Abstract
The aim of this study was to evaluate female urethral cancer (UCa) patients treated and followed-up during a time period spanning more than 20 yr at single institution in Korea. We reviewed medical records of 21 consecutive patients diagnosed with female UCa at our institution between 1991 and 2012. After exclusion of two patients due to undefined histology, we examined clinicopathological variables, as well as survival outcomes of 19 patients with female UCa. A Cox proportional hazards ratio model was used to identify significant predictors of prognosis according to variables. The median age at diagnosis was 59 yr, and the median follow-up duration was 87.0 months. The most common initial symptoms were voiding symptoms and blood spotting. The median tumor size was 3.4 cm, and 55% of patients had lesions involving the entire urethra. The most common histologic type was adenocarcinoma, and the second most common type was urothelial carcinoma. Fourteen patients underwent surgery, and 7 of these patients received adjuvant radiation or systemic chemotherapy. Eleven patients experienced tumor recurrence after primary therapy. Patients with high stage disease, advanced T stage (≥T3), and positive lymph nodes had worse survival outcomes compared to their counterparts. Particularly, lymph node positivity and advanced T stage were significant predictive factors for all survival outcomes. Tumor location was the only significant predictor for recurrence-free survival. Although our study included a small number of patients, it conveys valuable information about this rare female urologic malignancy in a Korean population.
Graphical Abstract
Keywords: Female, Urethral Neoplasms, Survival, Outcome Assessment
INTRODUCTION
Female urethral cancer (UCa) is a rare malignancy; it accounts for approximately 1% of all female urological cancer (1). Initial signs of UCa, such as voiding symptoms and bloody spotting, are ignored in most patients with female UCa because they are not pathognomonic. In fact, voiding symptoms and bloody spotting are common indicators of non-malignant conditions in normal healthy females. Despite improvements in diagnostic imaging, delayed diagnosis remains a challenge in these patients (2). Because initial diagnosis is delayed, female UCa patients often present with large and advanced lesions at initial diagnosis, which results in poor prognosis (3). More importantly, because female UCa is rare, the key prognostic factors remain poorly defined, and optimal therapeutic strategies are primarily based on limited evidence (4).
Given these challenges, all available evidence should be shared and discussed so that the current knowledge base about female UCa is improved. Additionally, previous studies were largely based on Western populations, so more evidence is required to improve our understanding of female UCa in Asian countries. In this study, we evaluated 19 cases of female UCa in Korea and reported useful information about clinical features, treatment, and prognostic factors.
MATERIALS AND METHODS
We reviewed medical records of 21 consecutive patients diagnosed with female UCa at our institution between August 1991 and April 2012. After two cases were excluded due to undefined histologic features, we finally examined 19 patients of female UCa. We evaluated the following variables associated with patient characteristics: age, initial presenting symptoms, procedure type, whether or not the patient received adjuvant therapy, histologic type, tumor size and location, recurrence and sites, whether or not the patient received salvage therapy, and recurrence-free (RFS), cancer-specific (CSS) and overall survival (OS). Tumor location was classified as proximal, distal, and whole urethral involvement, as in a previous report (5). Proximal tumors were defined as tumors limited to the proximal two-thirds of the urethra, while tumors were classified as "distal" if they were limited to the distal one-third of the urethra. The surgical modalities at presentation included mass excision, urethrectomy, and pelvic exenteration. Pathological stages were determined according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system (6). Stage Ta, T1, and T2 tumors with N0 and M0 were defined as low stage tumors, while stage T3 and T4 tumors with N1-2 and M1 were classified as high stage tumors (6). The pathological T stage was primarily used to determine T stage. However, in cases where there was no documentation of pathological T stage, clinical T stage was used. Local recurrence was defined as cancer reappearance at the operative sites or in the pelvic cavity, and distant recurrence was tumor relapse at other sites besides the pelvic cavity.
The Kaplan-Meier method was used to calculate survival outcomes (RFS, CSS, and OS) after initial treatment, and log-rank tests were used to determine statistically significant differences between survival curves. We used a Cox proportional hazards model to identify significant predictors of survival outcomes according to clinicopathological variables. We rejected the 2-sided null hypothesis that there was no significant difference in cases where the P value was less than 0.05. Consistently, we rejected the null hypothesis if the 95% confidence interval (CI) of point estimates excluded 1. All statistical analyses were performed using SPSS software, version 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
Ethics statements
The institutional review board (IRB) of Seoul National University Hospital approved this study (approval number: H-1406-072-588). Because this study was conducted retrospectively, the IRB waived the requirement for documentation of written informed consent from patients.
RESULTS
Patient characteristics and overall prognoses are summarized in Table 1. Seven patients were diagnosed by physical examination only, not by imaging studies. The median patient age at the time of diagnosis was 59.0 yr, and the median follow-up duration was 87.0 months. Initial presenting symptoms were varied. Voiding symptoms and blood spotting were the most common and only 1 patients had no symptoms. The median tumor size was 3.4 cm. With regard to tumor staging, 9 patients (47.4%) had low stage tumors and 10 patients (52.6%) had high stage tumors. Additionally, the pathological findings were histologically diverse. Adenocarcinoma was the most common type and urothelial carcinoma was the second most common.
Table 1. Patient characteristics.
| Characteristics | Total patients (n = 19) |
|---|---|
| Median age (yr) (IQR) | 59.0 (52.0-72.0) |
| Body mass index (kg/m2) (IQR) | 21.90 (20.80-25.79) |
| Initial symptoms, No. (%) | |
| Asymptomatic | 1 (5.2) |
| Voiding symptoms | 6 (31.6) |
| Blood spotting | 6 (31.6) |
| Gross hematuria | 2 (10.5) |
| Others | 4 (21.1) |
| Median tumor size (cm) (IQR) | 3.4 (1.3-4.5) |
| Size classification, No. (%) | |
| <2 cm | 5 (27.7) |
| 2-4 cm | 7 (36.8) |
| > 4 cm | 6 (31.6) |
| Tumor location, No. (%) | |
| Proximal urethra | 2 (11.1) |
| Distal urethra | 6 (33.3) |
| Entire urethra | 10 (55.6) |
| Surgery type, No. (%) | |
| Mass excision | 6 (42.8) |
| Urethrectomy | 1 (7.1) |
| Pelvic exenteration | 7 (50.1) |
| Tumor stage, No. (%) | |
| Low stage (stage I-II) | 9 (47.4) |
| High stage (stage III-IV) | 10 (52.6) |
| Histologic type, No. (%) | |
| Adenocarcinoma | 9 (47.3) |
| Urothelial carcinoma | 6 (31.5) |
| Squamous cell carcinoma | 3 (15.8) |
| Mixed type* | 1 (5.2)* |
| Adjuvant therapy, No. (%) | |
| None | 9 (47.4) |
| Radiation therapy | 5 (26.3) |
| Chemotherapy | 3 (15.8) |
| Combined | 2 (10.5) |
| Recurrence, No. (%) | 11 (57.9) |
| Salvage therapy, No. (%) | 8 (42.1) |
| Death, No. (%) | |
| All-cause | 10 (52.6) |
| Disease-specific | 9 (47.4) |
| Median F/U duration, months (95% CI) | 87.0 (61.74-202.77) |
*One case of mixed tumor was combined with granulosa cell tumor and urothelial carcinoma. IQR, interquartile range; CI, Confidence interval; F/U, follow-up.
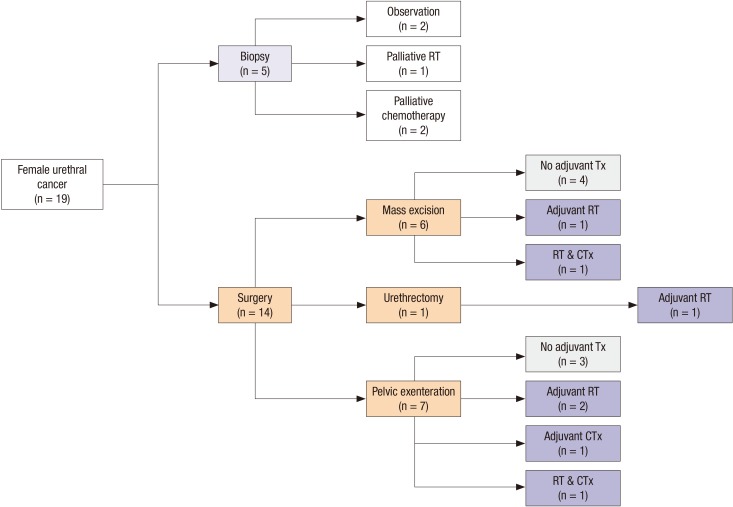
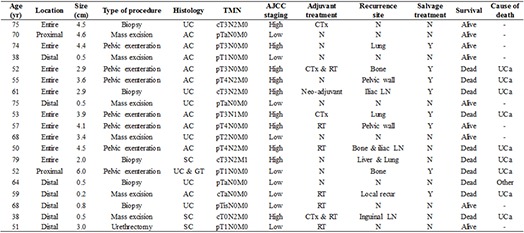
Fig. 1 is a flow chart of the types of primary and adjuvant therapy used to treat patients. While 5 patients underwent biopsies without other surgical procedures, 14 patients underwent surgery including pelvic exenteration, urethrectomy, and local mass excision. For the 7 patients who received adjuvant therapy, such as radiation and systemic chemotherapy, there was no dominantly employed modality. Overall, 57.9% of these patients (n=11) experienced tumor recurrence after primary therapy. The rates of lymph node and bone metastases were the same (27.7%), and these were the most common sites of recurrence. Summarized information for all 19 patients evaluated in this study is also provided in Table 2.
Fig. 1. Flow-chart of the treatment courses of the 19 patients with female urethral cancer in this study. The number of patients who were treated with specific modalities is described for each respective step. RT, radiation therapy; Tx, therapy; CTx, chemotherapy.
Table 2. Cancer associated information.
| Age (yr) | Location | Size (cm) | Type of procedure | Histology | TMN | AJCC staging | Adjuvant treatment | Recurrence site | Salvage treatment | Survival | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 75 | Entire | 4.5 | Biopsy | UC | cT3N2M0 | High | CTx | N | N | Alive | - |
| 70 | Proximal | 4.6 | Mass excision | AC | pTaN0M0 | Low | N | N | N | Alive | - |
| 74 | Entire | 4.4 | Pelvic exenteration | AC | pT3N0M0 | High | N | Lung | Y | Alive | - |
| 38 | Distal | 0.5 | Mass excision | AC | pT1N0M0 | Low | N | N | N | Alive | - |
| 52 | Entire | 2.9 | Pelvic exenteration | AC | pT3N0M0 | High | CTx & RT | Bone | Y | Dead | UCa |
| 55 | Entire | 3.6 | Pelvic exenteration | AC | pT4N2M0 | High | N | Pelvic wall | Y | Dead | UCa |
| 61 | Entire | 2.9 | Biopsy | UC | cT3N2M0 | High | Neo-adjuvant | Iliac LN | Y | Dead | UCa |
| 75 | Distal | 0.5 | Mass excision | UC | pTaN0M0 | Low | N | N | N | Alive | - |
| 53 | Entire | 3.9 | Pelvic exenteration | AC | pT3N1M0 | High | CTx | Lung | Y | Dead | UCa |
| 57 | Entire | 4.1 | Pelvic exenteration | AC | pT4N0M0 | High | RT | Pelvic wall | Y | Alive | - |
| 68 | Entire | 3.4 | Mass excision | UC | pT2N0M0 | Low | N | N | N | Alive | - |
| 50 | Entire | 4.5 | Pelvic exenteration | AC | pT4N2M0 | High | RT | Bone & iliac LN | N | Dead | UCa |
| 79 | Entire | 2.0 | Biopsy | SC | cT3N2M1 | High | N | Liver & Lung | N | Dead | UCa |
| 52 | Proximal | 6.0 | Pelvic exenteration | UC & GT | pT1N0M0 | Low | N | Bone | Y | Dead | UCa |
| 64 | Distal | 0.5 | Biopsy | UC | pTaN0M0 | Low | N | N | N | Dead | Other |
| 59 | Distal | 0.2 | Mass excision | AC | cTaN0M0 | Low | RT | Local recur | Y | Dead | UCa |
| 68 | Distal | 0.8 | Biopsy | UC | pTisN0M0 | Low | RT | N | N | Alive | - |
| 38 | Distal | 0.5 | Mass excision | SC | cT0N2M0 | High | CTx & RT | Inguinal LN | N | Dead | UCa |
| 51 | Distal | 3.0 | Urethrectomy | SC | pT1N0M0 | Low | RT | N | N | Alive | - |
UC, urothelial cancer; AC, adenocarcinoma; SC, squamous cell carcinoma; GT, granulosa cell tumor; CTx, chemotherapy; RT, radiotherapy; N, negative; LN, lymph node; UCa, urethral cancer; Y, yes; N, No.
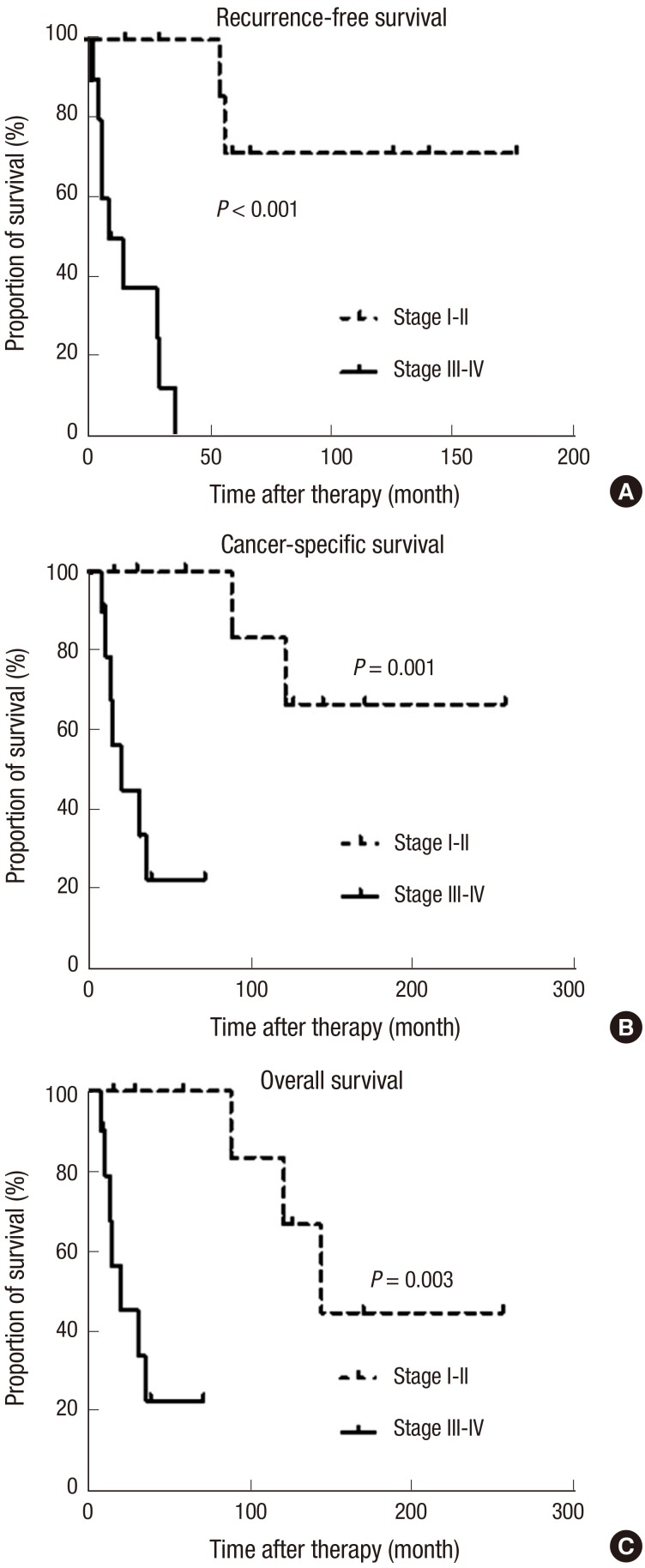
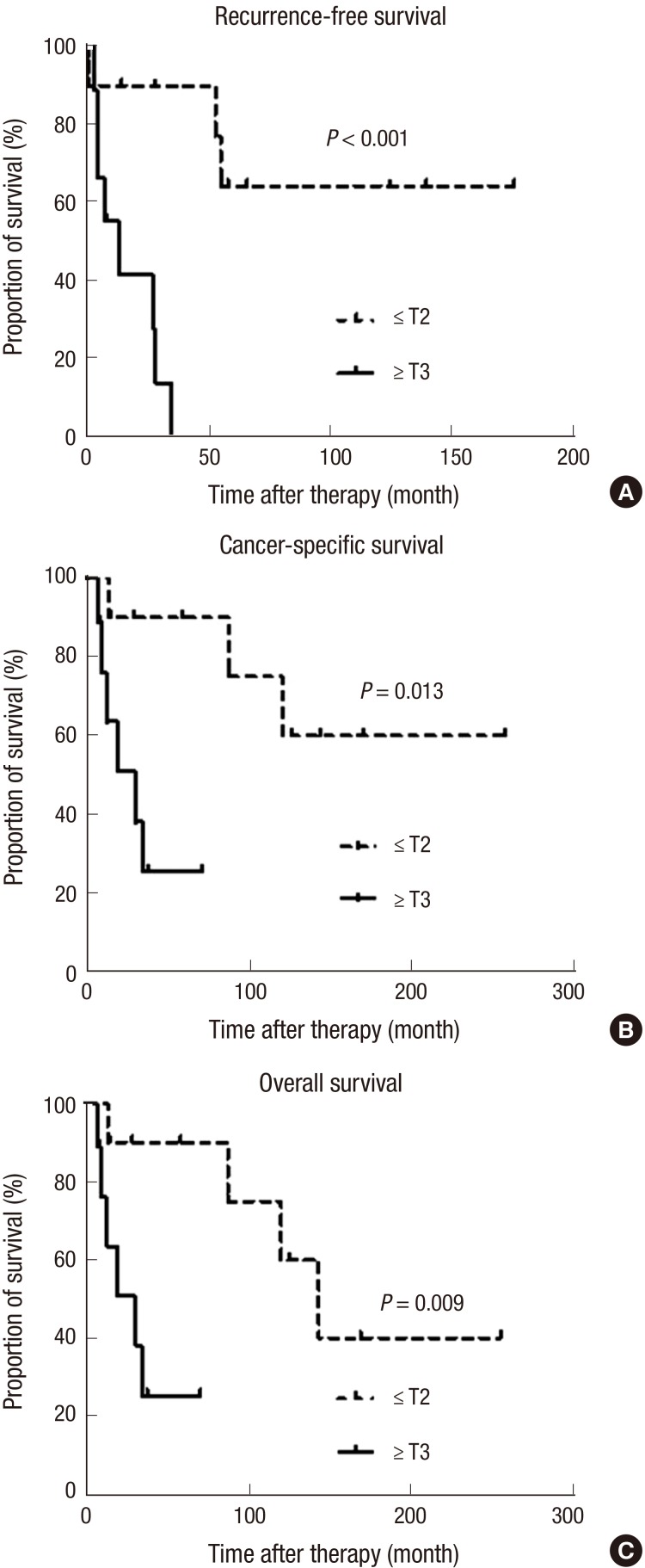
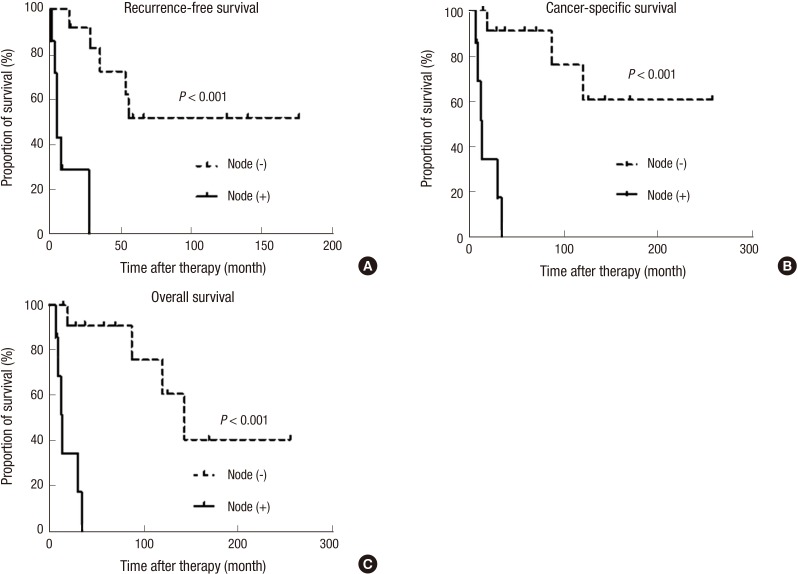
Fig. S1 shows the oncological outcomes (RFS, CSS, and OS) of the patients in this study. The 5-yr RFS, CSS, and OS rates were 34.8% (standard error [SE] 22.7%-46.9%), 58.9% (SE 46.8%-71.0%), and 58.9% (SE 46.8%-71.0%). All oncological outcomes were significantly different between patients with low stage (stage I-II) and high stage cancer (stage III-IV) (Fig. 2). When patients were classified by T stage, patients with advanced T stages (≥T3) had poor survival outcomes for RFS, CSS and OS compared to patients with localized tumors (≤T2) (Fig. 3). Patients with positive lymph nodes also had worse survival outcomes by all measurements compared to node-negative patients (Fig. 4). However, only RFS, but not CSS and OS, was worse in patients with tumors involving the entire urethra compared to patients with proximal or distal tumors (Fig. S2).
Fig. 2. Comparison of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients according to the American Joint Committee on Cancer staging guidelines analyzed by the Kaplan-Meier method and log-rank tests.
Fig. 3. Comparison of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients according to T stage analyzed by the Kaplan-Meier method and log-rank tests.
Fig. 4. Comparison of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival in female urethral cancer patients according to lymph node status analyzed by the Kaplan-Meier method and log-rank tests.
To identify predictive factors for oncological outcomes, we used a Cox proportional hazards ratio regression model (Table 3). In this analysis, positive lymph node status and advanced T stage (≥T3) were the significant factors for prediction of all oncological outcomes. Additionally, tumor location (entire urethral involvement) was the significant predictor only for RFS, and not for CSS or OS.
Table 3. Cox proportional hazard ratio analysis to identify predictors of oncological outcomes in the patients with female urethral cancer.
| Variables | RFS | CSS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Tumor location | |||||||||
| Non-entire urethra | Reference | Reference | Reference | ||||||
| Entire urethra | 4.63 | 1.13-18.90 | 0.033 | - | - | - | - | - | - |
| T stage | |||||||||
| ( ≤ ) T2 | Reference | Reference | Reference | ||||||
| ( ≥ ) T3 | 18.16 | 2.15-153.55 | 0.008 | 10.12 | 1.21-84.60 | 0.033 | 11.31 | 1.35-94.58 | 0.025 |
| Lymph node metastases | |||||||||
| Negative | Reference | Reference | Reference | ||||||
| Positive | 26.96 | 2.98-243.38 | 0.003 | 24.83 | 2.89-213.46 | 0.003 | 27.20 | 3.16-234.15 | 0.003 |
RFS, recurrence-free survival; CSS, cancer-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer.
DISCUSSION
In the present study, we examined the clinical experiences of 19 patients with female UCa at a single tertiary institution. Despite the small sample size and retrospective nature of this study, it is important to report all available evidence so that we can improve our understanding of this rare malignancy. Notably, we found that cancer stage based on the AJCC classification, T stage, and lymph node status were associated with remarkable differences in RFS, CSS, and OS between patient groups in Kaplan-Meier analysis. Patients with high stage tumors (stage III-IV), advanced T stage (≥T3), and positive lymph nodes had poor outcomes as indicated by all analyzed survival outcomes (RFS, CSS, and OS) compared to their counterparts. Importantly, lymph node positivity and advanced T stage were significantly predictive for poor outcomes in all survival estimates in Cox regression analysis. Additionally, involvement of the entire urethra remained as a predictor of RFS, but not CSS or OS.
Other studies support our results regarding the key prognostic factors for female UCa. Champ et al. (7) analyzed the female UCa patients in the Surveillance, Epidemiology, and End Results (SEER) database and reported that older age, pathological T stage, lymph node positivity, and non-squamous histology were significantly related to poor CSS. Dimarco et al. (5) suggested that pathological T stage was a strong predictive factor for RFS and CSS. Pathological findings about lymph node status were also shown to predict survival in their study. A single center study by Thyavihally et al. (8) reported that patients with early stage cancer (stage I-II) according to the AJCC staging guidelines for female UCa had better survival outcomes compared to patients with advanced stage tumors (stage III-IV), regardless of the treatment modality.
Although other variables such as tumor size, histologic type, and treatment modality were not associated with any oncological outcomes in the present study, these factors have been reported as significant prognostic factors for female UCa by other studies. Bracken et al. (3) evaluated 81 cases of female UCa and found that patients with large tumors (>4 cm) had a lower 5-yr survival rate (about 13%) compared to patients with small tumors (<2 cm), (about 60%). Proximal tumors and lesions that involve the whole urethra have commonly been shown to be associated with poor oncological outcomes compared to distal lesions (9). Benson et al. (10) reported that all patients with proximal urethral tumors died, while 50% of patients with distal urethral tumors survived. The histologic type of female UCa has also been suggested as a key determinant of prognosis (2,7,11). The predominant tumor types are squamous cell carcinoma and urothelial carcinoma, and localized tumors of these histologic types are associated with better survival outcomes compared to tumors of less common histologic types, such as adenocarcinoma (2,12,13). In a study by Meis et al. (14), most patients with adenocarcinoma had locally advanced disease involving adjacent tissues or lymph node metastasis, and approximately 60% of these patients experienced cancer-related death within 2 yr of diagnosis. In contrast to these findings, Dalbagni et al. (1) examined 72 females with primary UCa and reported that primary stage, lymph node status, and tumor site were key independent predictors of survival, but histologic type was not. Dimarco et al. (5) also found that tumor histology was not a significant predictor of recurrence or survival for female UCa patients. They noted that pathological stage was the most important factor for prediction of prognosis. In our analysis, nearly half of the patients (n=9; 42.9%) were diagnosed with adenocarcinoma at the time of presentation; these patients tended to have poor oncological outcomes, though the results were not statistically significant (data not shown).
As discussed in previous studies, therapeutic policies are typically determined by tumor size, location, and aggressiveness (4). For example, surgical resection or radiation can be applied to small (<2 cm) distal lesions, while a combination of radical surgery and radiation therapy is recommended for more advanced stage disease (15,16). Our results consistently demonstrated that patients who underwent aggressive surgery and received adjuvant radiation had mainly larger tumors (≥3-4 cm) and tumors that involved the entire urethra. Although multimodal treatment with surgery, radiation, and systemic chemotherapy could be beneficial for selected patients with advanced stage cancer, the role of adjuvant chemotherapy has not yet been definitively shown to improve oncological outcomes (2,5). In the present study, 2 patients with advanced stage lesions received chemoradiotherapy. Despite the use of combination therapy, these patients experienced tumor recurrence to the bone and inguinal lymph node, respectively, and eventually died as a result of their UCa.
We should consider the limitations of our study. First, the data were collected and reviewed retrospectively. Second, 5 urologic surgeons managed female UCa patients at our institution during the long study period. Therefore, there was some difference in the treatment and follow-up protocols for the patients with this rare malignancy. Finally, although this is the largest report of female UCa in Korea, the statistical power of this study was limited by insufficient number of cases.
In conclusion, high stage, advanced T stage, and lymph node positivity are associated with poor prognosis for all analyzed survival outcomes (RFS, CSS, and OS) in female UCa patients after primary treatment. Furthermore, lymph node status and T stage are significantly predictive of all survival outcomes. Therefore, early diagnosis and management with early identification of this rare malignancy can improve the overall prognosis of UCa patients. Despite the limited case number, our results provide valuable information that expands the current knowledge about clinicopathological characteristics and factors that predict long-term prognosis of UCa patients.
Footnotes
DISCLOSURE: The authors have no conflicts of interest.
AUTHOR CONTRIBUTION: Study concept and design: Kang M, Ku JH. Data acquisition: Kang M. Data review and analysis: Kang M, Jeong CW, Ku JH. Statistical analysis: Kang M, Jeong CW, Ku JH. Manuscript preparation: Kang M, Ku JH. Manuscript revision: Kwak C, Kim HH. Manuscript approval: all authors.
Supplementary Material
Kaplan-Meier analysis of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients.
Comparison of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients according to tumor location analyzed by the Kaplan-Meier method and log-rank tests.
References
- 1.Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Female urethral carcinoma: an analysis of treatment outcome and a plea for a standardized management strategy. Br J Urol. 1998;82:835–841. doi: 10.1046/j.1464-410x.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 2.Derksen JW, Visser O, de la Rivière GB, Meuleman EJ, Heldeweg EA, Lagerveld BW. Primary urethral carcinoma in females: an epidemiologic study on demographical factors, histological types, tumour stage and survival. World J Urol. 2013;31:147–153. doi: 10.1007/s00345-012-0882-5. [DOI] [PubMed] [Google Scholar]
- 3.Bracken RB, Johnson DE, Miller LS, Ayala AG, Gomez JJ, Rutledge F. Primary carcinoma of the female urethra. J Urol. 1976;116:188–192. doi: 10.1016/s0022-5347(17)58741-8. [DOI] [PubMed] [Google Scholar]
- 4.Gakis G, Witjes JA, Compérat E, Cowan NC, De Santis M, Lebret T, Ribal MJ, Sherif AM European Association of Urology. EAU guidelines on primary urethral carcinoma. Eur Urol. 2013;64:823–830. doi: 10.1016/j.eururo.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Dimarco DS, Dimarco CS, Zincke H, Webb MJ, Bass SE, Slezak JM, Lightner DJ. Surgical treatment for local control of female urethral carcinoma. Urol Oncol. 2004;22:404–409. doi: 10.1016/S1078-1439(03)00174-1. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 7.Champ CE, Hegarty SE, Shen X, Mishra MV, Dicker AP, Trabulsi EJ, Lallas CD, Gomella LG, Hyslop T, Showalter TN. Prognostic factors and outcomes after definitive treatment of female urethral cancer: a population-based analysis. Urology. 2012;80:374–381. doi: 10.1016/j.urology.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 8.Thyavihally YB, Wuntkal R, Bakshi G, Uppin S, Tongaonkar HB. Primary carcinoma of the female urethra: single center experience of 18 cases. Jpn J Clin Oncol. 2005;35:84–87. doi: 10.1093/jjco/hyi024. [DOI] [PubMed] [Google Scholar]
- 9.Garden AS, Zagars GK, Delclos L. Primary carcinoma of the female urethra. Results of radiation therapy. Cancer. 1993;71:3102–3108. doi: 10.1002/1097-0142(19930515)71:10<3102::aid-cncr2820711034>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Benson RC, Jr, Tunca JC, Buchler DA, Uehling DT. Primary carcinoma of the female urethra. Gynecol Oncol. 1982;14:313–318. doi: 10.1016/0090-8258(82)90105-6. [DOI] [PubMed] [Google Scholar]
- 11.Visser O, Adolfsson J, Rossi S, Verne J, Gatta G, Maffezzini M, Franks KN RARECARE working group. Incidence and survival of rare urogenital cancers in Europe. Eur J Cancer. 2012;48:456–464. doi: 10.1016/j.ejca.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Kobayashi S, Islam AM, Nishizawa O. Female para-urethral adenocarcinoma: histological and immunohistochemical study. Int J Urol. 2005;12:117–119. doi: 10.1111/j.1442-2042.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 13.Ouzaid I, Hermieu JF, Dominique S, Fernandez P, Choudat L, Ravery V. Management of adenocarcinoma of the female urethra: case report and brief review. Can J Urol. 2010;17:5404–5407. [PubMed] [Google Scholar]
- 14.Meis JM, Ayala AG, Johnson DE. Adenocarcinoma of the urethra in women. A clinicopathologic study. Cancer. 1987;60:1038–1052. doi: 10.1002/1097-0142(19870901)60:5<1038::aid-cncr2820600519>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Grigsby PW, Corn BW. Localized urethral tumors in women: indications for conservative versus exenterative therapies. J Urol. 1992;147:1516–1520. doi: 10.1016/s0022-5347(17)37614-0. [DOI] [PubMed] [Google Scholar]
- 16.Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP., Jr Management of primary urethral cancer. Urology. 1998;52:487–493. doi: 10.1016/s0090-4295(98)00199-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier analysis of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients.
Comparison of survival outcomes. (A) Recurrence-free survival, (B) cancer-specific survival, and (C) overall survival of female urethral cancer patients according to tumor location analyzed by the Kaplan-Meier method and log-rank tests.