Abstract
The study aimed to verify the prognostic utility, therapeutic application and clinical benefits of tumor substaging and HER2 status in papillary non-muscle invasive bladder cancer (NMIBC). Select NMIBC transurethral resection specimens from 141 patients were used to construct tissue microarrays for assessing the substaging, HER2 protein expression by immunohistochemistry (HER2-IHC) and gene amplification by dual-color silver in situ hybridization (HER2-SISH). Substages were identified by the differing depth of tumor invasion (pTa / pT1a / pT1b / pT1c). HER2 protein expression was semiquantitatively analyzed and grouped into negative (score 0, 1+) and positive (score 2+, 3+). Other clinicopathological variables were also investigated. For NMIBC, HER2-IHC and HER2-SISH showed positive results in 6/141 (4.3%) and 4/141 (2.8%) respectively, which correlated well with tumor substaging. In multivariate analysis, substaging, HER2-IHC, and HER2-SISH were found to be independent predictors of progression-free survival (P < 0.001, P < 0.001, P = 0.031). HER2-IHC was the sole independent predictor of recurrent free survival in NMIBC (P = 0.017). It is suggested that tumor substaging and HER2 status are independent predictive markers for tumor progression or recurrence, and thus could be included in diagnostic and therapeutic management for NMIBC.
Graphical Abstract
Keywords: Bladder Cancer, Cancer Staging, HER2 Gene, Immunohistochemistry, In Situ Hybridization
INTRODUCTION
More than 75% of bladder cancer is classified as nonmuscle-invasive bladder cancer (NMIBC), which is confined to the mucosal and stroma or lamina propria (1,2). The tumor recurrence of NMIBC varies from 30% to 80%; and 1% to 45% is known to progress into muscle invasive bladder cancer within 5 yr (1,2). Therefore, NMIBC is considered to be a heterogeneous disease, some of which might show the risk of progression to muscle invasion applicable for cystectomy during follow-up. However, there are no available clinical and pathological factors and is substantial need to find new predictive markers that indicate tumor recurrence or progression to effective treatment of the NMIBC patients.
During recent years, some literature described clinical and molecular predictive factors as accurate prognostic assessment for NMIBC patients (3,4,5,6). European Organization for Research and Treatment of Cancer (EORTC) proposed an NMIBC calculator that could predict the short- and long-term risks of recurrence and progression based on six clinicopathologic variables including grade, stage, concomitant carcinoma in situ (CIS), multiplicity, tumor size, and previous recurrence rate (2,6). Regarding molecular predictive factors, there are many studies that investigate various markers such as p53, Cyclin D1, FGFR-3, Ki-67, Cathepsin E, and maspin as significant predictors of recurrence or progression (4,5,7,8,9). However, overestimated risks of both recurrence and progression were reported when applying the EORTC tables to BCG-treated patients (9) and no molecular markers are currently in clinical use as predictive or prognostic marker in NMIBC.
The human epidermal growth factor receptor 2 (HER2) expression has been extensively investigated as a target therapy and is known to be a useful prognostic and therapeutic marker in breast cancer and advanced gastric cancer (10,11). HER2 is a 185-kDa transmembrane tyrosine kinase receptor of epidermal growth factor receptor family located on chromosome 17q21 and its intrinsic tyrosine kinase activity involves cell proliferation and survival via the RAS-MARK pathway (10,11,12). There have been several studies that elaborated on HER2 status in bladder cancers (12,13,14,15). However, HER2 status in bladder cancer has been reported to vary from 9% to 80% in regards to protein overexpression and 0% to 32% regarding gene amplification of HER2 (4,12,13,14,15). Therefore, the predictive value of HER2 status in bladder cancer remains controversial. Moreover, there are only a few studies of HER2 status in NMIBC (14,15).
NMIBC is approximately composed of 70% pTa, 20% pT1 and 10% CIS (1,2). Although tumor substaging still remains an issue of controversy, many publications have shown substaging of pT1 tumors correlates with clinical outcome such as progression free time, recurrent free time or disease specific survival (3,15,16,17). Hence, the aim of this study was to identify the potential impact on the clinical outcome of tumor substaging and HER2 status in NMIBC to verify their prognostic and therapeutic utility.
MATERIALS AND METHODS
Case selection
A retrospective study was conducted on patients who underwent transurethral resection (TUR) of bladder tumor and were subsequently diagnosed as NMIBCs between 1998 and 2012 at the Departments of Pathology, Asan Medical Center Seoul, Korea. It comprised of 300 patients who were diagnosed as pTa or pT1 papillary urothelial tumors based on primary TUR specimens, according to the 7th edition American Joint Committee on Cancer TNM system (18).
All histological sections were reviewed again and graded as papillary urothelial neoplasm of low malignant potential (LMP), low grade and high grade papillary urothelial carcinomas according to 2004 WHO classification (19). Tumor substaging was divided into four categories as follows; pTa (no invasion into the stroma), pT1a (invasion into the stroma but not to muscularis mucosa (MM), pT1b (invasion into MM but not beyond MM), pT1c (invasion beyond the MM but not to muscularis propria). pT1 tumors samples from TUR specimens that contained proper muscle were incorporated in this study and their substaging rate was 81.4% (144/177 pT1 tumors). Concomitant CIS and lymphovascular invasion were also included during review. Clinicopathologic data including age, sex, tumor size, and multifocality were collected from medical reports. After re-evaluating specimens and reviewing the hospital records, 141 NMIBCs were deemed fit for the final study because specimens were missing or not applicable for tissue microarray (TMA) construction and HER2 assessment as well as insufficient clinicopathologic information in the hospital records.
Recurrence was defined as occurrence of a new tumor confirmed by biopsy after three months of the first TUR of bladder tumor during follow-up. Progression was defined as recurrence confirmed by biopsy showing invasion into the muscularis propriae or more or distant metastasis, and or death due to the disease during follow-up.
Construction of tissue microarray
TMAs were constructed using a manual tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA). Three randomly representative 2 mm cores from the circled cancer were obtained from the most representative cancer areas of formalin-fixed paraffin-embedded tissue blocks and were arranged in TMA blocks. Hematoxylin and eosin staining of the TMA sections was performed for tissue confirmation.
Immunohistochemistry
TMAs paraffin blocks were sectioned into 4 µm slices, deparaffinized, and antigens demasked in EDTA buffer, pH 8.4. HER2 immunohistochemistry (IHC) was performed using PATHWAY anti-HER2/neu (4B5; rabbit monoclonal; pre-dilution; Ventana Medical Systems, Tucson, AZ, USA) antibody and ultraView Universal DAB kit (Ventana Medical Systems) on an automatic immunostainer (BenchMark XT, Ventana Medical Systems), according to the manufacturer's instructions. Primary antibody omission was used for the negative control and breast cancer previously confirmed by HER2-IHC showing 3+ immunopositivity was used for positive control. IHC scoring was independently performed by two pathologists without prior knowledge of clinicopathological information or molecular results obtained via other methods. The scoring was first semiquantitatively analyzed and grouped into 4 categories as follows: 0, no staining; 1+, faint/barely partial membrane staining less than 50% of tumor area; 2+, variable weak-to-moderate complete membrane staining in ≥50% of tumor area; 3+, strong complete membrane staining in all tumor area. The two pathologists completely agreed on all the IHC scoring and IHC 2+ and 3+ were considered to indicate HER2-IHC positive group for statistical reason.
Dual-color silver in-situ hybridization
Bright-field dual-color in-situ hybridization (SISH) analysis was performed on TMAs of NMIBCs using the automatic SISH staining device (BenchMark XT, Ventana Medical Systems), according to the manufacturer's protocols for INFORM HER2 DNA and INFORM Chromosome 17 (CEP17) probes (Ventana Medical Systems). HER2/CEP17 SISH signals were counted according to the interpretive guideline for Ventana INFORM HER2 DNA probe staining of gastric cancer cells (Ventana Medical Systems) (11). Tumor cells were scanned for hot spots by using 20× or 40× objectives, and the area with the highest signals was selected. The signals were counted in 20 nonoverlapping tumor cell nuclei from each case using 60× or 100× objectives by two pathologists who were blinded to HER2 status by other detection methods and clinical information. Small or large clusters were counted as 8 signals and 16 signals, respectively. HER2 gene amplification was defined as a HER2/CEP17 ratio of ≥2.0 in 20 tumor nuclei. The equivocal cases (ratio: 1.8 to 2.2) were recounted in at least 20 non-overlapping nuclei of different tumor cells at a second target area. Normal HER2 signals (1 to 2 copies per cell) are used as internal positive controls for each case as well as breast cancer tissue previously confirmed by 3+ HER2 gene amplification.
Statistical analysis
The chi-square or Fisher exact test was used to evaluate the stastistical significance of the associations between clinicopathologic parameters. Survival analysis was performed using the Kaplan-Meier method and the significance of differences in survival between the groups was determined using the log-rank test. Multivariate analysis was done to identify variables with independent prognostic relevance using the Cox proportional hazard model. A P value of <0.05 (two-tailed) was used to establish statistical significance. All statistical analyses were conducted using SPSS v. 19.0 (SPSS Inc., Chicago, IL, USA) and dBSTAT v. 5 (dBSTAT Co., Chuncheon, Korea).
Ethics statement
This study was approved by the institutional review boards of Asan Medical Center (2013-107). Informed consents were waived by the board.
RESULTS
The clinicopathological characteristics of the 141 patients with NMIBC are summarized in Table 1. The patients included 122 men (86.3%) and 19 women (13.5%). The mean age of the patients was 68.9 yr (range 20-93 yr). Mean follow duration was 73.3 months (range 3.9-187.9 months). Tumor recurrence and progression were found in 68 (48.2%) and 23 (16.3%) patients during follow-up. 3 cm or more sized tumor was found in 60 (42.6%); and 2 or more of multifocality was found in 66 cases (46.8%). Concomitant CIS was noted in 31 (22%) cases and lymphovascular invasion was present in 3 (2.1%). Tumors were graded to LMP in 14 (9.9%), low grade in 59 (41.8%), and high grade in 68 (48.2%). Pathologic stage consisted of pTa in 65 (46.1%) and pT1 in 76 (53.9%). Substaging of pT1 was made as follows; pT1a in 50, pT1b in 9, and pT1c in 17 (Table 1).
Table 1. Clinicopathologic characteristics of the 141 patients with NMIBC.
| Characteristics | No. (%) of cases |
|---|---|
| Sex | |
| Male | 122 (86,5) |
| Female | 19 (13.5) |
| Age (mean, range, yr) | |
| All | 68.9 (20-93)±13.6 |
| Male | 68.3 (20-93)±13.6 |
| Female | 72.7 (48-91)±13.5 |
| Follow up (months) for survival | |
| Range | 3.9-187.9 |
| Mean S.D | 73.3±44.3 |
| Recurrence | |
| Absent | 73 (51.8) |
| Present | 68 (48.2) |
| Progression | |
| Absent | 118 (83.7) |
| Present | 23 (16.3) |
| Tumor size (n=187) | |
| <3 cm | 81 (57.4) |
| ≥3 cm | 60 (42.6) |
| Multifocality (n=195) | |
| Single | 75 (53.2) |
| Multiple | 66 (46.8) |
| pT stage | |
| Ta | 65 (46.1) |
| T1a | 50 (35.5) |
| T1b | 9 (6.4) |
| T1c | 17 (12.1) |
| Tumor grade | |
| LMP | 14 (9.9) |
| Low grade | 59 (41.8) |
| High grade | 68 (48.2) |
| Concomitant carcinoma in situ | |
| Absent | 110 (78) |
| Present | 31 (22) |
| Lymphovascular invasion | |
| Absent | 138 (97.9) |
| Present | 3 (2.1) |
| HER2 protein expression (IHC) | |
| 0 | 127 (90.1) |
| 1 | 8 (5.7) |
| 2 | 3 (2.1) |
| 3 | 3 (2.1) |
| HER2 gene amplification (SISH) | |
| Absent | 137 (97.2) |
| Present | 4 (2.8) |
NMIBC, nonmuscle-invasive bladder cancer; LMP, low malignant potential; IHC, immunohistochemistry; SISH, Silver In-Situ Hybridization.
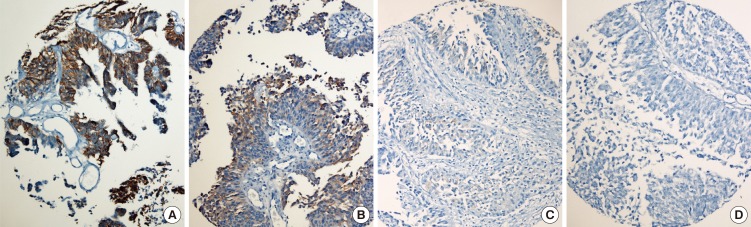
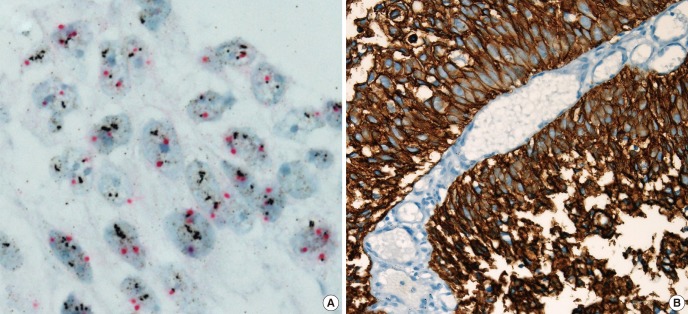
HER2 protein expression was semiquantatively scored to 0 in 127 (90.1%), 1 in 8 (5.7%), 2 in 3 (2.1%), and 3 in 3 (2.1%) cases, respectively. Score 2+ and 3+ were considered to indicate HER2-IHC positive in 6 of all 141 NMIBCs (4.3%) (Fig. 1 A-D), one of which is pTa tumor and 5 of which is pT1 tumors (6.6% of 76 pT1 tumors). All HER2-IHC positives were high grade NMIBC and accounted for 8.8% (6/68) of high grade NMIBC and 8.9% (5/56) of high grade pT1 tumors (Table 2). HER2 gene amplification was observed in 4 of all 141 NMIBC (2.8%), all of which were pT1 tumors (5.3%, 4/76 pT1 tumors) and high grade NMIBC (5.9%, 4/68 high grade tumors) (Tables 1, 2, 3) (Fig. 2A, B). HER2 gene amplification was 7.1% (4/56) of high grade pT1 tumors (Table 2)
Fig. 1. HER2 immunohistochemistry (HER2-IHC). (A) 3+, moderate-to-strong complete membrane staining in all tumor area. (B) 2+, variable weak-to-moderate complete membrane staining in ≥ 50% of tumor area. (C) 1+, faint/barely partial membrane staining less than 50% of tumor area. (D) 0, no staining. (magnification × 200).
Table 2. Comparison of substage and other clinicopathologic parameters in NMIBC.
| Parameters | pTa | pT1a | pT1b | pT1c | P value |
|---|---|---|---|---|---|
| Total (n = 141), (%) | 65 (46.1) | 50 (35.5) | 9 (6.4) | 17 (12.1) | |
| Tumor grade | < 0.001 | ||||
| LMP | 14 (21.5) | 0 (0) | 0 (0) | 0 (0) | |
| Low grade | 39 (60)) | 15 (30) | 1 (11.1) | 4 (23.5) | |
| High grade | 12 (18.5) | 35 (70) | 8 (88.9) | 13 (76.5) | |
| Multifocality | 0.084 | ||||
| Single | 39 (60) | 28 (56) | 3 (33.3) | 5 (29.4) | |
| Multiple | 26 (40) | 22 (44) | 6 (66.7) | 12 (70.6) | |
| Tumor size | 0.393 | ||||
| <3 | 41 (63.1) | 24 (48) | 6 (66.7) | 10 (58.8) | |
| ≥3 | 24 (36.9) | 26 (52) | 3 (33.3) | 7 (41.2) | |
| Concomitant CIS | 0.005 | ||||
| Absent | 59 (90.8) | 33 (66) | 5 (55.6) | 13 (76.5) | |
| Present | 6 (9.2) | 17 (34) | 4 (44.4) | 4 (23.5) | |
| LVI | 0.109 | ||||
| Absent | 65 (100) | 49 (98) | 8 (88.9) | 16 (94.1) | |
| Present | 0 (0) | 1 (2) | 1 (11.1) | 1 (5.9) | |
| HER2 protein expression (IHC) | 0.038 | ||||
| Negative | 64 (98.5) | 48 (96) | 7 (77.8) | 16 (94.1) | |
| Positive | 1 (1.5) | 2 (4) | 2 (22.2) | 1 (5.9) | |
| HER2 gene amplification (SISH) | 0.002 | ||||
| Absent | 65 (100) | 49 (98) | 7 (77.8) | 16 (94.1) | |
| Present | 0 (0) | 1 (2) | 2 (22.2) | 1 (5.9) | |
| Recurrence | 0.993 | ||||
| Absent | 33 (50.8) | 26 (52) | 5 (55.6) | 9 (51.8) | |
| Present | 32 (49.2) | 24 (48) | 4 (44.4) | 8 (47.1) | |
| Progression | < 0.001 | ||||
| Absent | 62 (95.4) | 43 (86) | 4 (44.4) | 9 (52.9) | |
| Present | 3 (4.6) | 7 (14) | 5 (55.6) | 8 (47.1) |
LMP, low malignant potential; CIS, carcinoma in situ; LVI, lymphovascular invasion; NMIBC, nonmuscle invasive bladder cancer; IHC, immunohistochemistry; SISH, silver in-situ hybridization.
Table 3. Comparison of tumor grade and HER status with other clinicopathologic parameters in NMIBC.
| Parameters Total (n=141) | Tumor grade | HER2 IHC | HER2 SISH | ||||
|---|---|---|---|---|---|---|---|
| LMP | Low grade | High grade | Negative | Positive | Absent | Present | |
| Tumor grade | |||||||
| LMP | 14 (10.4) | 0 (0) | 14 (10.2) | 0 | |||
| Low | 59 (43.7) | 0 (0) | 59 (43.1) | 0 (0) | |||
| High | 62 (45.9) | 6 (100) | 64 (46.7) | 4 (100) | |||
| Multifocality | |||||||
| Single | 9 (64.3) | 37 (62.7) | 29 (42.6) | 73 (54.1) | 2 (33.3) | 73 (53.3) | 2 (50) |
| Multiple | 5 (35.7) | 22 (37.3) | 39 (57.4) | 62 (45.9) | 4 (66.7) | 64 (46.7) | 2 (50) |
| Tumor size | |||||||
| <3 | 11 (78.6) | 32 (54.2) | 38 (55.9) | 78 (57.8) | 3 (50.0) | 79 (57.7) | 2 (50) |
| ≥3 | 3 (21.4) | 27 (45.8) | 30 (44.1) | 57 (42.2) | 3 (50.0) | 58 (42.3) | 2 (50) |
| Concomitant CIS | |||||||
| Absent | 14 (100) | 54 (91.5) | 42 (61.8) | 106 (78.5) | 4 (66.7) | 108 (77.4) | 4 (100) |
| Present | 0 (0) | 5 (8.5) | 26 (38.2) | 29 (21.5) | 2 (33.3) | 31 (22.6) | 0 (0) |
| LVI | |||||||
| Absent | 14 (100) | 58 (98.3) | 66 (97.1) | 134 (99.3) | 4 (66.7) | 136 (99.3) | 2 (50) |
| Present | 0 (0) | 1 (1.7) | 2 (2.9) | 1 (0.7) | 2 (33.3) | 1 (0.7) | 2 (50) |
| HER2 IHC | |||||||
| Negative | 14 (100) | 59 (100) | 62 (91.2) | 135 (98.5) | 0 (0) | ||
| Positive | 0 (0) | 0 (0) | 6 (8.8) | 2 (1.5) | 4 (100) | ||
| HER2 SISH | |||||||
| Absent | 14 (100) | 59 (100) | 64 (94.1) | 135 (100) | 2 (33.3) | ||
| Present | 0 (0) | 0 (0) | 4 (5.9) | 0 (0) | 4 (66.7) | ||
| Recurrence | |||||||
| Absent | 10 (71.4) | 24 (40.7) | 39 (57.4) | 71 (52.6) | 2 (33.3) | 71 (51.8) | 2 (50) |
| Present | 4 (28.6) | 36 (59.3) | 29 (42.6) | 64 (47.4) | 4 (66.7) | 66 (48.2) | 2 (50) |
| Progression | |||||||
| Absent | 14 (100) | 55 (93.2) | 49 (72.1 ) | 118 (87.4) | 0 (0) | 118 (86.1) | 0 (0) |
| Present | 0 (0) | 4 (6.8) | 19 (27.9) | 17 (12.6) | 6 (100) | 19 (13.9) | 4 (100) |
Bold, P<0.005. LMP, low malignant potential; CIS, carcinoma in situ; LVI, lymphovascular invasion; NMIBC, nonmuscle invasive bladder cancer; IHC, immunohistochemistry; SISH, silver in-situ hybridization.
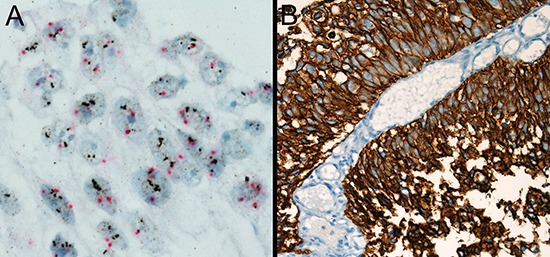
Fig. 2. HER2 gene amplification by dual-color silver in situ hybridization (HER2-SISH). HER2-SISH positive case (A) matched with HER2-IHC 3+ (B). (magnification: (A) × 1,000; (B) × 400).
Substaging was significantly associated with tumor grade (P<0.001), concomitant CIS (P=0.005), HER2 protein expression (P=0.038), and HER2 gene amplification (P=0.002) (Table 2). Substaging is also associated with tumor progression (P<0.001) but not in tumor recurrence (P=0.993) (Table 2).
Tumor grade correlated with concomitant CIS (P<0.001), HER2 protein expression (P=0.035), and tumor progression (P<0.001) (Table 3). HER2-IHC positive was significantly associated with lymphovascular invasion (P=0.004) and all 6 HER2-IHC positive showed tumor progression (P<0.001) (Table 3). A firm correlation was observed between HER2 protein expression by immunohistochemistry and gene amplification by SISH (P<0.001). Two HER2-IHC score 2+ cases had absence of HER2 gene amplification. HER2-SISH correlated with lymphovascular invasion (P=0.002) and tumor progression (P=0.001) (Table 3). There is no association of age, sex, tumor size and multifocality with other clinicopathologic parameters.
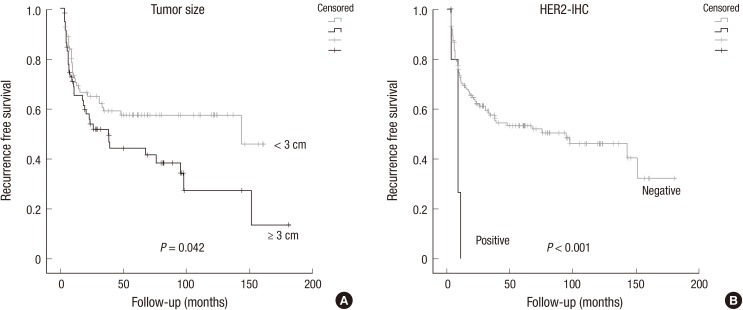
The average period until the first recurrence was 44.4 months (range, 3.2 to 180.4 months) (Table 4). Univariate analysis demonstrated that tumor size and HER2-IHC positive significantly correlated with recurrence-free survival (P=0.042, P<0.001, respectively) (Fig. 3A and B). Only HER2-IHC positive NMIBC showed shorter recurrence time with multivariate analysis (P=0.019) (Table 4).
Table 4. Univariate and multivariate analyses for recurrence and progression-free survival.
| Variables | Recurrence free survival | Progression free survival | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||
| P value | Hazard ratio (95% C.I) | P value | P value | Hazard ratio (95% C.I) | P value | |
| Substage | 0.704 | N/A | N/A | 0 | 1.999 (1.355-2.949) | < 0.001 |
| Tumor size | 0.042 | 1.595 (0.989-2.572) | 0.055 | 0.317 | N/A | N/A |
| Multifocality | 0.22 | N/A | N/A | 0.041 | 1.360 (0.490-3.775) | 0.555 |
| Tumor grade | 0.168 | N/A | N/A | 0 | 2.009 (0.660-6.114) | 0.219 |
| CIS | 0.569 | N/A | N/A | 0.95 | N/A | N/A |
| LVI | 0.616 | N/A | N/A | 0 | 1.861 (0.177-19.611) | 0.605 |
| HER2 IHC | 0 | 3.459 (1.223-9.781) | 0.019 | 0 | 84.642 (11.661-614.393) | < 0.001 |
| HER2 SISH | 0.195 | N/A | N/A | 0 | 0.086 (0.009-0.800) | 0.031 |
Only risk factors with P values<0.05 were included in the multivariate analysis. IHC, immunohistochemistry; SISH, silver in-situ hybridization; CI, confidence interval; N/A, not available.
Fig. 3. Kaplan Meier analysis for recurrence free survival in NMIBC. Tumor size (A) and HER2-IHC positive (B) significantly correlated with recurrence-free survival.
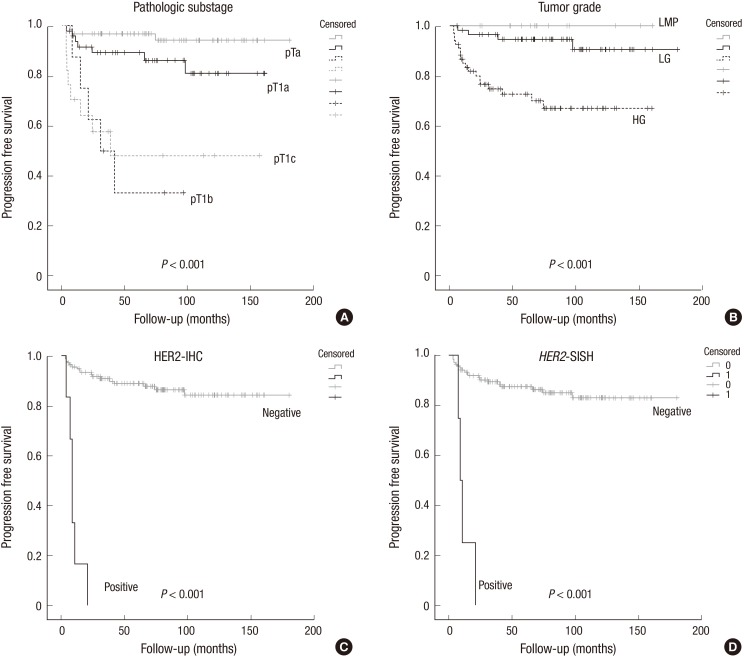
The average period until the first tumor progression was 69.9 months (range, 3.4 to 180.4 months) (Table 4). Substage, multifocality, tumor grade, lymphovascular invasion, HER2 protein expression and gene amplification correlated with progression-free survival (P<0.001, P=0.041, P<0.001, P<0.001, P<0.001, P<0.001, respectively) by univariate analysis (Table 4) (Fig. 4A-D). Multivariate analysis further demonstrated that substaging, HER2-IHC and HER2-SISH were an independent predictive factor of tumor progression for patients with NMIBC (P<0.001, P<0.001, P=0.031, respectively) (Table 4).
Fig. 4. Kaplan Meier analysis for progression free survival in NMIBC. Pathologic substage (A), tumor grade (B), HER2-IHC (C) and HER2-SISH (D) correlated with progression-free survival.
DISCUSSION
The therapeutic guidelines for NMIBC remain controversial; and conservative management may allow tumor to progress to muscle invasion, which requires cystectomy (1,2). The pathologic stage assessed by the severity of invasion depth is one of the most important prognostic predictors of NMIBC (3,16). Several literatures focusing on clinical significance of substaging pT1 tumors have been published, and their results still remain controversial (3,16,17). However, the necessity of substaging was further highlighted in several recent articles which showed strong correlation between progression free survival or overall survival and substaging in pT1 tumors (3,16,17). In the current study, the authors reached a consensus to conduct substaging, which yielded meaningful results in establishing the method as a significant prognostic predictor in NMIBC. In addition, higher substage was closely linked to higher tumor grade and greater likelihood of concomitant CIS. Hence, substaging could be recommended to be included in clinical therapeutic guideline for NMIBC.
However, several challenges in substaging have hindered its adoption in clinical guidelines for NMIBC management (16,17,20). First, the substaging rate of pT1 tumors varied from 58% to 100% in the literature (17,20) and was 81.4% in our study. We only utilized samples that contained proper muscle tissue for pT1 cases because the incidence of understaging is reported to be 14% when proper muscle tissue is present, while when proper muscle tissue is absent, the incidence is 49% (20). One persistent problem is low tissue quality, which is caused by damaged TUR specimen and unclear presence of muscularis mucosae. Moreover, there is lack of consensus among pathologists regarding substaging methodology and interobserver variability of its interpretation. In addition to the substaging method covered in this study, several studies have laid out different methods of substaging, which also showed good correlation with outcome (16,17,20). Brimo et al. (16) illustrated the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion for substaging. Hu et al. (17) measured the dimension of invasive component in pT1 urothelial carcinoma in TUR specimens. Their respective methods showed strong correlation with progression free survival or recurrent free survival. Therefore, further studies will be needed to come to a consensus on methodology and interpretation criteria of substaging.
Recently, there have been a number of articles which noted HER2 overexpression or amplification in many cancers, and these reports led to targeted therapy using trastuzumab (10,11,21). As widely known, the therapy is currently used worldwide for treating breast and gastric cancers (10,11). There are well established diagnostic guidelines that identify HER2 positive breast and gastric cancers according to either the ASCO/CAP or ToGA scoring schemes (10,11). Whereas HER2-IHC score 3+ is considered to be HER2 protein overexpression, HER2-IHC score 2+ should be confirmed using ISH by either SISH or fluorescent ISH (FISH) as HER2 positive for both breast and gastric cancers (10). HER2-SISH is known to be more useful method than HER2-FISH in a clinical setting as it is fully automated and viewable by bright-field microscopy (22).
The prognostic values of HER2 expression in bladder cancer have remained unclear due to inconsistent results (4,12,23). The figures previously reported in the literature showed a wide range from 9.2% to 85% HER2 expression in bladder cancers (4,12,13,14,15). One reason for this variation is the fact that studies used bladder cancer samples with a varying degree of tumor stages and histological grades. Another reason could be attributed to technical limitations in IHC, and also the fact that there were no clear guidelines for assessing HER2 status for bladder cancer (4,12). Nevertheless, the most recent papers have presented HER2 overexpression was significantly associated with poor clinicopathological factors including lymph node metastasis and poor prognosis in bladder cancer (12,13,24). HER2 gene amplification was also frequently found in the micropapillary variant of urothelial carcinoma of the bladder, which is a rare but aggressive subtype (25).
Lae et al. (12) investigated HER2 status using very well calibrated IHC and FISH in a large cohort of 1,005 invasive urothelial bladder cancers. They reported HER2 protein overexpression was 9.2% and HER2 gene amplification was 5.1%. This is a lower frequency compared to previously reported figures in the literature (4,13). Lae et al. (12) pointed out that the major cause of this large variation was the technical heterogeneity in IHC and FISH assays in the earlier articles. The most recent article by cancer genomic atlas network also reported HER2 was a potential therapeutic target in 9% of urothelial carcinoma of the bladder (26). Therefore, the frequency of HER2 overexpression could be approximately 5%-10% in bladder cancer, which was suggested to be a potential candidate for target therapy.
As of now, there are only a few studies that investigated HER2 status in NMIBC, and they have yielded controversial results. Olsson et al. (15) reported HER2 protein was overexpressed in 12.4% of primary stage T1 bladder urothelial carcinomas, and there was no significant association between tumor HER2 status and prognosis including tumor progression and recurrence. Olsson et al. (15) used whole sections of pT1 tumors and analyzed HER2-IHC according to ASCO/CAP guidelines presented for breast cancers. On the other hand, a recent similar study had been conducted by Chen et al. (14) that investigated HER status of NMIBCs on TMA using IHC and FISH. They demonstrated that HER2 gene amplification was 9% and correlated remarkably well with aggressive clinical outcome in high grade NMIBC, and suggested that HER2 status would be valuable for distinguishing patients with NMIBC who require diligent surveillance.
Our study concurred with the previous finding that HER2 gene amplification is an independent predictor of tumor progression (14). HER2 gene amplification was 2.8% in NMIBC, 5.3% of pT1 tumors, 5.9% in high grade NMIBC, and 7.1% of high grade pT1 tumors in current study. No amplification was observed in pTa tumors. This figure is a little lower than the statistics recorded in the previous reports (12,14), which was 5.1% in invasive bladder carcinomas (12), 4% in NMIBC, 9% in high grade NMIBC and 2.2% in pTa tumors (14). HER2 status of bladder cancer may differ among different populations. Ethnic or geographic differences in HER2 expression were documented in several literatures (21). Our results highlight a good concordance between HER2-IHC and HER2-SISH in NMIBC and HER2-SISH positive cases might be applicable for HER2 target therapy.
In the current study, HER2-IHC positive correlated well with recurrence or progression free survival, accounted for 4.3% of NMIBC, 6.6% of pT1 tumors, 8.8% of high grade NMIBC, and 8.9% of high grade pT1 tumors. Though tumor progression in NMIBC ranges widely from 1% to 45% (1,2), it was found to be 16.3% in the current study. An interesting finding was that all six HER2-IHC positive patients showed tumor progression. Although HER2-IHC positive cases take up a small percentage of NMIBC cases, we believe HER2-IHC positive patients should receive more aggressive surveillance and monitoring with respect to personalized medicine. HER2-IHC turned out to be more informative than HER2-SISH in our study. HER2-IHC is cheaper and technically more convenient than HER2-SISH, and could be used in a routine diagnostic practice if it is well calibrated at the laboratory.
We were uncertain whether the guideline for breast cancer and gastric cancer of HER2 expression would apply to urothelial carcinoma, especially since small 2 mm core of NMIBC on TMA. As mentioned in the materials and methods, scoring of HER2-IHC was grouped into four patterns; and several statistical analyses concluded that HER2 positive group with score 2+ and 3+ was statistically the most significant for survival analyses. Chen et al. (14) also ran a comparative test on 30%, 40%, and 50% cut off criteria of HER2 expression analysis and reported IHC and FISH results were in closest agreement when overexpression was defined as 50% of tumor cells showing immunoreactivity. Although they did not report the detail of HER2 IHC results with regard to tumor grade or stage, HER2 protein expression was 4.2% (50% criterion) in NMIBCs in Table 2 of their article, which was the same with this study. In fact, one out of three cases with 2+ HER2-IHC showed gene amplification by HER2-SISH. Therefore, we classified HER2 score 2+ and 3+ as HER2-IHC positive group.
This study is also the first attempt to observe the changes in HER2 status using IHC and SISH depending on substage in NMIBC. Besides clinical and histopathological risk factors such as tumor grade, stage, concomitant CIS, multiplicity, tumor size, and previous recurrence rate, HER2 status and substage may be included as prognostic factors to the risk tables for bladder cancer (2,6). Granted, there are limitations in this study. The treatment has not been uniform for the NMIBC patients, whose therapy period ranged from 1998 to 2012. However, the tumor recurrence and progression data were from patients treated by a single institution. Therefore, HER2 assessment merits consideration in NMIBC diagnosis as well as prognostic and therapeutic management. Another limitation was the issue of tumor heterogeneity due to use of TMA tissue instead of whole sections of NMIBC. Intratumoral heterogeneity of HER2 expression was reported in 35% of invasive urothelial bladder carcinomas (12), but it was not investigated in earlier NMIBCs study using whole sections of tumor. In this study, the frequency of HER2-IHC positive (6.6% of pT1 tumors) was found to be lower than Olsson et al. (15) report (12.4% of pT1 tumors), which used whole tissue sections, but comparable to Chen et al. study (14), which used TMA tissue. On the other hand, Burandt et al. (27) identified homogeneously distributed HER2 overexpression/amplification in bladder cancers and demonstrated that tissue microarray based screening for therapeutic target genes represents a feasible approach suitable for routine application. Therefore, a future validation study with a set of reference samples of known HER2 status is necessary to attain the best protocol for HER2 status in NMIBC.
In conclusion, this study demonstrated that substaging, HER2 protein expression, and gene amplification are independent predictors of tumor progression in NMIBC. We recommend NMIBC should be tested for HER2 status as a predictive marker in practice, and therapeutic strategies of HER2 expression could be considered for the management of NMIBC.
Footnotes
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: A111345).
DISCLOSURE: There are no potential conflicts of interest in this article.
AUTHOR CONTRIBUTION: Conception & design of experiments: Lim SD, Yoon G, Cho YM, Choi GS. Performing experiments: Lim SD, Kim WY, Yoon G. Data analysis: Lim SD, Cho YM, Yoon G. Statistical analysis: Kim SN, Lim SD. Material contribution: Cho YM, Yoon G. Writing first draft and paper: Lim SD, Yoon G, Paick SH, Park HK, Choi GS. Review and revising the manuscript: Lim SD, Yoon G. Manuscript approval: all authors.
References
- 1.Hong SJ, Cho KS, Han M, Rhew HY, Kim CS, Ryu SB, Sul CK, Chung MK, Park TC, Kim HJ, et al. Korean Urological Oncology Society. Nomograms for prediction of disease recurrence in patients with primary Ta, T1 transitional cell carcinoma of the bladder. J Korean Med Sci. 2008;23:428–433. doi: 10.3346/jkms.2008.23.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, et al. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Lee JY, Joo HJ, Cho DS, Kim SI, Ahn HS, Kim SJ. Prognostic significance of substaging according to the depth of lamina propria invasion in primary T1 transitional cell carcinoma of the bladder. Korean J Urol. 2012;53:317–323. doi: 10.4111/kju.2012.53.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Xu W, Zhang Z, Song R, Zeng S, Sun Y, Xu C. Prognostic role of HER2 expression in bladder cancer: a systematic review and meta-analysis. Int Urol Nephrol. 2015;47:87–94. doi: 10.1007/s11255-014-0866-z. [DOI] [PubMed] [Google Scholar]
- 5.Ha YS, Yan C, Jeong P, Kim WT, Yun SJ, Kim IY, Moon SK, Kim WJ. GSTM1 tissue genotype as a recurrence predictor in non-muscle invasive bladder cancer. J Korean Med Sci. 2011;26:231–236. doi: 10.3346/jkms.2011.26.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A, Portillo J, Montesinos M, Gonzalez M, Pertusa C, et al. Club Urológico Español de Tratamiento Oncológico. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: external validation of the EORTC risk tables. Eur Urol. 2011;60:423–430. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Lee K, Jung ES, Choi YJ, Lee KY, Lee A. Expression of pRb, p53, p16 and cyclin D1 and their clinical implications in urothelial carcinoma. J Korean Med Sci. 2010;25:1449–1455. doi: 10.3346/jkms.2010.25.10.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fristrup N, Ulhøi BP, Birkenkamp-Demtröder K, Mansilla F, Sanchez-Carbayo M, Segersten U, Malmström PU, Hartmann A, Palou J, Alvarez-Múgica M, et al. Cathepsin E, maspin, Plk1, and survivin are promising prognostic protein markers for progression in non-muscle invasive bladder cancer. Am J Pathol. 2012;180:1824–1834. doi: 10.1016/j.ajpath.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 9.van Rhijn BW, Zuiverloon TC, Vis AN, Radvanyi F, van Leenders GJ, Ooms BC, Kirkels WJ, Lockwood GA, Boevé ER, Jöbsis AC, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol. 2010;58:433–441. doi: 10.1016/j.eururo.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, Bangs CD, Cherry AM, Pai RK. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20:13–24. doi: 10.1097/PAI.0b013e31821c821c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815–819. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolenz C, Shariat SF, Karakiewicz PI, Ashfaq R, Ho R, Sagalowsky AI, Lotan Y. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010;106:1216–1222. doi: 10.1111/j.1464-410X.2009.09190.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen PC, Yu HJ, Chang YH, Pan CC. Her2 amplification distinguishes a subset of non-muscle-invasive bladder cancers with a high risk of progression. J Clin Pathol. 2013;66:113–119. doi: 10.1136/jclinpath-2012-200944. [DOI] [PubMed] [Google Scholar]
- 15.Olsson H, Fyhr IM, Hultman P, Jahnson S. HER2 status in primary stage T1 urothelial cell carcinoma of the urinary bladder. Scand J Urol Nephrol. 2012;46:102–107. doi: 10.3109/00365599.2011.637955. [DOI] [PubMed] [Google Scholar]
- 16.Brimo F, Wu C, Zeizafoun N, Tanguay S, Aprikian A, Mansure JJ, Kassouf W. Prognostic factors in T1 bladder urothelial carcinoma: the value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum Pathol. 2013;44:95–102. doi: 10.1016/j.humpath.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Hu Z, Mudaliar K, Quek ML, Paner GP, Barkan GA. Measuring the dimension of invasive component in pT1 urothelial carcinoma in transurethral resection specimens can predict time to recurrence. Ann Diagn Pathol. 2014;18:49–52. doi: 10.1016/j.anndiagpath.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 497–502. [Google Scholar]
- 19.Lopez-Beltran A, Sauter G, Gasser T, Hartmann A, Schmitz-Drager BJ, Helpap B, Ayala AG, Tamboli P, Knowles MA, Sidransky D, et al. Infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC Press; 2004. pp. 93–109. (World Health Organization Classification of Tumors; vol 6) [Google Scholar]
- 20.van Rhijn BW, van der Kwast TH, Alkhateeb SS, Fleshner NE, van Leenders GJ, Bostrom PJ, van der Aa MN, Kakiashvili DM, Bangma CH, Jewett MA, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012;61:378–384. doi: 10.1016/j.eururo.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, Selvarajah S, Barletta JA, Berman DM, de Muga S, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015;4:844–852. doi: 10.1002/cam4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim SJ, Cantillep A, Carpenter PM. Validation and workflow optimization of human epidermal growth factor receptor 2 testing using INFORM HER2 dual-color in situ hybridization. Hum Pathol. 2013;44:2590–2596. doi: 10.1016/j.humpath.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Caner V, Turk NS, Duzcan F, Tufan NL, Kelten EC, Zencir S, Dodurga Y, Bagci H, Duzcan SE. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol Oncol Res. 2008;14:261–266. doi: 10.1007/s12253-008-9027-y. [DOI] [PubMed] [Google Scholar]
- 24.Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60:350–357. doi: 10.1016/j.eururo.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Schneider SA, Sukov WR, Frank I, Boorjian SA, Costello BA, Tarrell RF, Thapa P, Houston Thompson R, Tollefson MK, Jeffrey Karnes R, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol. 2014;27:758–764. doi: 10.1038/modpathol.2013.201. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burandt E, Schreiber M, Stein A, Minner S, Clauditz TS, Bokemeyer C, Jänicke F, Fisch M, Izbicki JR, Knecht R, et al. Continuous tissue microarray based identification of cancers with homogeneous target expression for successful targeted therapy in clinical routine practice. Genes Chromosomes Cancer. 2014;53:228–239. doi: 10.1002/gcc.22130. [DOI] [PubMed] [Google Scholar]