423 (2.9) | 28,126 (8.8) | 1,370 (3.3) | 18,072 (8.4) | 2,793 (3.1) | 46,198 (8.7) |
*Inhaled corticosteroid; †Specialties with total ICS prescription less than 1,000 cases.
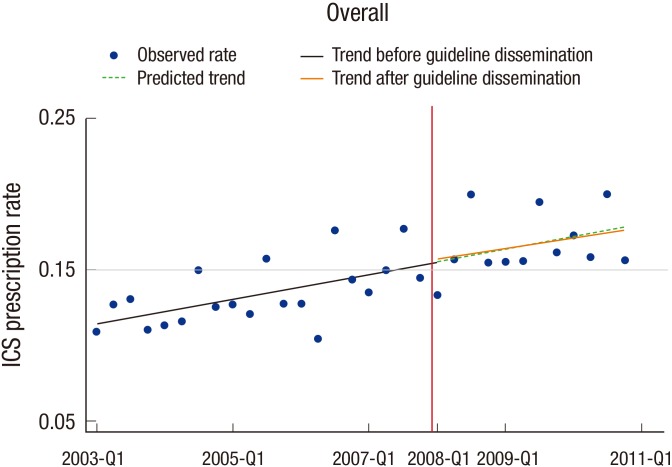
In segmented regression, the overall trend of ICS prescription rate did not change. The trend observed after guideline dissemination was almost the same as the prediction using the trend before guideline dissemination. Moreover, level change at the point of guideline dissemination was not observed (Fig. 2).
Fig. 2. Segmented regression of the inhaled corticosteroid prescription rate. The vertical redline indicates the point of the guideline dissemination.
In the multilevel analysis, adjusted for random effects for 251 municipal districts, there was no significant guideline effect on ICS prescription (odds ratio, 1.03; 95% CI, 1.00-1.06); we also calculated the ICC for ICS prescription (Table 2). The overall ICC was 0.040 (95% CI, 0.033-0.050). There was no significant ICC change after guideline dissemination, from 0.045 (95% CI, 0.036-0.055) to 0.040 (95% CI, 0.033-0.050), indicating that the regional variation of ICS prescription did not change significantly after guideline dissemination.
Table 2. Multilevel logistic regression for guideline effect on inhaled corticosteroid (ICS) prescription.
| Variables | Odds ratio for ICS prescription (95% CI) |
|---|---|
| Overall* | 1.03 (1.00-1.06) |
| Grade of hospital† | |
| Primary clinic | 0.98 (0.94-1.02) |
| Secondary care hospital | 1.15 (1.02-1.30) |
| General hospital | 1.10 (1.04-1.16) |
Before guideline dissemination as the reference group. *Adjust as fixed effect; adjusted by age, sex, year of visit, grade of hospital and specialty of physician; †Subgroup analyses stratified by grade of hospital and adjusted by age, sex, year of visit and specialty of physician. Adjust as random effect; 251 municipal districts. CI, confidential interval.
As mentioned above, since there was a statistically significant interaction between guideline and grade of hospital in the multilevel logistic regression model, we performed subgroup analysis stratified by grade of hospitals. Although guideline dissemination made no significant change in primary clinics (odds ratio, 0.98; 95% CI, 0.94-1.02), the ICS prescriptions increased significantly in both secondary care hospitals (odds ratio, 1.15; 95% CI, 1.02-1.30) and general hospitals (odds ratio, 1.10; 95% CI, 1.04-1.16) (Table 2).
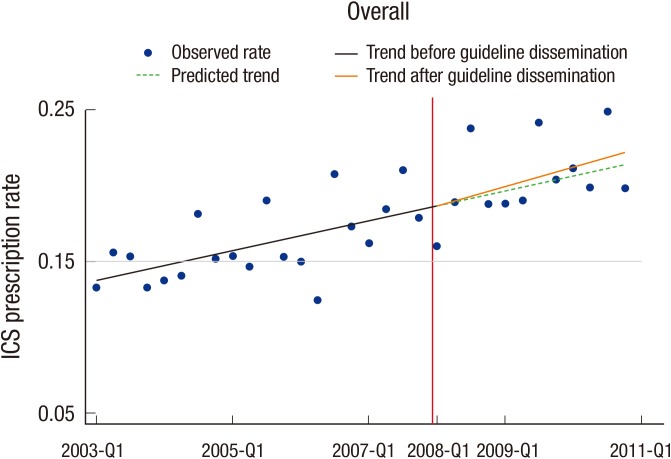
In the sensitivity analysis, total 397,206 quarterly visits (159,478 subjects) for asthma were observed. The observed trend for ICS prescription was not different from that of the main result (Fig. 3). In multilevel logistic regression, the overall ICS prescription rate was slightly increased after the guideline dissemination (odds ratio, 1.05; 95% CI, 1.01-1.09). However, guideline dissemination made no significant changes in primary clinics (odds ratio, 0.98; 95% CI, 0.94-1.02) and secondary care hospitals (odds ratio, 1.15; 95% CI, 1.00-1.33), but the ICS prescription rate increased significantly in general hospitals (odds ratio, 1.12; 95% CI, 1.05-1.19) (Table 3).
Fig. 3. Sensitivity analysis using segmented regression of the inhaled corticosteroid prescription rate. The vertical redline indicates the point of the guideline dissemination.
Table 3. Sensitivity analysis using multilevel logistic regression for guideline effect on inhaled corticosteroid (ICS) prescription.
| Variables | Odds ratio for ICS prescription (95% CI) |
|---|---|
| Overall* | 1.05 (1.01-1.09) |
| Grade of hospital† | |
| Primary clinic | 1.01 (0.96-1.05) |
| Secondary care hospital | 1.15 (1.00-1.33) |
| General hospital | 1.12 (1.05-1.19) |
Before guideline dissemination as the reference group. *Adjust as fixed effect; adjusted by age, sex, year of visit, grade of hospital and specialty of physician; †Subgroup analyses stratified by grade of hospital and adjusted by age, sex, year of visit and specialty of physician. Adjust as random effect; 251 municipal districts. CI, confidential interval.
As described above, we conducted in-depth interviews to uncover the barriers to the guideline in primary clinics. All of the participating physicians were aware of the "Korean Asthma Management Guideline for Adults 2007." Seven out of 8 primary physicians agreed that the observed ICS prescription rate for primary clinics was too low. All physicians complained of the gap between the guideline recommendations and the reimbursement criteria of the "Health Insurance Review and Assessment Service (HIRA)", which reviews the cost of health care benefits and evaluates the reasonableness of the health care services provided. They said that the criteria of HIRA were too strict to prescribe ICS, considering the financial penalties. The physicians who participated in the interviews emphasized patients' preferences for oral drugs rather than inhalers and difficulty educating patients about ICS usage as other barriers to adherence to the asthma guideline in primary clinics. They also mentioned the rejection of elderly patients for being prescribed ICS.
DISCUSSION
To our knowledge, this is the first study to examine the effects of dissemination of the "Korean Asthma Management Guideline for Adults 2007" on the ICS prescription rate for asthma in the general population using NHI claims data. Other strengths of this study include the large sample size and regularly collected data over time with equally spaced intervals, allowing us to conduct segmented regression, the results of which were consistent with those of a multilevel logistic regression. Additionally, the very wide population coverage of the NHI makes it possible to generalize the results to the general population (12).
Overall, we found that there was no significant guideline effect on ICS prescription. Although the overall odds ratio was slightly increased in the sensitivity analysis, it is still under expectation. Also, the trend of the ICS prescription rate was not changed in analysis using segmented regression. Moreover, the guideline had no significant effect especially in the primary clinics, which covered most of the asthma cases, consistently in both main and sensitivity analysis. As the main targets of the clinical guideline were primary physicians, the result is indicative of the presence of some barriers to the guideline.
There are many studies to identify the barriers to ICS prescription for asthma according to the clinical asthma guideline's recommendations. These barriers can be subdivided into three categories. First, there are patient-level barriers. Age and comorbidities are important patient-level barriers (16,17). ICSs are more inconvenient to use than oral medications (18), and there might be some concerns about the side effects of ICS (19). Therefore, patients might be more compliant with oral medication than with ICS (20). Additionally, patients' mistaken belief that ICS is unnecessary during asymptomatic periods might have resulted in a decreased prescription rate (21). Second, there are physician-level barriers. There might not be enough time for the clinician to educate the patient about asthma, including ICS usage (22). Lack of familiarity with the guideline's recommendations about ICS use and less expertise about ICS management are important barriers to ICS prescribing (23). Furthermore, some physicians disagree with the guideline's recommendation about ICS (24). Additionally, there is a lack of awareness about the existence of the clinical guidelines (18). Finally, socio-environmental barriers also exist. Poverty and the high cost of ICS are socio-environmental barriers to ICS prescribing as recommended in the asthma guideline (18,25). In some instances, health care systems can restrict physicians from prescribing ICS (26).
The subgroup analyses showed that the guideline dissemination increased the ICS prescription in secondary care hospitals and general hospitals, but this effect was not observed within primary clinics, which encompassed most of the asthma cases in this study. From the in-depth interview, we can identify some barriers for ICS prescription. First of all, the reimbursement criteria of the HIRA are the most important barriers for doctor to prescribe ICS. HIRA requires medical records about asthma severity as measured by a pulmonary function test (PFT) when prescribing ICS, in spite of the fact that most of the primary clinics in Korea cannot perform PFT within their clinics. When the prescription of ICS does not meet the criteria, HIRA imposes financial penalties on the clinics (12). So, it is hard for most of the primary clinics to prescribe ICS. Also, the patient's preference of oral drugs rather than inhaler is another barrier for ICS prescription. So the education for general population and asthma patients about the benefits of ICS in asthma is essential to increase ICS prescription in asthma. While the aforementioned barriers could explain some of our results, a thorough, well-designed study is required to identify further barriers to prescribing ICS.
There are some limitations in our study. First, we did not take into account other comorbidities (27). Even though there are some potential but small risks of side effects associated with ICS use in some comorbid conditions, there are no absolute contraindications for ICS (6). Additionally, evidence supports the idea that the proven effectiveness of ICS treatment outweighs the possible risks (28). Hence, there may be little, if any, effect of comorbid conditions influencing the outcomes reflected in our study results.
Second, we could not consider the exact severity of asthma. Severe asthma attacks require different treatment strategies than mild-to-moderate asthma (13). Therefore, we excluded J46 (status asthmaticus) codes and emergency department visits, which might have included some of the severe asthma cases. At the same time, as in other studies using NHI claims data, we could not exclude any mild asthma cases that only require a rapid-acting β2-agonist as needed (11,29,30), since it is impossible to distinguish mild asthma cases from NHI claims database alone. Finally, there is also the possibility that the ICD codes from the claims database may not be accurate. There can be some biases according to the definition of the asthma cases. However, this possible bias due to inaccuracy would be of little significance, because we compared the trends before and after the dissemination of the guidelines, meaning that the same inaccuracy bias would have affected both periods equally. The similar patterns of ICS prescription in segmented regression and multilevel logistic regression also support our results. Also the consistent patterns from both main and sensitivity analysis support the validity of the asthma case definition used in this study.
In conclusion, we find out that the "Korean Asthma Management Guideline for Adults 2007" has made no significant effects on ICS prescription for asthma management, especially in primary clinics. The discrepancy between the clinical guideline and the reimbursement criteria of HIRA and patient's preference for oral drugs are important barriers for prescribing ICS. To increase the ICS prescription rate for asthma patients, continuous efforts to reduce the patients' resistance about ICS and political support to eliminate discrepancy between clinical guidelines and reimbursement criteria of HIRA are essential.
Footnotes
This study was supported by a grant of Korea Centers for Disease Control and Prevention (KCDC), Republic of Korea.
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception of the study: Cho BL, Shin DW. Acquisition of data: Kim SH, Yun JM, Chung YH. Statistical analysis: Kim SH, Yun JM, Hwang SS. Manuscript preparation: Kim SH, Ahn EM, Lee HJ, Nam YS. Manuscript approval: all authors.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 3.Dal Negro R, Berto P, Tognella S, Quareni L Global Outcomes in Lung Disease Study Group. Cost-of-illness of lung disease in the TriVeneto Region, Italy: the GOLD Study. Monaldi Arch Chest Dis. 2002;57:3–9. [PubMed] [Google Scholar]
- 4.Kim CY, Park HW, Ko SK, Chang SI, Moon HB, Kim YY, Cho SH. The financial burden of asthma: a nationwide comprehensive survey conducted in the republic of Korea. Allergy Asthma Immunol Res. 2011;3:34–38. doi: 10.4168/aair.2011.3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YH, Yoon SJ, Kim EJ, Kim YA, Seo HY, Oh IH. Economic burden of asthma in Korea. Allergy Asthma Proc. 2011;32:35–40. doi: 10.2500/aap.2011.32.3479. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention (updated 2012) 2012. [accessed on June 29 2015]. Available at http://www.ginasthma.org.
- 7.Adams RJ, Fuhlbrigge A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol. 2002;110:58–64. doi: 10.1067/mai.2002.125489. [DOI] [PubMed] [Google Scholar]
- 8.Cazzoletti L, Marcon A, Janson C, Corsico A, Jarvis D, Pin I, Accordini S, Almar E, Bugiani M, Carolei A, et al. Therapy and Health Economics Group of the European Community Respiratory Health Survey. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120:1360–1367. doi: 10.1016/j.jaci.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, Trung PL, Zhong NS, Zainudin N, Zainudin BM, et al. Asthma Insights and Reality in Asia-Pacific Steering Committee. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111:263–268. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- 10.Korean Academy of Medical Sciences, Korean Academy of Tuberculosis and Respiratory Diseased & Korean Academy of Asthma, Allergy and Clinical Immunology. Korean Asthma Management Guideline for Adults 2007. [accessed on June 29 2015]. Available at http://guideline.or.kr/guideline/search.php?mode=search&type=2&Msid=28&Fsid=667.
- 11.Kim S, Kim J, Kim K, Kim Y, Park Y, Baek S, Park SY, Yoon SY, Kwon HS, Cho YS, et al. Healthcare use and prescription patterns associated with adult asthma in Korea: analysis of the NHI claims database. Allergy. 2013;68:1435–1442. doi: 10.1111/all.12256. [DOI] [PubMed] [Google Scholar]
- 12.Chun CB, Kim SY, Lee JY, Lee SY. Republic of Korea: health system review. Health Syst Transit. 2009;11:1–184. [Google Scholar]
- 13.Lazarus SC. Clinical practice. Emergency treatment of asthma. N Engl J Med. 2010;363:755–764. doi: 10.1056/NEJMcp1003469. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization, Ministry of Health and Welfare Republic of Korea. Republic of Korea health service delivery profile. 2012. [accessed on Jung 29 2015]. Available at http://www.wpro.who.int/health_services/service_delivery_profile_republic_of_korea.pdf.
- 15.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest. 2001;119:720–725. doi: 10.1378/chest.119.3.720. [DOI] [PubMed] [Google Scholar]
- 17.Bae YJ, Kim TB, Jee YK, Park HW, Chang YS, Cho SH, Cho YS, Moon HB. Severe asthma patients in Korea overestimate their adherence to inhaled corticosteroids. J Asthma. 2009;46:591–595. doi: 10.1080/02770900902980908. [DOI] [PubMed] [Google Scholar]
- 18.Lopez G, Wyatt L. Asthma prevalence, barriers to effective treatment, and current policy recommendations for the State of California. California. 2002 Educational Tour Series. California Research Bureau. Sacramento: California State Library, California Research Bureau; 2003. [accessed on June 29 2015]. Available at https://www.library.ca.gov/crb/03/04/03-004.pdf. [Google Scholar]
- 19.Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130:65s–72s. doi: 10.1378/chest.130.1_suppl.65S. [DOI] [PubMed] [Google Scholar]
- 20.Kelloway JS, Wyatt RA, Adlis SA. Comparison of patients' compliance with prescribed oral and inhaled asthma medications. Arch Intern Med. 1994;154:1349–1352. [PubMed] [Google Scholar]
- 21.Chambers CV, Markson L, Diamond JJ, Lasch L, Berger M. Health beliefs and compliance with inhaled corticosteroids by asthmatic patients in primary care practices. Respir Med. 1999;93:88–94. doi: 10.1016/s0954-6111(99)90296-2. [DOI] [PubMed] [Google Scholar]
- 22.Sun YH, Eun BW, Sim SY, Cho KH, Ryoo E, Cho DY, Son DW, Tchah H, Jeon IS. Poor adherence and reasons for nonadherence to the asthma guidelines among pediatricians in Korea. Asian Pac J Allergy Immunol. 2010;28:147–154. [PubMed] [Google Scholar]
- 23.Wisnivesky JP, Lorenzo J, Lyn-Cook R, Newman T, Aponte A, Kiefer E, Halm EA. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Ann Allergy Asthma Immunol. 2008;101:264–270. doi: 10.1016/S1081-1206(10)60491-7. [DOI] [PubMed] [Google Scholar]
- 24.Cabana MD, Ebel BE, Cooper-Patrick L, Powe NR, Rubin HR, Rand CS. Barriers pediatricians face when using asthma practice guidelines. Arch Pediatr Adolesc Med. 2000;154:685–693. doi: 10.1001/archpedi.154.7.685. [DOI] [PubMed] [Google Scholar]
- 25.Cho SH, Park HW, Rosenberg DM. The current status of asthma in Korea. J Korean Med Sci. 2006;21:181–187. doi: 10.3346/jkms.2006.21.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho SH, Kim YK, Chang YS, Kim SS, Min KU, Kim YY. Asthma insights and reality in Korea. Korean J Med. 2006;70:69–77. [Google Scholar]
- 27.Milchak JL, Carter BL, James PA, Ardery G. Measuring adherence to practice guidelines for the management of hypertension: an evaluation of the literature. Hypertension. 2004;44:602–608. doi: 10.1161/01.HYP.0000144100.29945.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American College of Chest Physicians, American Academy of Allergy, Asthma and Immunology, American College of Allergy Asthma & Immunology. Quick reference guide for clinicians: systematic review of the evidence regarding potential complications of inhaled steroid use in asthma. Ann Allergy Asthma Immunol. 2004;92:291–293. doi: 10.1016/S1081-1206(10)61565-7. [DOI] [PubMed] [Google Scholar]
- 29.Kang HY, Park CS, Bang HR, Sazonov V, Kim CJ. Effect of allergic rhinitis on the use and cost of health services by children with asthma. Yonsei Med J. 2008;49:521–529. doi: 10.3349/ymj.2008.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park CS, Kang HY, Kwon I, Kang DR, Jung HY. Cost-of-illness study of asthma in Korea: estimated from the Korea National Health insurance claims database. J Prev Med Public Health. 2006;39:397–403. [PubMed] [Google Scholar]