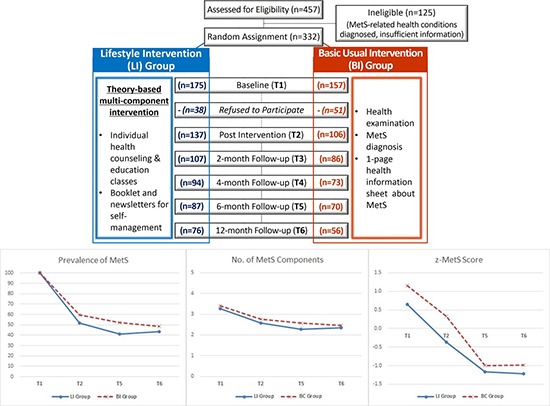
Abstract
This randomized controlled trial study aimed to investigate the effects of a lifestyle intervention on metabolic syndrome (MetS) among middle-aged Koreans. A total of 243 middle-aged Koreans with MetS were randomly assigned to either of 2 types of lifestyle intervention for MetS and followed for 12 months. Health examinations and interventions were implemented at 16 regional branch facilities of a Korean medical institution from 2010, following the NCEP-ATP III criteria and recommendations. Lifestyle intervention (LI) group (n = 137) participated in a 12-week multi-component intervention including individual counseling, group sessions, and self-help materials. Basic usual intervention (BI) group (n = 106) was provided with one-page health information sheet on MetS and MetS management at baseline. Prevalence of MetS and each of MetS components, except for low HDL-cholesterol, in both groups were significantly reduced and maintained after the intervention. Notably, prevalence of hypertension and abdominal obesity continued to improve during the follow-up period. Between-group differences in results were not found. Both interventions were effective when they were accompanied with repeated check-ups and notification of MetS status. It is recommended to design clear guidelines for the notification of MetS after MetS screening and to encourage checking MetS status periodically for effective MetS management (KCT 0000446).
Graphical Abstract
Keywords: Metabolic Syndrome, Lifestyle Intervention, Randomized Controlled Trial, Middle-aged Adults
INTRODUCTION
Metabolic syndrome (MetS) is a cluster of risk factors for diabetes (1), cardiovascular disease, and all-cause mortality (2). The prevalence of MetS has been increasing globally with widely varying pace by demographic factors such as age, sex, and ethnicity. The significant increase of MetS in western countries during the last decades has been a very serious public health concern (3,4). The increase of MetS is responsible for high socioeconomic cost due to higher prevalence of comorbidities (5).
The epidemic of MetS has been considered as an important public health problem in most Asian countries as well with prevalence higher than 20% (6,7). Asians are likely to carry more body fat at a lower body weight or smaller waist circumference than Caucasians (8,9). The prevalence of MetS has been increasing in Korea, especially in middle-aged adults. The age-adjusted prevalence of MetS increased significantly from 24.9% in 1998 to 31.3% in 2007 (10). The National Health Insurance Service (NHIS) Korea reports that about 30% of Koreans aged 30 yr and older have MetS (11). About 3 quarters (73.7%) of Korean adults have at least one of five parameters composing the MetS at an abnormal level. From 1998 to 2007, the prevalence of low HDL-cholesterol increased the most (+ 13.8%) among Koreans, followed by abdominal obesity (+ 8.7%) and hypertriglyceridemia (+ 4.9%).
With an increased concern, control of MetS has been one of the main targets of public health interventions in Asian countries (12). For example, Japanese government introduced a national strategy to prevent the obesity and MetS which is called as 'Metabo-Law'. The Metabo-Law requires waist circumferences of all Japanese adults aged 40-74 to be measured at annual health check-ups performed via local governments and employers. According to the Metabo-Law, those with abdominal obesity (waist circumference > 85 cm for male and > 90 cm for female) are referred to counseling sessions with phone calls and e-mail correspondence, and motivational support (13). In Korea, the metropolitan government of Seoul has launched the "5-Rock" MetS control and prevention program since 2011 (http://www.5check.or.kr/). Citizens of Seoul can participate in the program via local public health centers in all 25 districts in Seoul. Those diagnosed with MetS receive counseling services in this program on a regular basis to reduce the MetS risks through lifestyle changes.
Most interventions on MetS prevention and management consist of lifestyle modification strategies. The U.S. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) emphasized lifestyle modification as an essential strategy for the risk management for cardiovascular diseases (14). The Korean Academy of Family Medicine also endorses lifestyle changes, dietary therapies, and physical activity as non-pharmaceutical strategies for prevention and control of MetS (15). The positive impact of lifestyle modification interventions on MetS management including dietary changes and physical activity have been reported by several empirical studies (16,17,18).
MetS intervention studies in Korea so far (19,20) have been implemented to limited target population, few of which have evaluated long-term effects of the interventions. In the 5-Rock project, for example, the group with 6-month intervention resulted in significant improvements in four of five components of MetS except for the HDL-cholesterol (19). However, the evaluation was limited as there was not a control group in the study, the intervention programs were not standardized across the public health centers, and the program participation was on a voluntary basis.
The present study was an attempt to investigate the effects of lifestyle interventions on MetS in Korean middle-aged adults in a randomized controlled trial with multiple follow-up assessments. A theory-based nationwide intervention program conducted by the Korea Association of Health Promotion (KAHP) was followed multiple times for 12 months to identify long-term effects of the intervention on MetS in comparison with conventional basic care for MetS at KAHP.
MATERIALS AND METHODS
Study design and participants
Effective lifestyle interventions for MetS are recommended to combine guidance for nutrition and exercise with behavioral and cognitive strategies (21). This study employed an 'alternative-treatments design with pretest' (22) in order to determine the effectiveness of a 12-week multi-component lifestyle intervention versus a one-time, single-component usual care for MetS. This design was particularly useful because it allowed us: 1) to compare the effectiveness of an alternative intervention to that of a usual care; and 2) to stay ethical to provide care for all participants in this study.
A total of 457 Korean men and women aged 40-60 yr were recruited nationwide after having been identified with MetS at one of 16 regional branch health facilities of the KAHP in 2010. MetS was defined by the NCEP -ATP III criteria for MetS and the Asia-Pacific standard for abdominal obesity (23). That is, middle-aged Koreans who have 3 or more of the five conditions described in Table 1 are classified as having MetS.
Table 1. Definition of metabolic syndrome.
| Risk factors | Components | Defining level |
|---|---|---|
| Hypertension | Blood pressure | Systolic BP ≥ 130 mmHg or Diastolic BP ≥ 85 mmHg |
| Abdominal obesity | Waist circumference | Male > 90 cm or Female > 80 cm |
| Hyperglycemia | Fasting glucose | ≥ 100 mg/dL |
| Hypertriglyceridemia | Triglycerides | ≥ 150 mg/dL |
| Low HDL-cholesterol | HDL-cholesterol | Male < 40 mg/dL or Female < 50 mg/dL |
BP, blood pressure; HDL-cholesterol, high-density lipoprotein cholesterol.
Primary outcome of this study was the prevalence of MetS. We assumed improvement rates of 65% for intensive intervention and 44% for basic usual intervention based on our previous studies on lifestyle interventions for MetS (24,25) for sample size calculation. Similarly, 30% attrition was considered. With 80% power and 5% level of significance, the sample size required per group was 111.
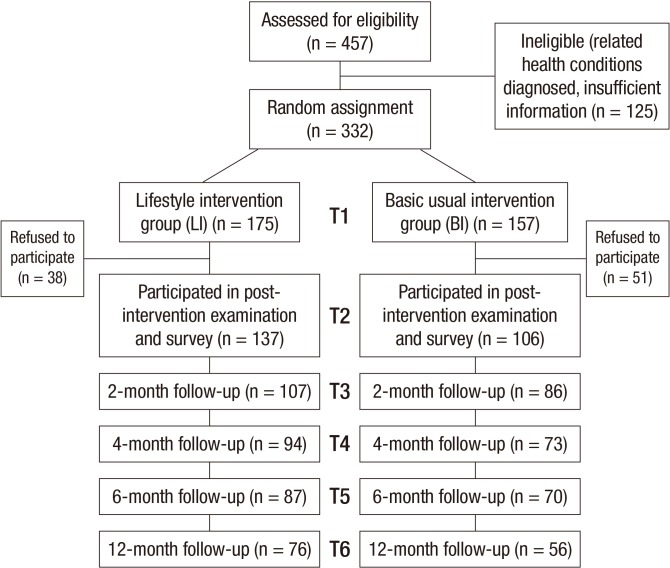
Of these 457 participants with MetS, 125 people were excluded: who were in treatment or on medication for MetS-related health conditions such as hypertension, dyslipidemia, and diabetes; who provided incomplete information to core study questions in the survey; and who had not signed consent for participation in the study (Fig. 1). Using a random number table, the 332 eligible people who agreed to participate were randomly assigned either to a 12-week intensive lifestyle intervention (LI) group or to a basic usual intervention (BI) group. Both participants and researchers were blinded to be unaware of intervention allocations. During the intervention, 38 participants in LI group and 51 participants in BI group discontinued participating. Thereby, 137 participants in LI group and 106 participants in BI group were included in post-intervention health examination and survey, and followed for multiple post assessments for 12 months. There were no significant differences in socio-demographic and MetS characteristics between the intervention participants and the dropouts at baseline (data not shown). This study is registered in the Clinical Research Information Service by Korea Centers for Diseases Control and Prevention, Republic of Korea (#KCT 0000446).
Fig. 1. Flow chart of participants.
Intervention program
The LI group received a theory-based 12-week multi-component intervention after having been diagnosed of MetS (Table 2). The multi-component intervention program included individual health counseling, health education classes - group sessions, and a booklet and newsletters for self-management. One-on-one health counseling was provided every week by health counselors, most of whom were trained dietitians, at the KAHP health facilities where the participants had been screened for MetS. The Intervention Mapping Approach (26) was employed as a framework for the development of the intensive lifestyle intervention. A matrix of change objectives was structured for each MetS-related health behavior and appropriate theoretical methods and strategies were matched. Based on the Transtheoretical Model (27), assessments were conducted on individual participants' MetS status and related health behavior problems including stages of changes for healthy diet, physical activity, and weight control. Then the participants established behavioral objectives and strategies for dietary behaviors, physical activity, smoking, alcohol drinking, and stress management guided by health counselors. They were advised to monitor their own behaviors every day by keeping a health diary and to check with their health counselors to modify the lifestyle change strategies. In addition, the LI group was invited to two health education classes on prevention and management of hypertension, diabetes, and MetS. A handbook and monthly newsletters on healthy lifestyle were sent to LI group to promote self-management. The aforementioned strategies were applied to enhance the participants' self-efficacy and motivation based on the Social Cognitive Theory (28).
Table 2. 12-week intensive lifestyle intervention program contents.
| Week | Session topics | TTM & SCT based methods and strategies |
|---|---|---|
| 1 | - Overview of the program | - Consciousness raising |
| - MetS and its relation to CVDs & diabetes | - Outcome expectation | |
| - Managing MetS through lifestyle change | - Self-liberation | |
| 2-7 | - Weight control | - Knowledge acquisition |
| - Balanced diet and fruits/vegetable intake | - Skill building | |
| - Reduction of total caloric intake and low fat diet | - Decisional balance | |
| - Special diet for risk factor management | - Goal setting | |
| - Physical activity guideline to manage MetS | - Self monitoring | |
| - Physically active lifestyle | - Self efficacy | |
| 8-9 | - Moderate alcohol drinking and smoking cessation | - Counterconditioning |
| - Managing stress and depressive mood | - Stimulus control | |
| 10 | - Regular health check-ups | |
| 11-12 | - Maintaining healthy lifestyle | - Feedback |
| - Self efficacy | ||
| - Reinforcement management |
MetS, metabolic syndrome; CVD, cardiovascular diseases; TTM, transtheoretical model; SCT, social cognitive theory.
The BI group, on the other hand, was informed about their MetS status once diagnosed and provided with a one-page health information sheet about MetS and self-management guidelines. During one-year follow up, both groups were screened for MetS for 4 times: at 2-, 4-, 6-, and 12-month post intervention. More detailed protocol and theoretical framework of the intervention is described elsewhere (25).
Before the onset of study, all intervention implementers attended a training session on study procedure based on a standardized protocol across intervention sites. Plus, 'intervention site' was included in the analysis as a variable for adjustment.
For participants in both groups, five clinical and anthropo-metric outcomes related to MetS were measured at baseline, post intervention (12 weeks from the baseline) and at 2-, 4-, 6-, and 12-month follow-ups post intervention. Trained research assistants performed clinical and anthropometric measurements with a standardized protocol. Blood pressure was measured with an automatic digital sphygmomanometer (TM-2655P, A&D, Tokyo, Japan). Measurements were made after participants being seated quietly for at least 5 min in a chair with feet on the floor. Determination of blood pressure is based upon the mean of two measurements separated by at least 2 min. Waist circumference was measured with a tape spring-tension measure at a level midway between the lowest rib and the iliac crest. Fasting blood samples were drawn and analyzed at KAHP laboratories. Fasting glucose, triglycerides and HDL-cholesterol were measured using HITACHI 7600-110 (HITACHI, Tokyo, Japan). Socio-demographic characteristics of the participants including age, sex, education level, marital status, employment status, and monthly income were surveyed at baseline by trained research assistants.
Statistical analysis
Baseline socio-demographic and MetS characteristics were reported and compared between the LI and BI groups with chi-square tests for discrete variables and t-tests for continuous variables. Binary outcome measures consisted of prevalence of the MetS and abnormality of each of five MetS parameters. Continuous outcome measures consisted of measured values of each MetS component, the number of MetS risks of each participant, that is, the number of MetS components at abnormal levels, and z-MetS score. The z-MetS score was calculated as below where SDSBP, SDDBP, SDWC, SDFG, SDTG, and SDHDL-C are gender-specific standard deviations for systolic blood pressure, diastolic blood pressure, waist circumference, fasting glucose, triglyceride, and HDL-cholesterol, respectively, from all participants at baseline (29). Z-MetS score can detect continuous changes in each MetS component even though the changes does not influence the binary MetS status.
| z-MetS score for male=(Systolic Blood Pressure-130)/SDSBP + (Diastolic Blood Pressure-85)/SDDBP + (Waist Circumference-90)/SDWC + (Fasting Glucose-100)/SDFG + (Triglyceride-150)/SDTG + (40-HDL-Cholesterol)/SDHDL-C |
| z-MetS score for female=(Systolic Blood Pressure-130)/SDSBP + (Diastolic Blood Pressure-85)/SDDBP + (Waist Circumfer-ence-80)/SDWC + (Fasting Glucose-100)/SDFG + (Triglyceride-150)/SDTG + (50-HDL-Cholesterol)/SDHDL-C |
Means and standard deviations (SDs) of the measures at each measured time point were calculated. Changes occurred in outcomes between baseline and post intervention were examined with McNemar's tests for discrete variables and paired t-tests for continuous variables. Effect size statistics with standardized mean differences for the continuous variables and odds-ratios for the binary variables were calculated to characterize the magnitude of the intervention program effect (29).
Post intervention follow-up measurements were taken multiple times up to the 12-month post intervention point to examine between-group and between-time differences in outcomes, respectively. Follow-up results were analyzed with the Generalized Estimating Equations (GEE) models for discrete variables and the mixed effects model for continuous variables. The long-term effects of interventions were analyzed comparing the outcome values of the intervention groups at post intervention (T2) with those at 6-month follow-up (T5) and at 12-month follow-up (T6). Intervention group by measured time point interaction was included in the analysis to determine whether the changes between both intervention groups were different. Missing values were imputed with the multiple imputation procedure, and analysis was performed according to the 'intention-to-treat' approach. All statistical analyses were performed using the SAS 9.3 statistical software package (SAS Institute, Cary, NC, USA).
Ethics statement
The study protocol was approved by the institutional review board of the KAHP (IRB No. 10-B-01). Informed consent was confirmed by the board and obtained from all study participants.
RESULTS
Participant characteristics
Table 3 presents baseline (T1) characteristics of the participants per intervention group. At baseline, participants in this study had 3.3 risks of MetS on average. Baseline prevalence of each of four MetS components except for low HDL-cholesterol (37.4%) was higher than 60%. Both LI and BI groups were similar in all reported characteristics except for the prevalence of low HDL-cholesterol, which was higher in BI group (P=0.026).
Table 3. Baseline (T1) characteristics according to the LI and the BI group.
| Characteristics | No. (%) of participants | P value* | |||
|---|---|---|---|---|---|
| LI group (n = 137) | BI group (n = 106) | All (n = 243) | |||
| Socio-demographic characteristics | |||||
| Age in years | Mean (SD) | 51.4 (5.5) | 51.6 (5.6) | 51.5 (5.5) | 0.730 |
| Sex | Male | 52 (38.0) | 40 (37.7) | 92 (37.9) | 0.972 |
| Female | 85 (62.0) | 66 (62.3) | 151 (62.1) | ||
| Education | Middle school | 35 (25.7) | 34 (32.4) | 69 (28.6) | 0.306 |
| High school | 53 (39.0) | 43 (41.0) | 96 (39.8) | ||
| College | 48 (35.3) | 28 (26.7) | 76 (31.5) | ||
| Marital status | Live with spouse | 116 (85.3) | 87 (82.9) | 203 (84.2) | 0.607 |
| Live without spouse | 20 (14.7) | 18 (17.1) | 38 (15.8) | ||
| Employment | Employed | 81 (60.0) | 55 (54.5) | 136 (57.6) | 0.630 |
| Housewives | 39 (28.9) | 35 (34.7) | 74 (31.4) | ||
| Others | 15 (11.1) | 11 (10.9) | 26 (11.0) | ||
| Monthly income | 1,000 USD > | 12 (9.3) | 14 (13.9) | 26 (11.3) | 0.120 |
| 1,000-3,000 USD | 50 (38.8) | 48 (47.5) | 98 (42.6) | ||
| ≥ 3,000 USD | 67 (51.9) | 39 (38.6) | 106 (46.1) | ||
| Metabolic syndrome characteristics, mean (SD) | |||||
| No. of risks meeting MetS | 3.3 (0.5) | 3.4 (0.6) | 3.3 (0.5) | 0.154 | |
| z-MetS score | 0.6 (2.2) | 1.2 (2.5) | 0.9 (2.3) | 0.087 | |
| Hypertension (No., %) | 101 (73.7) | 74 (69.8) | 175 (72.0) | 0.501 | |
| Systolic BP (mmHg) | 130.1 (12.3) | 130.2 (14.0) | 130.2 (13.0) | 0.933 | |
| Diastolic BP (mmHg) | 81.9 (8.8) | 83.5 (9.5) | 82.6 (9.1) | 0.179 | |
| Abdominal obesity (No., %) | 117 (85.4) | 95 (89.6) | 212 (87.2) | 0.328 | |
| Waist circumference (cm) | 88.8 (7.4) | 89.6 (8.2) | 89.1 (7.7) | 0.425 | |
| Hyperglycemia (No., %) | 91 (66.4) | 71 (67.0) | 162 (66.7) | 0.927 | |
| Fasting glucose (mg/dL) | 112.1 (36.2) | 110.1 (23.7) | 111.2 (31.3) | 0.615 | |
| Hypertriglyceridemia (No., %) | 96 (70.1) | 72 (67.9) | 168 (69.1) | 0.719 | |
| Triglycerides (mg/dL) | 198.0 (92.4) | 202.1 (100.7) | 199.8 (95.9) | 0.740 | |
| Low HDL-cholesterol (No., %) | 43 (31.4) | 48 (45.3) | 91 (37.4) | 0.026 | |
| HDL-cholesterol (mg/dL) | 52.2 (9.3) | 50.3 (9.4) | 51.4 (9.4) | 0.123 | |
*P value, based on a t-test for continuous variables and the chi-square test for categorical variables (Education and marital status include 2 missing values; employment includes 7 missing values; monthly income includes 13 missing values). LI, intensive lifestyle intervention; BI, basic usual intervention; SD, standard deviation; MetS, metabolic syndrome; BP, blood pressure; HDL-cholesterol, high-density lipoprotein cholesterol.
Effects of intervention on MetS
Table 4 shows the prevalence of MetS and measured values of the MetS components at baseline and post intervention. Prevalence of MetS and each of its components in both groups was significantly reduced after the interventions except for the prevalence of low HDL-cholesterol in the LI group. More than 40% of participants (48.2% of LI and 40.6% of BI) no longer had MetS post intervention (P<0.001). The average number of MetS risks per person was significantly decreased from 3.3 to 2.6 in the LI group and from 3.4 to 2.8 in the BI group after the intervention (P<0.001). Z-MetS scores were also reduced significantly in both groups after the intervention: from 0.6 to -0.4 in the LI group (P<0.001) and from 1.2 to 0.3 in the BI group (P=0.003). For the effect size of this study, the odds of being free of abdominal obesity for the LI group were 1.95 times greater than those for the BI group. Based on the standardized mean difference effect size (29), the LI group improved the number of MetS risk components by +0.19 standard deviation relative to the BI group.
Table 4. Changes of metabolic syndrome components at baseline and post intervention.
| Components | LI group (n = 137) | P value* | BI group (n = 106) | P value* | Effect size† (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Baseline (T1) | Post (T2) | Baseline (T1) | Post (T2) | ||||
| Prevalence of the MetS, No. (%) | 137 (100) | 71 (51.8) | < 0.001 | 106 (100.0) | 63 (59.4) | < 0.001 | 1.36 (0.85, 1.87) |
| Hypertension | 101 (73.7) | 70 (51.1) | < 0.001 | 74 (69.8) | 54 (50.9) | 0.001 | 0.99 (0.48, 4.50) |
| Abdominal obesity | 117 (85.4) | 98 (71.5) | < 0.001 | 95 (89.6) | 88 (83.0) | 0.035 | 1.95 (1.32, 2.58) |
| Hyperglycemia | 91 (66.4) | 72 (52.6) | < 0.001 | 71 (67.0) | 57 (53.8) | 0.003 | 1.05 (0.54, 1.56) |
| Hypertriglyceridemia | 96 (70.1) | 70 (51.1) | < 0.001 | 72 (67.9) | 58 (54.7) | 0.003 | 1.14 (0.63, 1.65) |
| Low HDL-cholesterol | 43 (31.4) | 44 (32.1) | 0.853 | 48 (45.3) | 36 (34.0) | 0.014 | 1.09 (0.55, 1.63) |
| MetS components, mean (SD) | |||||||
| Systolic BP (mmHg) | 130.1 (12.3) | 124.9 (12.5) | < 0.001 | 130.2 (14.0) | 127.0 (13.2) | 0.016 | 0.16 (-2.35, 2.26) |
| Diastolic BP (mmHg) | 81.9 (8.8) | 79.6 (9.1) | 0.002 | 83.5 (9.5) | 81.0 (11.3) | 0.016 | 0.14 (-2.01, 1.66) |
| Waist circumference (cm) | 88.8 (7.4) | 88.3 (8.0) | 0.139 | 89.6 (8.2) | 89.6 (8.2) | 0.826 | 0.16 (-1.40, 1.50) |
| Fasting glucose (mg/dL) | 112.1 (36.2) | 109.2 (35.8) | 0.092 | 110.1 (23.7) | 106.6 (20.5) | 0.033 | -0.09 (-3.99, 5.91) |
| Triglycerides (mg/dL) | 198.0 (92.4) | 172.6 (90.5) | 0.003 | 202.1 (100.7) | 189.1 (111.2) | 0.184 | 0.17 (-21.00, 15.32) |
| HDL-cholesterol (mg/dL) | 52.2 (9.3) | 51.4 (9.8) | 0.283 | 50.3 (9.4) | 50.7 (9.0) | 0.627 | -0.07 (-1.79, 1.57) |
| No. of risks meeting MetS | 3.3 (0.5) | 2.6 (1.1) | <0.001 | 3.4 (0.6) | 2.8 (1.0) | <0.001 | 0.19 (0.00, 0.37) |
| z-MetS score | 0.6 (2.2) | -0.4 (3.0) | <0.001 | 1.2 (2.5) | 0.3 (3.2) | 0.003 | 0.23 (-0.38, 0.73) |
*P value, based on a paired t-test for continuous variables and the McNemar test for categorical variables; †Cohen's d (standardized mean difference) for continuous variables and odds ratios for categorical variables. LI, intensive lifestyle intervention; BI, basic usual intervention; CI, confidence interval; SD, standard deviation; MetS, metabolic syndrome; BP, blood pressure; HDL-cholesterol, high-density lipoprotein cholesterol.
While the prevalence of MetS was reduced significantly, some of the average measured values of MetS components were improved. Systolic and diastolic blood pressure levels were reduced significantly in both LI and BI groups. Triglycerides level was significantly decreased only in the LI group (P=0.003), whereas fasting glucose level was significantly decreased only in the BI group (P=0.033). Average waist circumference and HDL-cholesterol level did not change in either group. In addition, there were no significant differences in all of the measured values by sex except for average waist circumference. The average waist circumference of men in the LI group became significantly smaller (P=0.034) from 93.6 cm at baseline to 92.6 cm at post intervention, while such a change was not observed in women (data not shown).
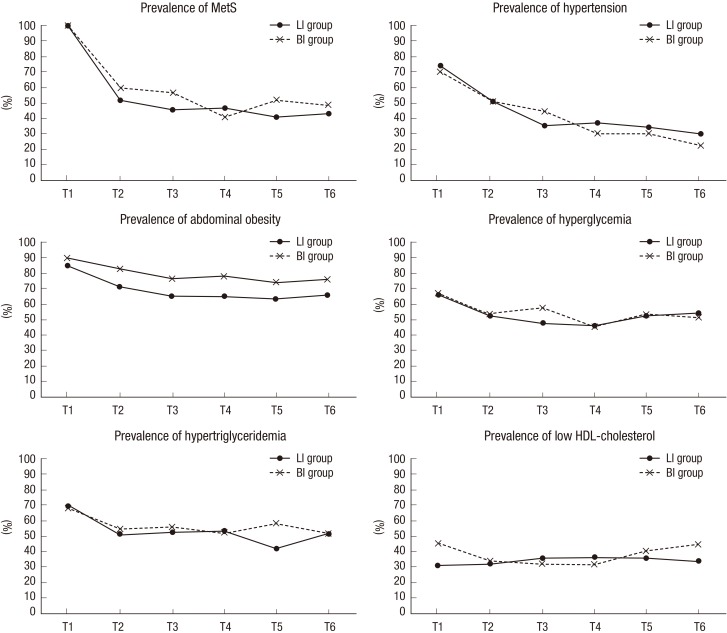
Fig. 2 shows the changes in prevalence of MetS and each MetS component during the follow-up period after adjusting for age, sex, education level, marital status, intervention site, frequency of visits, and the baseline values. Once the prevalence of MetS was reduced at post intervention (T2), the reduced prevalence of most MetS components was maintained during the 12-month follow-up period. The prevalence of hypertension and abdominal obesity, in particular, continued to decline after the interventions: from 51.1% at T2 to 34.1% at T5 (P=0.003) and to 29.7% at T6 (P=0.001) for hypertension; and from 71.5% to 63.6% at T5 (P=0.026) for abdominal obesity. Furthermore, the LI group participants were less likely to be obese in abdomen than the BI group participants after adjusting for the other variables (P= 0.045 at T5 and P=0.048 at T6). There were no differences between intervention groups in all measures except for abdominal obesity throughout the 12-month follow-up period. No effects were found for intervention group by time point interaction.
Fig. 2. Long-term effects of intervention on metabolic syndrome prevalence followed-up 12 months.
Table 5 presents the long-term effects of interventions on each component of the MetS, the number of MetS risks, and z-MetS score, which were adjusted for age, sex, education level, marital status, intervention site, frequency of visits, and the baseline values. The mean values of both systolic blood pressure (P<0.001 at both T5 and T6) and diastolic blood pressure (P=0.001 at both T5 and T6) continuously decreased during the follow-up period. The decreased mean values of triglycerides in the LI group and fasting glucose in the BI group at T2 were maintained throughout the follow-up period. Waist circumference of the LI group decreased from T2 to T5 (P=0.001), but the change was not sustained until T6. The fact that the number of MetS risks and z-MetS score had consistently improved one year after the intervention may infer a potential of long-term MetS control or even health improvement.
Table 5. Long-term effects of intervention on metabolic syndrome components followed-up after 6 and 12 months.
| Components/risks | Post intervention (T2) | 6 months after the intervention (T5) | 12 months after the intervention (T6) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LI group (n=137) | BI group (n=106) | LI group (n=88) | BI group (n=69) | Time effect | Group effect | Time×group effect | LI group (n=74) | BI group (n=58) | Time effect | Group effect | Time×group effect | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Estimates§ | Mean (SD) | Mean (SD) | Estimates∥ | |||||
| MetS components | ||||||||||||
| Systolic BP (mmHg) | 124.9 (12.5) | 127.0 (13.2) | 122.5 (12.2) | 118.8 (10.6) | -7.7100‡ | -1.6260 | 5.6649† | 118.6 (12.2) | 117.7 (12.0) | -8.6307‡ | -1.9257 | 2.6634 |
| Diastolic BP (mmHg) | 79.6 (9.1) | 81.0 (11.3) | 78.3 (8.2) | 76.7 (8.5) | -3.6190† | -0.5251 | 2.3390 | 77.7 (8.6) | 75.7 (7.8) | -4.2309† | -0.5529 | 2.4910 |
| Waist circumference (cm) | 88.3 (8.0) | 89.6 (8.2) | 86.9 (7.9) | 87.4 (7.7) | -1.4704† | -0.4044 | 0.2701 | 87.1 (7.8) | 88.6 (7.4) | -0.1910 | -0.2532 | -0.7514 |
| Fasting glucose (mg/dL) | 109.2 (35.8) | 106.6 (20.5) | 103.2 (16.6) | 105.4 (19.1) | 0.3068 | 1.7020 | -1.4848 | 105.2 (16.1) | 102.7 (13.9) | -0.783 | 1.4474 | -1.0324 |
| Triglycerides (mg/dL) | 172.6 (90.5) | 189.1 (111.2) | 152.0 (72.3) | 184.6 (116.5) | -3.4847 | -16.3580 | -10.7127 | 169.9 (90.1) | 202.8 (138.3) | 14.2499 | -12.5951 | -12.9605 |
| HDL-cholesterol (mg/dL) | 51.4 (9.8) | 50.7 (9.0) | 49.6 (9.1) | 49.5 (7.4) | 0.1896 | -0.0419 | -0.8878 | 50.1 (8.7) | 49.6 (12.3) | -0.4291 | -0.3818 | -0.0323 |
| No. of risks meeting MetS | 2.6 (1.1) | 2.8 (1.0) | 2.3 (1.1) | 2.6 (1.1) | -0.2415* | -0.0940 | -0.1059 | 2.4 (1.1) | 2.5 (1.0) | -0.3084* | -0.0564 | -0.0026 |
| z-MetS score | -0.4 (3.0) | 0.3 (3.2) | -1.2 (2.7) | -1.0 (2.8) | -1.2696‡ | -0.2606 | 0.5896 | -1.2 (2.7) | -1.0 (2.7) | -0.9612† | -0.2039 | 0.1437 |
*P<0.05; †P<0.01; ‡P<0.001; §Coefficients obtained by comparing differences in the variables between post intervention (T2) and 6 months after the intervention (T5) based on Mixed Effects Models for adjusted for age, sex, education level, income, marriage status, intervention site, frequency of visits and the baseline values; ∥Coefficients obtained by comparing differences in the variables between post intervention (T2) and 12 months after the intervention (T6) based on Mixed Effects Models for adjusted for age, sex, education level, income, marriage status, intervention site, frequency of visits and the baseline values. LI, intensive lifestyle intervention; BI, basic usual intervention; SD, standard deviation; MetS, metabolic syndrome; BP, blood pressure; HDL-cholesterol, high-density lipoprotein cholesterol.
DISCUSSION
We examined the effects of lifestyle interventions on MetS management among middle-aged Koreans. A 12-week theory-based intensive lifestyle intervention program was conducted nationwide, and compared with basic usual one-time intervention for 12 months. The overall prevalence of MetS was significantly reduced after the interventions, along with the improvements in individual MetS components except for the HDL-cholesterol. Reduced prevalence of MetS and improvements in the levels of fasting glucose and triglycerides were maintained throughout the follow-up period, and the prevalence of hypertension and abdominal obesity continued to decrease. Between intervention group differences were, however, not observed.
Several previous intervention studies also reported no significant differences between the intervention group and the control group (30,31). It should be noted though that the BI group in this study was not a 'non-treatment' control group, but a group with basic usual intervention. At baseline, the participants in both groups were informed that they had a condition called 'Metabolic Syndrome (MetS)' and explained what MetS was. Such notification made the participants pay attention to 'MetS', the health condition that was unfamiliar to many of the participants. An in-depth interview with Korean adults with MetS addressed that those diagnosed with the unfamiliar term 'MetS' took their health issues more seriously and had greater motivation to cope with MetS (32). Diagnosis and notification of the exact health status can raise awareness and motivation which facilitate self-management of MetS.
The BI group participants attended regular checkups as part of this study and were informed about their MetS status in the same frequency as the LI group. Participants in both intervention groups in this study might have been strongly motivated with elevated awareness and knowledge about their MetS status that they had not known before. Furthermore, repeated examinations for MetS in this study might have impacted both LI and BI group participants to be more conscious of their health status and management. Research participation or consequent awareness of being observed is likely to influence participants' behavior to demonstrate better performances that can be confounded with the outcomes being studied (33). It might have resulted in improvements of MetS status in all participants in the study and non-significant differences between groups. Further research is called for in order to examine intervention effects on MetS by intervention intensity, particularly from the standpoint of process evaluation.
Nearly half of the participants in this study no longer had MetS after the interventions. As the MetS is defined with the number of risk factors, small changes in the clinical values of MetS parameters near cut-off points could have affected the reduction in prevalence of MetS (34). In this study, 70% of participants had three MetS risks at baseline, thus improvement in one of the 3 risks that was slightly abnormal might have helped them being out of MetS. The GEE models and the mixed effects models in this study included baseline values to analyze adjusted long-term effects of interventions. The baseline values of all components affected the MetS status significantly (P<0.001, data now shown), which means that the participants who were with more severe MetS at baseline were less likely to demonstrate improvements in their MetS status, although overall intervention effects were observed in the study. Nevertheless, a consistent decrease in z-MetS score in this study may infer that improvements may be observed further.
In a recent systematic review and meta-analysis with eleven intervention studies with follow-up periods of 6 months or longer in eight randomized controlled trials from 1966 to 2011 (17), lifestyle modification interventions were concluded to be effective in reducing MetS and improving the levels of MetS components. However, lifestyle intervention effects on low HDL-cholesterol were non-significant. Indeed, lifestyle intervention effects on HDL-cholesterol have been reported as less effective (35,36). Results of this study on HDL-cholesterol corroborate such previous findings. It is possible that most lifestyle intervention programs employ fat reduction strategies in diet, which may affect adversely to raise the HDL-cholesterol level decided by ratio of polyunsaturated to saturated fats (37).
National Health Insurance Service (NHIS) Korea funds biannual health check-ups which includes all clinical tests for MetS for adults. Over 70% of NHIS beneficiaries participate in the national health screening program annually, and the screening rate has grown gradually (11). The examinees who are diagnosed with MetS by the national NHIS screening program are highly recommended to participate in the NHIS MetS management program. The NHIS MetS management program provides health consultation and MetS-related health information materials online. However, all such processes depend on voluntary participation and are limited to online services. Therefore, clients, especially elderly, could be challenged to sustain their efforts for self-management of MetS via the program. As we found in this study, regular check-ups and notification of MetS status motivated participants to manage and promote their health. Therefore, more concrete guidelines for MetS screening and lifestyle modifications and practicing the guidelines are required to achieve effective prevention and sustained management of MetS within the screening system.
There are some limitations in this study. First, although randomly selected, participation in this study was on a voluntary basis. Study participants might have been more health conscious and willing to modify their health behaviors than non-participants. Significant reduction of MetS prevalence observed in the BI group implies that relatively moderate efforts for promoting MetS management can give more motivation for a lifestyle change of the participants than expected. It is required to take more caution to develop universally effective strategies for a lifestyle modification intervention. Second, during the 1-yr follow-up period, 45.7% of participants dropped out of the study, and more than 20% of which occurred at T6. In previous intervention studies in Korea and Japan, about 80% of participants refused or gave up their participation during the follow-up period (20,31). Participants in this study had some difficulties with frequent visits to interventions which required fasting. Approximately 60% of the participants were employed, and half a day off from the work was needed for each visit to the intervention program. Reminder calls and personalized text messages were attempted in this study to keep the participants in the program. There were no significantly different characteristics between retained participants and dropouts in this study. Still, more effective strategies to lowering the dropout rates are needed in the future research. This study also leaves a room for further research to identify effects of behavior changes on MetS management, to determine effects of MetS intervention by its level of intensity, and to implement the interventions in younger people with MetS.
Despite some limitations, this study is still meaningful for evaluating the lifestyle intervention effects on MetS over one year. Lifestyle modification intervention was effective to reduce MetS risks among the participants, and continuous progress was also observed during the follow-up period. Regular check-ups and appropriate notifications of their own MetS status and progress is helpful for MetS risk groups to manage and promote their health. The overall results of such research will be helpful for establishing evidence for more concrete MetS prevention and management strategies in Korea.
ACKNOWLEDGMENTS
The authors thank the Korea Association of Health Promotion for participating in intervention development and implementation, facilitating the data collection process, and granting access to the data.
Footnotes
This study was supported by the National Research Foundation of Korea (Grant No. NRF-2010-0004016).
DISCLOSURE: The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTION: Conception and coordination of the study: Yoo S. Design of ethical issues: Yoo S, Kim H. Acquisition of data: Kim H, Han Y. Data review: Yoon N, Han Y, Kim H. Statistical analysis: Yoon N, Han Y, Yoo S. Manuscript preparation: Yoon N, Yoo S, Han Y. Manuscript approval: all authors
References
- 1.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 2.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999-2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitch K, Pyenson B, Iwasaki K. Metabolic syndrome and employer sponsored medical benefits: an actuarial analysis. Value Health. 2007;10:S21–S28. [Google Scholar]
- 6.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 7.Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013;57:867–871. doi: 10.1016/j.ypmed.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J. The metabolic syndrome: an Asian perspective. Diabetes Voice. 2006;51:18–20. [Google Scholar]
- 9.Patel A, Huang KC, Janus ED, Gill T, Neal B, Suriyawongpaisal P, Wong E, Woodward M, Stolk RP. Is a single definition of the metabolic syndrome appropriate?--A comparative study of the USA and Asia. Atherosclerosis. 2006;184:225–232. doi: 10.1016/j.atherosclerosis.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, Choi SH, Cho SI, Park KS, Lee HK, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Insurance Service. National Health Screening Statistical Yearbook. Seoul: National Health Insurace Service; 2012. [Google Scholar]
- 12.Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oda BT. An alternative perspective to battling the bulge: the social and legal fallout of Japan's anti-obesity legislation. Asian Pac Law Policy J. 2010;12:249–294. [Google Scholar]
- 14.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 15.The Korean Academy of Family Medicine. Clinical practice guidelines for prevention and treatment of metabolic syndrome among Korean adults. Seoul: Korean Medical Guideline Information Center; 2013. [Google Scholar]
- 16.Tajima M, Lee JS, Watanabe E, Park JS, Tsuchiya R, Fukahori A, Mori K, Kawakubo K. Association between changes in 12 lifestyle behaviors and the development of metabolic syndrome during 1 year among workers in the Tokyo metropolitan area. Circ J. 2014;78:1152–1159. doi: 10.1253/circj.cj-13-1082. [DOI] [PubMed] [Google Scholar]
- 17.Atlantis E, Taylor AW, Wittert G, Shi Z. Weight gain and lifestyle risk factors for developing metabolic syndrome. Circ J. 2014;78:1066–1068. doi: 10.1253/circj.cj-14-0324. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med. 2012;10:138. doi: 10.1186/1741-7015-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Yoon S, Lee K, Oh S, Ryu H, Choo J, Lee K, Ryu B, Lee D, Park D. Effects of a self-management program for metabolic syndrome-a metabolic syndrome management program in Seoul. Korean J Health Educ Promot. 2011;28:51–62. [Google Scholar]
- 20.Seo JR, Bae SS. The effect of metabolic syndrome management program in a public health center. J Agric Med Community Health. 2011;36:264–279. [Google Scholar]
- 21.Dalle Grave R, Calugi S, Centis E, Marzocchi R, El Ghoch M, Marchesini G. Lifestyle modification in the management of the metabolic syndrome: achievements and challenges. Diabetes Metab Syndr Obes. 2010;3:373–385. doi: 10.2147/DMSOTT.S13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Belmont, CA: Wadsworth Cengage Learning; 2001. pp. 261–262. [Google Scholar]
- 23.National Institutes of Health. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in Adults (Adult Treatment Panel III) Final Report. Washington, DC: Department of Health and Human Services; 2002. NHI Publication No. 02-5215. [PubMed] [Google Scholar]
- 24.Yoo S, Kim H, Cho HI. Improvements in the metabolic syndrome and stages of change for lifestyle behaviors in korean older adults. Osong Public Health Res Perspect. 2012;3:85–93. doi: 10.1016/j.phrp.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korea Association of Health Promotion, Health Promotion Research Institute. Lifestyle intervention study protocol. Seoul: Korea Association of Health Promotion; 2013. [Google Scholar]
- 26.holomew LK, Parcel GS, Kok G, Gottlieb NH, Fernández ME. Planning health promotion programs: an intervention mapping approach. 3rd ed. San Francisco, CA: Jossey-Bass; 2011. [Google Scholar]
- 27.Prochaska JO. Systems of psychotherapy : a transtheoretical analysis. Homewood, Ill: Dorsey Press; 1979. [Google Scholar]
- 28.Bandura A. Self-efficacy : the exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 29.Rossi PH, Lipsey MW, Freeman HE. Evaluation : a systematic approach. Thousand Oaks, CA: Sage; 2004. pp. 302–306. [Google Scholar]
- 30.Wang X, Hsu FC, Isom S, Walkup MP, Kritchevsky SB, Goodpaster BH, Church TS, Pahor M, Stafford RS, Nicklas BJ. Effects of a 12-month physical activity intervention on prevalence of metabolic syndrome in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67:417–424. doi: 10.1093/gerona/glr187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanri A, Tomita K, Matsushita Y, Ichikawa F, Yamamoto M, Nagafuchi Y, Kakumoto Y, Mizoue T. Effect of six months lifestyle intervention in Japanese men with metabolic syndrome: randomized controlled trial. J Occup Health. 2012;54:215–222. doi: 10.1539/joh.11-0238-oa. [DOI] [PubMed] [Google Scholar]
- 32.Sim SR. Experiences of diagnosis and lifestyle management in individuals with metabolic syndrome. Seoul: Seoul National University; 2013. Dissertation. [Google Scholar]
- 33.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bo S, Ciccone G, Baldi C, Benini L, Dusio F, Forastiere G, Lucia C, Nuti C, Durazzo M, Cassader M, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med. 2007;22:1695–1703. doi: 10.1007/s11606-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 36.Oh EG, Kim SH, Hyun SS, Kang MS, Bang SY. The analysis of intervention studies for patients with metabolic syndrome. J Korean Acad Nurs. 2007;37:72–80. doi: 10.4040/jkan.2007.37.1.72. [DOI] [PubMed] [Google Scholar]
- 37.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S Diabetes Prevention Program Research Group. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]