Abstract
Intraventricular schwannomas are rare primary brain tumors, with fewer than 25 cases reported in the literature. Here, we present the case of a 20-year-old male patient with a 2 year history of blurry vision and dysesthesia involving his right occiput and upper neck. Imaging demonstrated a homogeneously enhancing mass located within the atrium of the right lateral ventricle with associated right lateral ventricular entrapment. Pathology confirmed the tumor to be an intraventricular schwannoma. Imaging findings, presentation, complications, and treatment options for intraventricular schwannomas are described.
Keywords: ventricles, brain, schwannoma, hydrocephalus
Case Presentation
A 20-year-old male patient without significant medical history presented to an urgent care facility for the evaluation of intermittent blurry vision which had been present for approximately 2 years. The patient additionally complained of dysesthesia: a burning sensation, located at the upper neck and right occiput, intermittent in occurrence and lasting only a few seconds, but increasing in intensity and severity over the previous 2 months.
Physical examination found the patient to be awake, alert, and oriented. Pupils were equal, round, and reactive to light and accommodation. A fundoscopic examination was not performed. Cranial nerves II–XII were intact; specifically, extraocular motion was intact. Reflexes and range of motion were normal, and the strength of the bilateral upper and lower extremities was 5/5. Sensation, proprioception, muscle tone, and gait were intact.
Initial radiologic evaluation included an unenhanced computed tomography (CT) scan of the head which showed a right temporal horn intraventricular mass, with surrounding white matter edema and dilation of the right lateral ventricle. Subsequent evaluation with an enhanced magnetic resonance imaging (MRI) of the head included T2, T2*, fluid-attenuated inversion recovery, diffusion-weighted, and T1-unenhanced images in the axial plane. Unenhanced T1-weighted images were acquired in the sagittal plane, as well as enhanced T1-weighted images in the axial, coronal, and sagittal planes. In aggregate, these images showed a 2.1 × 1.9 × 2.6 cm (transverse × anterior-posterior × superior-inferior) mass, located within the atrium of the right lateral ventricle. The mass demonstrated mild T1 hypointensity (Fig. 1A) with areas of heterogeneous T2 hyperintensity (Fig. 2A).
Fig. 1.
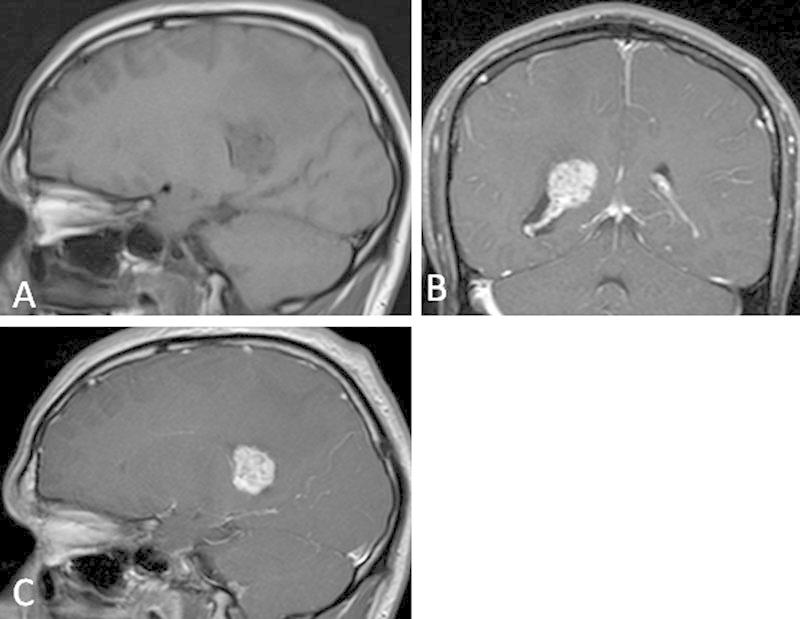
T1-weighted magnetic resonance imaging of the brain before (A) and after (B, C) the administration of gadolinium-based contrast demonstrate a midly T1 hypointense, avidly enhancing mass within the right lateral ventricle.
Fig. 2.
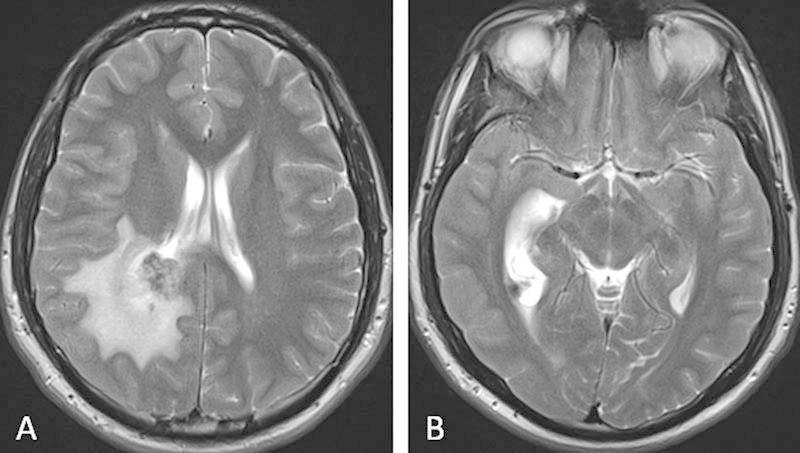
. T2-weighted magnetic resonance imaging of the brain demonstrate a heterogeneously hyperintense mass within the right lateral ventricle. Enlargement of the posterior horn of the right lateral ventricle with surrounding white matter edema is consistent with ventricular entrapment.
Foci of susceptibility artifact within the mass (Fig. 3) were suggestive of calcification or hemosiderin deposition from previous hemorrhage. Postcontrast images displayed homogeneous enhancement (Fig. 1B, C). Within the white matter of the surrounding right parietal and inferior right temporal lobes, there was a large amount of edema (Fig. 2A). Asymmetric enlargement of the right lateral ventricle (Fig. 2B), particularly at the level of the right temporal horn, was suggestive of ventricular entrapment. The third and fourth ventricles were normal in size. The primary radiographic consideration was a meningioma, although ependymoma and choroid plexus papilloma were in the differential.
Fig. 3.
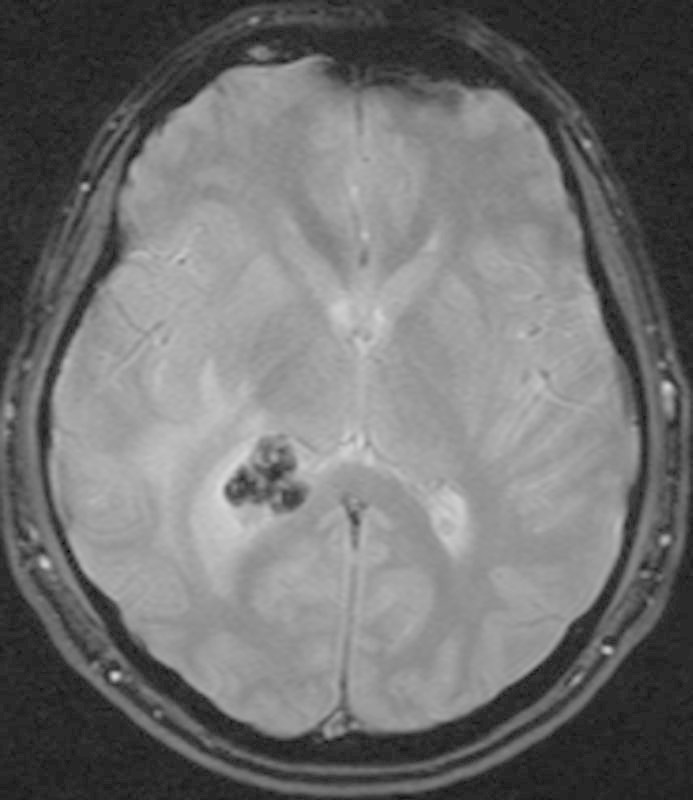
T2*-weighted magnetic resonance imaging of the brain demonstrates susceptibility artifact within the mass, representing foci of calcification.
The patient underwent surgical resection of the tumor through a right parietal craniotomy, with a transparietal approach, and intraventricular shunt placement for treatment of associated ventricular entrapment. Gross examination demonstrated the tumor to be well encapsulated and adherent to the medial aspect of the right lateral ventricle wall. Areas of moderate calcification were noted.
Pathologic evaluation demonstrated densely packed fascicles of spindle cells with elongated nuclei representing Antoni A tissue (Fig. 4). These were mixed with areas of loosely packed, hypocellular foci of spindle cells with small, round nuclei within myxoid matrix. This was consistent with Antoni B tissue (Fig. 5).
Fig. 4.
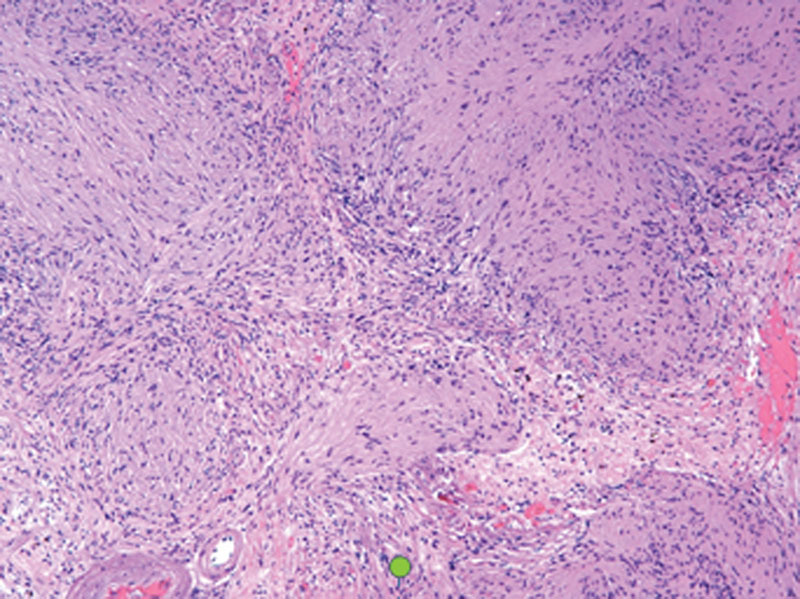
Hematoxylin-eosin staining viewed at ×200 magnification demonstrates densely packed fascicles of spindle cells with elongated nuclei (Antoni A).
Fig. 5.
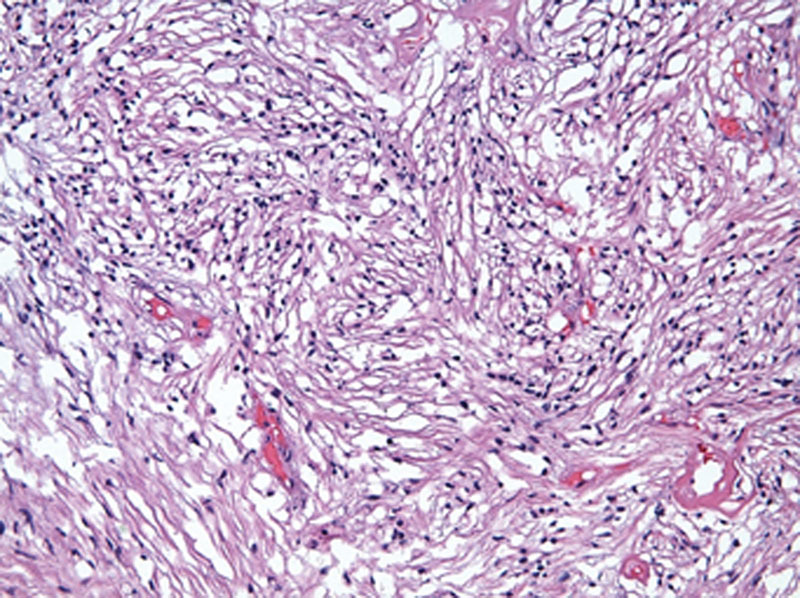
Hematoxylin-eosin staining viewed at ×200 magnification demonstrates a loosely packed, hypocellular focus of spindle cells with small, round nuclei in a myxoid matrix (Antoni B).
Positive S-100 staining confirmed the Schwann cell origin of the tumor while the negative epithelial membrane antigen confirmed that the tumor was not meningeal in origin. Glial fibrillary acidic protein was also positive, a marker which may be variably positive in schwannomas, but which is often positive in intracranial schwannomas. MIB-1 stain demonstrated low nuclear positivity, consistent with a benign process.
The patient is currently 9 months out from surgical resection of the tumor without radiographic evidence of residual or recurrent tumor.
Discussion
Schwannomas account for 8% of all primary brain tumors.1 Most schwannomas are associated with cranial nerves, most commonly cranial nerves VIII, V, and VII, and 80 to 90% are located in the region of the cerebellopontine angle.2 They have also been associated with spinal nerve roots and have been found in the retroperitoneum and mediastinum. Rarely, schwannomas have been noted to arise from intracranial locations, a subset of which have been described as intraventricular in location.
The earliest reported case of an intraventricular schwannoma was described by Marchand et al in 1957.3 Since then 23 cases have been reported in the literature (Table 1).
Table 1. Reported cases of intraventricular schwannoma.
| Age (y) | Gender | Location | Benign/malignant | Year | Citation |
|---|---|---|---|---|---|
| 44 | Male | Lateral (occipital) | Benign | 1990 | Ost et al5 |
| 21 | Male | Lateral (temporal) | Benign | 1975 | Van Rensburg12 |
| 63 | Female | Lateral | Benign | 1975 | Ghatak13 |
| 8 | Male | Lateral | Benign | 1988 | Pimentel et al14 |
| 40 | Male | Lateral (temporal) | Malignant | 1995 | Jung et al4 |
| 15 | Male | Lateral | Benign | 1965 | David et al15 |
| 7 | Male | Fourth | Benign | 1990 | Redekop et al16 |
| 16 | Male | Lateral (trigone) | Benign | 2004 | Dow et al7 |
| 15 | Male | Lateral (occipital) | Benign | 2008 | Benedict et al11 |
| 21 | Male | Lateral | Benign | 2003 | Erdogan et al17 |
| 21 | Female | Third | Benign | 2006 | Messing-Jünger et al18 |
| 71 | Female | Fourth | Benign | 2009 | Oertel et al10 |
| 36 | Female | Fourth | Benign | 2002 | Estrada19 |
| 16 | Male | Lateral | Benign | 2007 | Lévêque et al20 |
| 13 | Female | Lateral | Benign | 2001 | Barbosa et al21 |
| 43 | Male | Fourth | Malignant | 1957 | Marchand et al3 |
| 21 | Male | Lateral | Benign | 2013 | Luo et al2 |
| 61 | Male | Fourth | Benign | 1993 | Weiner et al22 |
| 78 | Female | Fourth | Benign | 1993 | Weiner et al22 |
| 16 | Male | Lateral (occipital) | Benign | 2013 | Jaimovich et al9 |
| 16 | Male | Lateral | Benign | 1993 | Casadei23 |
| 49 | Female | Lateral | Benign | 1993 | Casadei23 |
| 21 | Female | Lateral (occipital) | Benign | 2009 | Vasconcellos et al24 |
Among these cases there is almost a 2:1 male-to-female predominance. The median age of presentation is 31 years, with a reported age range of 7 to 78 years. Overall, 21 of the reported cases were benign in etiology with only 2 reported cases demonstrating malignant features.3 4 These lesions are most often located within the lateral ventricles (70%) but have also been found in the fourth (26%) and third (4%) ventricles. The most common complication associated with intraventricular schwannoma is the presence of hydrocephalus.
The clinical presentation of an intraventricular schwannoma often varies but most commonly involves headache, nausea, and vomiting. Brachial-crural hemiparesis, seizures, vertigo, and visual symptoms such as homonymous hemanopsia and transient scintillating scotomas have also been reported. These lesions may also be asymptomatic and only detected incidentally, through papilledema or retinal hemorrhage seen on an ophthalmic screening examination.
Currently, the etiology of intraventricular schwannomas is unknown, although three theories are often mentioned in the literature. The most popular theory postulates that intraventricular schwannomas are the result of hyperplastic transformation of Schwann cells associated with peripheral or autonomic nerve fibers located within the choroid plexus.5 This theory is supported by the identification of peripheral nerve fibers within the choroid plexus of the fourth ventricle.6 Another theory is neoplastic transformation of ectopic neural crest cells located within the ventricles due to disorganized embryogenesis,7 8 a theory that is supported by reported instances of schwannoma attachment to the choroid plexus. The final theory involves neoplastic transformation of pluripotent mesenchymal cells into Schwann cells after tissue injury.9 No association between intraventricular schwannomas and genetic syndromes has been identified.
The ubiquitous availability of CT means it is often the modality used for the initial evaluation of intraventricular schwannoma. Intraventricular schwannomas have been described as both hypodense and hyperdense, with possible cystic components. Calcifications are often present. On MRI, intracranial schwannomas are predominantly solid with cystic components. Solid components are most often homogeneously T1 hypointense with heterogeneous T2 hyperintensity. The cystic components mostly follow simple fluid characteristics (T1 hypointensity, T2 hyperintensity). The enhancement pattern has been described as heterogeneous both on CT and MR studies, and attachment to the choroid plexus and ventricle wall may be visualized. Associated periventricular edema may be visualized on both CT and MRI. Due to the rarity of these lesions, the differential diagnosis is mostly based on imaging findings and lesion location. The imaging differential diagnosis includes cystic astrocytomas, cystic meningiomas, ependymoma, choroid plexus papilloma, choroid plexus carcinoma, hemangioblastoma, and metastatic lesions.
Primary treatment is complete surgical resection. For tumors located within the lateral ventricles, a transcortical approach with osteoplastic craniotomy is favored.10 When the tumor is located in the third or fourth ventricles, a midline suboccipital approach is generally performed.10 Although complete surgical resection is always the preferred treatment, occasionally tumors may involve intracranial vessels or invade cerebral parenchyma, and complete resection may not be possible. In the single reported case where complete resection was not possible and pathology demonstrated malignancy, subsequent treatment with whole brain radiation was also performed (Jung et al). In cases of benign intraventricular schwannomas where complete resection is not possible, close radiologic follow-up is often pursued.11
Only two cases of malignant intraventricular schwannomas have been reported (Jung et al and Marchand et al). In the case of Jung et al, the schwannoma was incompletely resected due to tumor invasion and treated with subsequent whole brain radiation. Tumor recurrence with metastatic disease to the left cerebellopontine angle and cerebellar surface was diagnosed 4 months after the initial surgery.
Conclusions
Here, we present a case of right atrial intraventricular schwannoma causing right lateral ventricle entrapment. Although a rare cause of primary central nervous system neoplasm, with fewer than 25 cases reported in the literature, clinicians should keep intraventricular schwannoma in mind when evaluating enhancing intraventricular masses. These lesions are most often benign and may be fully cured with surgical resection.
References
- 1.Russell D S, Rubinstein L J. London: Edwards Arnold; 1977. Pathology of tumors of the nervous system. 4th ed; pp. 372–379. [Google Scholar]
- 2.Luo W, Ren X, Chen S, Liu H, Sui D, Lin S. Intracranial intraparenchymal and intraventricular schwannomas: report of 18 cases. Clin Neurol Neurosurg. 2013;115(7):1052–1057. doi: 10.1016/j.clineuro.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Marchand L, Koechlin P, Racine Y. Malignant neurinoma of the fourth ventricle with intrabulbar propagation in a schizophrenic; death during electroshock [in French] Ann Med Psychol (Paris) 1957;115(1):108–113. [PubMed] [Google Scholar]
- 4.Jung J M, Shin H J, Chi J G, Park I S, Kim E S, Han J W. Malignant intraventricular schwannoma. Case report. J Neurosurg. 1995;82(1):121–124. doi: 10.3171/jns.1995.82.1.0121. [DOI] [PubMed] [Google Scholar]
- 5.Ost A H, Meyer R. Cystic intraventricular schwannoma: a case report. AJNR Am J Neuroradiol. 1990;11(6):1262–1264. [PMC free article] [PubMed] [Google Scholar]
- 6.Benedickt M. Ueber die Innervation des Plexus choroideus. Virchows Arch Pathol Anat. 1874;59:395–400. [Google Scholar]
- 7.Dow G R, Hussein A, Robertson I J. Supratentorial intraventricular schwannoma. Br J Neurosurg. 2004;18(5):561–562. doi: 10.1080/02688690400012632. [DOI] [PubMed] [Google Scholar]
- 8.Ramamurthi B, Anguli V C, Iyer C GS. A case of intramedullary neurinoma. J Neurol Neurosurg Psychiatry. 1958;21(2):92–94. doi: 10.1136/jnnp.21.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaimovich R, Jaimovich S G, Arakaki N, Sevlever G. Supratentorial intraventricular solitary schwannoma. Case report and literature review. Childs Nerv Syst. 2013;29(3):499–504. doi: 10.1007/s00381-012-1977-4. [DOI] [PubMed] [Google Scholar]
- 10.Oertel M F, Nolte K W, Blaum M, Weis J, Gilsbach J M, Korinth M C. Primary intraventricular schwannomas. Clin Neurol Neurosurg. 2009;111(9):768–773. doi: 10.1016/j.clineuro.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Benedict W J Jr, Brown H G, Sivarajan G, Prabhu V C. Intraventricular schwannoma in a 15-year-old adolescent: a case report. Childs Nerv Syst. 2008;24(4):529–532. doi: 10.1007/s00381-007-0556-6. [DOI] [PubMed] [Google Scholar]
- 12.Van Rensburg M J, Proctor N S, Danziger J, Orelowitz M S. Temporal lobe epilepsy due to an intracerebral Schwannoma: case report. J Neurol Neurosurg Psychiatry. 1975;38(7):703–709. doi: 10.1136/jnnp.38.7.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghatak N R, Norwood G W, Davis G H. Intracerebral schwannoma. Surg Neurol. 1975;3:45–57. [PubMed] [Google Scholar]
- 14.Pimentel J, Tavora L, Cristina M L, Antunes J A. Intraventricular schwannoma. Childs Nerv Syst. 1988;4(6):373–375. doi: 10.1007/BF00270615. [DOI] [PubMed] [Google Scholar]
- 15.David M, Guyot J F, Ballivet J, Sachs M. Schwannoid tumor of the lateral ventricle [in French] Neurochirurgie. 1965;11(6):578–581. [PubMed] [Google Scholar]
- 16.Redekop G, Elisevich K, Gilbert J. Fourth ventricular schwannoma. Case report. J Neurosurg. 1990;73(5):777–781. doi: 10.3171/jns.1990.73.5.0777. [DOI] [PubMed] [Google Scholar]
- 17.Erdogan E, Ongürü O, Bulakbasi N, Baysefer A, Gezen F, Timurkaynak E. Schwannoma of the lateral ventricle: eight-year follow-up and literature review. Minim Invasive Neurosurg. 2003;46(1):50–53. doi: 10.1055/s-2003-37969. [DOI] [PubMed] [Google Scholar]
- 18.Messing-Jünger A M Riemenschneider M J Reifenberger G A 21-year-old female with a third ventricular tumor Brain Pathol 200616187–88., 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estrada M J, Sánchez Rodríguez G, Farías García R. Calderón Garcidue ñas AL. Schwannoma del cuarto ventrículo. Rev Med Inst Mex Seguro Soc. 2002;40(5):405–408. [Google Scholar]
- 20.Lévêque M, Gilliard C, Godfraind C, Ruchoux M M, Gustin T. Intraventricular schwannoma: a case report [in French] Neurochirurgie. 2007;53(5):383–386. doi: 10.1016/j.neuchi.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa M D, Rebelo O, Barbosa P, Gonçalves J, Fernandes R. Cystic intraventricular schwannoma: case report and review of the literature. Neurocirugia (Astur) 2001;12(1):56–60. doi: 10.1016/s1130-1473(01)70719-1. [DOI] [PubMed] [Google Scholar]
- 22.Weiner H L, Zagzag D, Babu R, Weinreb H J, Ransohoff J. Schwannoma of the fourth ventricle presenting with hemifacial spasm. A report of two cases. J Neurooncol. 1993;15(1):37–43. doi: 10.1007/BF01050261. [DOI] [PubMed] [Google Scholar]
- 23.Casadei G P, Komori T, Scheithauer W B, Miller G N, Parisi J E, Kelly P J. Intracranial parenchymal schwannoma. A clinicopathological and neuroimaging study of nine cases. J Neurosurg. 1993;79(2):217–222. doi: 10.3171/jns.1993.79.2.0217. [DOI] [PubMed] [Google Scholar]
- 24.Vasconcellos L P, Santos A R, Veiga J C, Schilemann I, Lancellotti C L. Supratentorial intraventricular schwannoma of the choroid plexus. Arq Neuropsiquiatr. 2009;67(4):1100–1102. doi: 10.1590/s0004-282x2009000600027. [DOI] [PubMed] [Google Scholar]


