Abstract
During the course of infection, many natural defenses are set up along the boundaries of the host-pathogen interface. Key among these is the host response to withhold metals to restrict the growth of invading microbes. This simple act of nutritional warfare, starving the invader of an essential element, is an effective means of limiting infection. The physiology of metal withholding is often referred to as “nutritional immunity,” and the mechanisms of metal transport that contribute to this host response are the focus of this review.
Keywords: iron, manganese, membrane protein, metal homeostasis, transport, Slc11a1, Slc11a2, Slc30a10, Slc39a14, Slc40a1, nutritional immunity, DMT1, ferroportin, Zip14, hypermanganesemia
Introduction
The rationale for host metal withholding against invading pathogens seems intuitive; restriction of any nutrient required for pathogen survival should combat infection. But why metals? The answer to this question becomes crystal clear when one recognizes that iron is required for nearly all life forms, including pathogenic bacteria, with a few rare exceptions that rely on manganese instead (1–7). Iron is essential for ATP production and other metabolic pathways, but its concentration is limited to avoid production of ROS2 catalyzed by excess levels of this redox-active metal (8). In some organisms, manganese can substitute for redox-active iron to protect against oxidative stress (9, 10). Moreover, most pathogens require manganese to produce their own superoxide dismutase activity to thwart oxidative killing mechanisms exerted by the host (11–13). Iron and manganese have similar ionic radii, have a divalent charge state under physiological conditions, have similar coordination chemistries, and have cellular concentrations in the micromolar range. Consequently, it comes as no surprise that these two metals also share membrane transporters, despite their different cellular functions and distributions. Restriction of iron and/or manganese provides a broad-spectrum metabolic “antidote” against all pathogenic infections, and the common transport pathways used by these metals present selective targets to limit their availability. Although many other immune responses contribute to the fight against infection, metal withholding represents a major force in host defense with combat controlled through transport.
The concept of nutritional immunity through metal withholding is largely based on the observations that the iron content of the human diet profoundly affects infectious diseases such as malaria, brucellosis, and tuberculosis (14, 15). Iron overload states such as sickle cell anemia and β-thalassemia increase the risk of infection (16–18). Hereditary hemochromatosis, an inherited disorder of iron metabolism, is also linked to susceptibility to some pathogens (17, 19–22). Iron deficiency, on the other hand, is associated with decreased survival of individuals infected with HIV and other agents (23). Still, high iron is positively associated with viral load and mortality in HIV (24–26), suggesting that an optimal balance of iron is necessary. In general terms, low iron status is protective, whereas elevated iron levels promote infection (27, 28). The complexity of host-pathogen interactions therefore presents a clinical conundrum; although iron supplements protect against iron deficiency, such measures could negatively impact infectious diseases (29, 30). This idea has been extended to suggest that iron deficiency frequently occurs in regions with endemic infectious diseases as a protective adaptation. Thus, metal restriction appears to play a key role in the human host's immune defense strategies. It is interesting to note that accumulating evidence shows that metal transporters are involved in host response to bacterial pathogens in plants, too.
A foundational discovery in nutritional immunity was the positional cloning of the murine Nramp allele. The natural resistance-associated macrophage protein locus was also called Bcg, Lsh, or Ity (31). Nramp1 (Slc11a1) was linked to immunity because mutation of its gene conferred susceptibility to intracellular pathogens. Allelic variations profoundly affect resistance to Salmonella, Leishmania, and Mycobacteria (32). The Nramp1 protein was initially characterized as a manganese transporter (33), consistent with studies demonstrating that this metal is a virulence factor for certain pathogens, including Yersinia (34) and Salmonella (35). Nramp1 expression is up-regulated by cytokines (36), and its function helps to produce nitric oxide along with other pro-inflammatory responses (37, 38). Loss of function not only reduces the inflammatory response to infection (39), but it also impedes iron recycling by macrophages (40). Greater insight about the metal transport role of Nramp1 was achieved when its paralog Nramp2 (also called DMT1, DCT1 (divalent cation transporter-1), or Slc11a2) was tightly linked to iron homeostasis (41, 42). Both members of the mammalian Nramp/Slc11 family are thought to combat infection by limiting availability of iron and manganese, and possibly other metals, to microbes.
Iron and Manganese Transport: Roles of Nramp1 and Nramp2 (DMT1)
Nramp1 is expressed in monocytes and macrophages, cell types that function in the front line immune response to invading pathogens. Recently, Nramp1 was found to be expressed in lymphocytes, and in particular, a subset of these cells that are responsible for interferon-γ production (43). Nramp1 shares ∼65% amino acid sequence identity with DMT1, which is ubiquitously expressed with higher levels found in the kidney and intestine (42). These proteins are members of a large and evolutionarily conserved family of divalent metal transporters (44). In macrophages, Nramp1 is associated with lysosomes and late endosomes and is found on maturing phagosomes (45, 46). Its cytolocalization is consistent with the transporter's identified role in the resistance to infection by obligate intracellular pathogens, which are known to reside in such intracellular compartments. Susceptibility is acquired by a single G169D substitution in the predicted fourth transmembrane-spanning domain; therefore mice with the Nramp1G169 allele are resistant (31). The Nramp1D169 allele appears to encode a non-functional protein that does not mature properly (47).
The RAW264.7 macrophage cell line derives from a mouse strain carrying the Nramp1D169 allele and therefore displays Nramp1 deficiency. These particular cells proved useful in early transport studies of Nramp1 properties and characteristics. Iron transport into phagosomes containing latex beads (48) or mycobacteria (48, 49) was first shown to be higher in RAW264.7 cells transfected with Nramp1G169 as compared with Nramp1D169. These observations led to the hypothesis that Nramp1 transports iron into the bacterium-containing phagosome, thereby limiting mycobacterial growth by catalyzing ROS. In contrast, other studies suggested that Nramp1 transported metals out of the phagosome, a function that could restrict pathogen growth by limiting the availability of the essential nutrient metals (33, 50, 51). Controversy surrounding the directionality of transport was further heightened by the proposal that the function of Nramp1 was bidirectional depending on pH (52).
More information about the function of Nramp1 function came from biochemical comparison with its counterpart Nramp2 (DMT1). Although discovered after Nramp1, DMT1 has been much better characterized at the molecular level (42). The latter functions as a proton symporter with a selectivity rank of Cd2+>Fe2+>Co2+ ∼ Mn2+≫Zn2+ (53). Other interacting metals include Ni2+, Pb2+, and possibly Cu2+ and Ag2+ (42, 54–56); although not all metals have been tested experimentally, a few have been ruled out as transport substrates (Fig. 1A). Comparative studies of Nramp1 and DMT1 expressed at the cell surface with an epitope tag have identified both transporters to have similar structural topology and cellular membrane distribution (57). Studies in HEp-2, HeLa, and COS-7 cells localized DMT1 to recycling endosomes, where it transports iron from transferrin into the cytosol (58, 59). DMT1 also becomes associated with phagosomes in J774 macrophages along with Nramp1, suggesting that the transporters pump metals out into the cytosol and sequester them from pathogens that attempt to take up intracellular residence in this compartment (60). Although it is clear that the H+ gradient is important to the cotransport of metals by DMT1, it has yet to be rigorously determined whether Nramp1 is a cotransporter (32, 57) or an exchanger (52, 61).
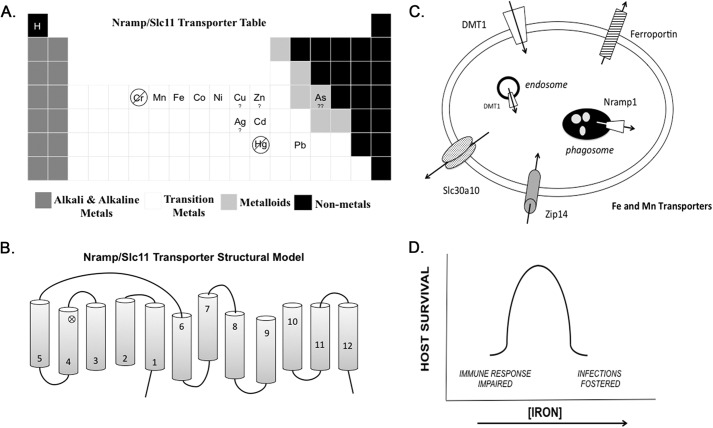
FIGURE 1.
Metal transporters combat infection. Panel A highlights metals within the periodic table of elements that have been studied as potential substrates for Nramp1 and/or DMT1. Panel B shows a structural homology model with 12 transmembrane-spanning domains representing two pseudo-symmetric halves. The loop between membrane domains 7–8 has been topologically determined to face the extracellular milieu. Also indicated is the proximity of Gly-169 and Gly-185 residues in the fourth transmembrane domain (cross-mark). Amino acid substitutions at this site in mice and rats disable transport function and stability of the membrane transporters Nramp1 and DMT1, respectively. Panel C depicts the cellular localizations of iron and manganese transporters that may provide host resistance as discussed in the text. Panel D outlines the double-edged sword of iron transport. As iron levels increase, the susceptibility to pathogen growth and virulence is known to increase with decreasing survival of host. Conversely, iron depletion disables the appropriate inflammatory response necessary for NO production and cytokine expression.
Structural modeling predicts that the Slc11 transporters have 12 membrane-spanning domains (Fig. 1B). Based on homology with members of the LeuT superfamily, Nramp1 and DMT1 most likely have pseudo-symmetry with domains 1–6 and domains 7–12 representing N- and C-terminal halves, respectively (44). The loop between domains 7 and 8 is extracellular and glycosylated (57, 62). In a curiosity of nature, DMT1 allelic variants have been found in mice (41) and rats (58) with the same G185R substitution in the predicted fourth transmembrane-spanning domain. In structural models, these mutations are proximal to the Nramp1 G169D mutation that confers susceptibility to infections. Like the Nramp1 mutant, DMT1G185R also appears to be an unstable protein of impaired function (63). Both microcytic anemia (mk) mice and Belgrade (b) rats that carry the mutant DMT1 allele display iron deficiency phenotypes with limited iron uptake from the diet and impaired heme metabolism. It has also been demonstrated that Belgrade rats have perturbed manganese transport and metabolism (64, 65). Studies of mk mice support an important role for DMT1 in intestinal iron absorption (41), whereas studies using Belgrade rat reticulocytes confirm that DMT1 functions to export iron from the endosome to the cytosol, where it can be utilized for heme synthesis and other metabolic purposes (66). It is important to note that cellular localization studies of DMT1 are consistent with the transporter's function in intestinal iron uptake and release of iron from endosomes after endocytic uptake of transferrin (63). Impairment of both pathways leads to systemic iron deficiency in the host, ultimately limiting pathogen nutrient availability. Fig. 1C depicts the relationship between cellular localization of Nramp1 and DMT1 and their functions.
Roles of Other Transporters
The iron export protein ferroportin was identified in zebrafish (67) and mice (68, 69). Also known as MTP1 (metal-transporting protein-1) or Ireg-1 (iron-regulated transporter-1), this transporter was referred to as HFE4 due to its association with hereditary hemochromatosis type 4 or “ferroportin disease” (70, 71). Initially termed Slc11a3 as a third member of solute carrier family 11 (proton-coupled divalent metal ion transporter), its function and most likely its mechanism are quite different. It is now recognized as the first member of solute carrier family 40 (iron-regulated transporter or Slc40a1).
Activation of macrophages with LPS or interferon-γ enhances ferroportin expression (72). When challenged with Salmonella typhimurium or Mycobacterium tuberculosis, macrophages also up-regulate ferroportin expression (73, 74). In turn, this iron exporter reduces intracellular iron otherwise available to pathogens. As a sequella of infection, release of the inflammatory cytokine IL-6 induces the expression of hepcidin, an iron regulatory hormone, which binds to ferroportin and promotes its internalization and degradation in lysosomes (56). This mechanism ultimately diminishes the release of iron from macrophages, which play an important role in recycling the metal from senescent red cells after erythrophagocytosis. In addition to macrophages, ferroportin is also found on the basolateral surface of enterocytes, where it mediates dietary iron uptake by exporting the metal across the intestinal epithelium to circulation. Thus, increased hepcidin reduces systemic iron by controlling iron absorption and recycling to promote hypoferremia. This hypoferremia or “anemia of inflammation” ultimately affords systemic protection against infection (75).
In hereditary hemochromatosis type 1, disruption of hepcidin regulation leads to higher-than-normal ferroportin levels; consequently, excess iron accumulates in the host. Individuals with hemochromatosis are susceptible to infections (Vibrio, Yersinia, E. coli), but the overexpression of ferroportin reduces iron in macrophages such that resistance to obligate intracellular pathogens is observed (Mycobacteria, Salmonella, Legionella) (76). In vitro studies show that overexpression of ferroportin disrupts M. tuberculosis growth (77), diminishes growth of Salmonella (78), and reduces HIV replication (79). Conversely, studies of flatiron mice, which have ferroportin deficiency (70, 76, 80), demonstrate that loss of its export activity confers greater susceptibility to intracellular pathogens (81). Macrophages from these mice support greater growth of Chlamydophila psittaci, and this effect was lost upon iron chelation (81). Using flatiron macrophages, studies have also revealed that Leishmania amazonensis blocks ferroportin expression to inhibit iron export as a strategy to promote its intracellular growth (82). Because ferroportin has been implicated in Mn2+ transport (83, 84), it is possible that it exerts antimicrobial roles to block pathogen acquisition of this metal as well. Interestingly, DMT1 is also implicated in dietary Mn2+ (65). Hepcidin does appear to play a role in regulating intake of this metal (85), but whether it exerts effects on ferroportin or DMT1 (or both) is unclear (86).
The zinc transporter Zip14 (Slc39a14) also plays a role in iron homeostasis (87–89). Zip14 is expressed in liver, heart, and pancreas (90), the tissues most affected by iron loading, and in vitro studies have shown that Zip14 imports iron in addition to zinc (87, 91). Zip14 appears to function in both transferrin-bound iron uptake and non-transferrin-bound iron transport. The latter plays a role in clearing excess iron from circulation in conditions such as hemochromatosis, and it has been proposed that Zip14, rather than DMT1, is the predominant transport pathway for iron in hepatocytes (92).
Also like Nramp1, the inflammatory cytokine IL-6 up-regulates expression of Zip14 (87). This observation is important because infections are not only associated with hypoferremia, but also with hypozincemia. Lower availability of zinc negatively impacts pathogen growth in much of the same way iron and manganese withdrawal combats infection (11). Notably, Zip14 knock-out mice have reduced circulating zinc after LPS injection, but they do not display hypoferremia. Thus, regulation of metal metabolism by the immune response can be exerted via multiple transport pathways to target specific metals. Interestingly, LPS-induced inflammation is not only associated with increased hepatic zinc accumulation, but cadmium levels in the liver also rise, suggesting a role for Zip14 in this mechanism, too (93).
Another zinc transporter, Slc30a10, has been implicated in manganese export. Slc30a10 is highly expressed in the liver and the brain. Recent genetic studies identified mutations in the human gene that were associated with hypermanganesemia. Loading of this metal is observed in both tissues, resulting in cirrhosis of the liver and Parkinson-like motor impairments (94, 95). Due to the roles of manganese (and zinc) in combating infection, one speculation is that this transporter may also participate in the host immune response. Further study of this transporter and patients affected by mutations in the SLC30A10 gene is warranted to better understand how disruptions in manganese metabolism may affect the course of microbial infection.
Table 1 outlines known characteristics of Nramp1 in comparison with other transporters known to combat infection. Fig. 1C shows how their cellular distribution and transport activity help to move metals to and from various cellular compartments.
TABLE 1.
Cellular distribution and function of metal transporters
| Transporter | Localization | Function |
|---|---|---|
| Nramp1 (Slc11a1) | Late endosome/phagosome (macrophages) | Export divalent metals |
| Nramp2/DMT1 (Slc11a2) | Apical membrane (intestine) | Absorb divalent metals (from diet) |
| Endosome (reticulocytes/other cells) | Export divalent metals (from endosome) | |
| Kidney | Metal reabsorption? | |
| Ferroportin (Slc40a1) | Basolateral membrane (intestine) | Absorb divalent metals (from intestinal enterocyte) |
| Macrophages (spleen, liver) | Recycle iron (after erythrophagocytosis) | |
| Hepatocytes | Release iron from stores | |
| Zip 14 (Slc39a14) | Liver, heart, pancreas | Clearance of non-transferrin-bound iron (liver) |
| Slc30a10 | Liver, brain | Excrete excess manganese (biliary pathway in liver) |
Resistance versus Risk
Despite our expanding knowledge about the regulation of metal transport during infection, precisely how the activity of Nramp1 combats infection remains to some extent uncertain. This membrane protein most likely has many functions that contribute to multiple pathways. It has been proposed that the transporter's mechanistic effects simply reflect changes in the level of iron and other metals to alter ROS-regulated signaling and pro-inflammatory pathways that are important for antimicrobial defense mechanisms. It is known that impaired Nramp1 limits NO production and alters cytokine expression in macrophages (96). These responses could reflect changes in iron content at the cellular level because studies have shown that macrophage cytokine translation is altered by iron homeostasis (97). Studies in Nramp1 knock-out mice suggest that its key role might be to enable a faster pro-inflammatory response associated with increased cytokine gene expression (39). Biochemical studies have suggested that Nramp1 regulates protein tyrosine phosphatase activity, and changes in the transporter's activity may modulate signal transduction pathways involved in the macrophage inflammatory response (98). Its function in this signaling pathway may in fact play a key role in lymphocyte activation, too (43).
It is possible that the influence of Nramp1 on the divalent metal content of phagosomes and inflammatory responses is yet still secondary to dysregulation of iron homeostasis through effects on other transporters. Early studies suggested that Nramp1 impairment reduced iron release from macrophages (99). Later investigations of knock-out mice showed that iron accumulates in liver and spleen during erythrophagocytosis (40). Both DMT1 and ferroportin are up-regulated upon loss of Nramp1 function; therefore Nramp1−/− mice have increased transferrin saturation presumably due to enhanced dietary uptake as well as impaired macrophage iron recycling. Because increased iron is associated with susceptibility to infections, these changes would shift the balance to confer susceptibility.
Such additional roles of metal transport beyond simple nutrient limitation must be better appreciated in the inflammatory response to infection because there is a need to balance resistance to infection with risk imparted by disturbances in immunostasis. As an interesting example, human mutations in the gene for Nramp1 influence tuberculosis infection and rheumatic disease with pleiotropic effects (100). Several human polymorphisms in the SLC11A1 gene harbor 5′-(GT)n microsatellite repeats with the two most frequent alleles differing by one GT repeat, which affects the gene's promoter activity. Allele 3 appears to yield higher expression of Nramp1, whereas allele 2, which is proposed to promote Z-DNA formation, yields lower expression. A model has been proposed wherein low-expressing allele 2 induces chronic infection with low macrophage killing, whereas allele 3 is associated with acute infection and greater pro-inflammatory potential. Human studies suggest that the latter is associated with increased risk of tuberculosis and other infectious diseases (Table 2). The balance reflects the critical importance of metal homeostasis diagrammed in Fig. 1D. Too little iron affects our ability to promote the appropriate inflammatory response and may be associated with auto-immune and other diseases, whereas too much iron fosters human susceptibility to infectious disease. Achieving the optimum level of iron and other metals is the necessary and coordinated task of transporters such as Nramp1, DMT1, ferroportin, and others. Although metal transporters help combat infection, we must remember that they have essential roles that maintain human health that are not limited to infectious disease.
TABLE 2.
Risk of chronic and infectious disease associated with human alleles encoding Nramp1
See Ref. 100 and references therein.
| SLC11A1 | |
|---|---|
| Inflammatory disease | |
| Rheumatoid arthritis | Allele 2, allele 3 |
| Inflammatory bowel disease | Allele 7 |
| Type 1 diabetes | Allele 3 |
| Multiple sclerosis | Allele 3, allele 5 |
| Infectious disease | |
| Tuberculosis | Allele 2 |
| Leprosy | Other polymorphisms |
| HIV | Allele 3 |
| Leishmania | Allele 3 |
| Meningococcal meningitis | Allele 3 |
This work was supported by National Institutes of Health Grants R01ES0146380 and R01DK064570 (to M. W. R.). This is the sixth article in the Thematic Minireview series “Metals at the Host-Pathogen Interface.” The author declares that she has no conflicts of interest with the contents of this article.
- ROS
- reactive oxygen species
- DMT1
- divalent metal transporter-1.
References
- 1. Posey J. E., Gherardini F. C. (2000) Lack of a role for iron in the Lyme disease pathogen. Science 288, 1651–1653 [DOI] [PubMed] [Google Scholar]
- 2. Lisher J. P., Giedroc D. P. (2013) Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell. Infect. Microbiol. 3, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguirre J. D., Clark H. M., McIlvin M., Vazquez C., Palmere S. L., Grab D. J., Seshu J., Hart P. J., Saito M., Culotta V. C. (2013) A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288, 8468–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Troxell B., Ye M., Yang Y., Carrasco S. E., Lou Y., Yang X. F. (2013) Manganese and zinc regulate virulence determinants in Borrelia burgdorferi. Infect. Immun. 81, 2743–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holland R., Pritchard G. G. (1975) Regulation of the l-lactase dehydrogenase from Lactobacillus casei by fructose-1,6-diphosphate and metal ions. J. Bacteriol. 121, 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stetter K. O., Kandler O. (1973) Manganese requirement of the transcription processes in Lactobacillus curvatus. FEBS Lett. 36, 5–8 [DOI] [PubMed] [Google Scholar]
- 7. Culotta V. C., Daly M. J. (2013) Manganese complexes: diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid. Redox Signal. 19, 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberg E. D. (2010) The hazards of iron loading. Metallomics 2, 732–740 [DOI] [PubMed] [Google Scholar]
- 9. Sobota J. M., Imlay J. A. (2011) Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. U.S.A. 108, 5402–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anjem A., Varghese S., Imlay J. A. (2009) Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72, 844–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kehl-Fie T. E., Skaar E. P. (2010) Nutritional immunity beyond iron: a role for manganese and zinc. Curr. Opin. Chem. Biol. 14, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguirre J. D., Culotta V. C. (2012) Battles with iron: manganese in oxidative stress protection. J. Biol. Chem. 287, 13541–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaharik M. L., Finlay B. B. (2004) Mn2+ and bacterial pathogenesis. Front. Biosci. 9, 1035–1042 [DOI] [PubMed] [Google Scholar]
- 14. Gangaidzo I. T., Moyo V. M., Mvundura E., Aggrey G., Murphree N. L., Khumalo H., Saungweme T., Kasvosve I., Gomo Z. A., Rouault T., Boelaert J. R., Gordeuk V. R. (2001) Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 184, 936–939 [DOI] [PubMed] [Google Scholar]
- 15. Lounis N., Truffot-Pernot C., Grosset J., Gordeuk V. R., Boelaert J. R. (2001) Iron and Mycobacterium tuberculosis infection. J. Clin. Virol. 20, 123–126 [DOI] [PubMed] [Google Scholar]
- 16. Onwubalili J. K. (1983) Sickle cell disease and infection. J. Infect. 7, 2–20 [DOI] [PubMed] [Google Scholar]
- 17. Moyo V. M., Gangaidzo I. T., Gordeuk V. R., Kiire C. F., Macphail A. P. (1997) Tuberculosis and iron overload in Africa: a review. Cent. Afr. J. Med. 43, 334–339 [PubMed] [Google Scholar]
- 18. Wanachiwanawin W. (2000) Infections in E-β thalassemia. J. Pediatr. Hematol. Oncol. 22, 581–587 [DOI] [PubMed] [Google Scholar]
- 19. Höpfner M., Nitsche R., Rohr A., Harms D., Schubert S., Fölsch U. R. (2001) Yersinia enterocolitica infection with multiple liver abscesses uncovering a primary hemochromatosis. Scand. J. Gastroenterol. 36, 220–224 [DOI] [PubMed] [Google Scholar]
- 20. Gerhard G. S., Levin K. A., Price Goldstein J., Wojnar M. M., Chorney M. J., Belchis D. A. (2001) Vibrio vulnificus septicemia in a patient with the hemochromatosis HFE C282Y mutation. Arch. Pathol. Lab. Med. 125, 1107–1109 [DOI] [PubMed] [Google Scholar]
- 21. Barton J. C., Acton R. T. (2009) Hemochromatosis and Vibrio vulnificus wound infections. J. Clin. Gastroenterol. 43, 890–893 [DOI] [PubMed] [Google Scholar]
- 22. Galan S. R., Kann P. H., Gress T. M., Michl P. (2011) Listeria monocytogenes-induced bacterial peritonitis caused by contaminated cheese in a patient with haemochromatosis. Z. Gastroenterol. 49, 832–835 [DOI] [PubMed] [Google Scholar]
- 23. McDermid J. M., Prentice A. M. (2006) Iron and infection: effects of host iron status and the iron-regulatory genes haptoglobin and NRAMP1 (SLC11A1) on host-pathogen interactions in tuberculosis and HIV. Clin. Sci. 110, 503–524 [DOI] [PubMed] [Google Scholar]
- 24. Friis H., Gomo E., Nyazema N., Ndhlovu P., Krarup H., Madsen P. H., Michaelsen K. F. (2003) Iron, haptoglobin phenotype, and HIV-1 viral load: a cross-sectional study among pregnant Zimbabwean women. J. Acquir. Immune Defic. Syndr. 33, 74–81 [DOI] [PubMed] [Google Scholar]
- 25. Gordeuk V. R., Onojobi G., Schneider M. F., Dawkins F. W., Delapenha R., Voloshin Y., von Wyl V., Bacon M., Minkoff H., Levine A., Cohen M., Greenblatt R. M. (2006) The association of serum ferritin and transferrin receptor concentrations with mortality in women with human immunodeficiency virus infection. Haematologica 91, 739–743 [PubMed] [Google Scholar]
- 26. McDermid J. M., Jaye A., Schim van der Loeff M. F., Todd J., Bates C., Austin S., Jeffries D., Awasana A. A., Whittlex A. A., Prentice A. (2007) Elevated iron status strongly predicts mortality in West African adults with HIV infection. J. Acquir. Immune Defic. Syndr. 46, 498–507 [DOI] [PubMed] [Google Scholar]
- 27. Moalem S., Weinberg E. D., Percy M. E. (2004) Hemochromatosis and the enigma of misplaced iron: implications for infectious disease and survival. Biometals 17, 135–139 [DOI] [PubMed] [Google Scholar]
- 28. Denic S., Agarwal M. M. (2007) Nutritional iron deficiency: an evolutionary perspective. Nutrition 23, 603–614 [DOI] [PubMed] [Google Scholar]
- 29. Sazawal S., Black R. E., Ramsan M., Chwaya H. M., Stoltzfus R. J., Dutta A., Dhingra U., Kabole I., Deb S., Othman M. K., Kabole F. M. (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367, 133–143 [DOI] [PubMed] [Google Scholar]
- 30. Soofi S., Cousens S., Iqbal S. P., Akhund T., Khan J., Ahmed I., Zaidi A. K., Bhutta Z. A. (2013) Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 382, 29–40 [DOI] [PubMed] [Google Scholar]
- 31. Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. (1993) Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73, 469–485 [DOI] [PubMed] [Google Scholar]
- 32. Forbes J. R., Gros P. (2001) Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9, 397–403 [DOI] [PubMed] [Google Scholar]
- 33. Jabado N., Jankowski A., Dougaparsad S., Picard V., Grinstein S., Gros P. (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192, 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Champion O. L., Karlyshev A., Cooper I. A., Ford D. C., Wren B. W., Duffield M., Oyston P. C., Titball R. W. (2011) Yersinia pseudotuberculosis mntH functions in intracellular manganese accumulation, which is essential for virulence and survival in cells expressing functional Nramp1. Microbiology 157, 1115–1122 [DOI] [PubMed] [Google Scholar]
- 35. Zaharik M. L., Cullen V. L., Fung A. M., Libby S. J., Kujat Choy S. L., Coburn B., Kehres D. G., Maguire M. E., Fang F. C., Finlay B. B. (2004) The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect. Immun. 72, 5522–5525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Govoni G., Gauthier S., Billia F., Iscove N. N., Gros P. (1997) Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J. Leukoc. Biol. 62, 277–286 [DOI] [PubMed] [Google Scholar]
- 37. Blackwell J. M., Goswami T., Evans C. A., Sibthorpe D., Papo N., White J. K., Searle S., Miller E. N., Peacock C. S., Mohammed H., Ibrahim M. (2001) SLC11A1 (formerly NRAMP1) and disease resistance. Cell. Microbiol. 3, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wyllie S., Seu P., Goss J. A. (2002) The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect. 4, 351–359 [DOI] [PubMed] [Google Scholar]
- 39. Valdez Y., Grassl G. A., Guttman J. A., Coburn B., Gros P., Vallance B. A., Finlay B. B. (2009) Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Microbiol. 11, 351–362 [DOI] [PubMed] [Google Scholar]
- 40. Soe-Lin S., Apte S. S., Andriopoulos B., Jr., Andrews M. C., Schranzhofer M., Kahawita T., Garcia-Santos D., Ponka P. (2009) Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 5960–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fleming M. D., Trenor C. C., 3rd, Su M. A., Foernzler D., Beier D. R., Dietrich W. F., Andrews N. C. (1997) Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 16, 383–386 [DOI] [PubMed] [Google Scholar]
- 42. Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 [DOI] [PubMed] [Google Scholar]
- 43. Hedges J. F., Kimmel E., Snyder D. T., Jerome M., Jutila M. A. (2013) Solute carrier 11A1 is expressed by innate lymphocytes and augments their activation. J. Immunol. 190, 4263–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cellier M. F. (2012) Nramp: from sequence to structure and mechanism of divalent metal import. Curr. Top. Membr. 69, 249–293 [DOI] [PubMed] [Google Scholar]
- 45. Gruenheid S., Pinner E., Desjardins M., Gros P. (1997) Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 185, 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Searle S., Bright N. A., Roach T. I., Atkinson P. G., Barton C. H., Meloen R. H., Blackwell J. M. (1998) Localisation of Nramp1 in macrophages: modulation with activation and infection. J. Cell Sci. 111, 2855–2866 [DOI] [PubMed] [Google Scholar]
- 47. Vidal S. M., Pinner E., Lepage P., Gauthier S., Gros P. (1996) Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 157, 3559–3568 [PubMed] [Google Scholar]
- 48. Kuhn D. E., Baker B. D., Lafuse W. P., Zwilling B. S. (1999) Differential iron transport into phagosomes isolated from the RAW264.7 macrophage cell lines transfected with Nramp1Gly169 or Nramp1Asp169. J. Leukoc. Biol. 66, 113–119 [DOI] [PubMed] [Google Scholar]
- 49. Zwilling B. S., Kuhn D. E., Wikoff L., Brown D., Lafuse W. (1999) Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect. Immun. 67, 1386–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Atkinson P. G., Barton C. H. (1999) High level expression of Nramp1G169 in RAW264.7 cell transfectants: analysis of intracellular iron transport. Immunology 96, 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barton C. H., Biggs T. E., Baker S. T., Bowen H., Atkinson P. G. (1999) Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens. J. Leukoc. Biol. 66, 757–762 [DOI] [PubMed] [Google Scholar]
- 52. Goswami T., Bhattacharjee A., Babal P., Searle S., Moore E., Li M., Blackwell J. M. (2001) Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 354, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Illing A. C., Shawki A., Cunningham C. L., Mackenzie B. (2012) Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 287, 30485–30496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arredondo M., Mendiburo M. J., Flores S., Singleton S. T., Garrick M. D. (2014) Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals 27, 115–123 [DOI] [PubMed] [Google Scholar]
- 55. Jiang L., Garrick M. D., Garrick L. M., Zhao L., Collins J. F. (2013) Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J. Nutr. 143, 1927–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Garrick M. D., Singleton S. T., Vargas F., Kuo H. C., Zhao L., Knöpfel M., Davidson T., Costa M., Paradkar P., Roth J. A., Garrick L. M. (2006) DMT1: which metals does it transport? Biol. Res. 39, 79–85 [DOI] [PubMed] [Google Scholar]
- 57. Forbes J. R., Gros P. (2003) Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102, 1884–1892 [DOI] [PubMed] [Google Scholar]
- 58. Fleming M. D., Romano M. A., Su M. A., Garrick L. M., Garrick M. D., Andrews N. C. (1998) Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. U.S.A. 95, 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tabuchi M., Yoshimori T., Yamaguchi K., Yoshida T., Kishi F. (2000) Human NRAMP2/DMT1, which mediates iron transport across endosomal membranes, is localized to late endosomes and lysosomes in HEp-2 cells. J. Biol. Chem. 275, 22220–22228 [DOI] [PubMed] [Google Scholar]
- 60. Gruenheid S., Canonne-Hergaux F., Gauthier S., Hackam D. J., Grinstein S., Gros P. (1999) The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J. Exp. Med. 189, 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Techau M. E., Valdez-Taubas J., Popoff J. F., Francis R., Seaman M., Blackwell J. M. (2007) Evolution of differences in transport function in Slc11a family members. J. Biol. Chem. 282, 35646–35656 [DOI] [PubMed] [Google Scholar]
- 62. Picard V., Govoni G., Jabado N., Gros P. (2000) Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J. Biol. Chem. 275, 35738–35745 [DOI] [PubMed] [Google Scholar]
- 63. Su M. A., Trenor C. C., Fleming J. C., Fleming M. D., Andrews N. C. (1998) The G185R mutation disrupts function of the iron transporter Nramp2. Blood 92, 2157–2163 [PubMed] [Google Scholar]
- 64. Thompson K., Molina R. M., Donaghey T., Schwob J. E., Brain J. D., Wessling-Resnick M. (2007) Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 21, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chua A. C., Morgan E. H. (1997) Manganese metabolism is impaired in the Belgrade laboratory rat. J. Comp. Physiol. B 167, 361–369 [DOI] [PubMed] [Google Scholar]
- 66. Garrick M. D., Gniecko K., Liu Y., Cohan D. S., Garrick L. M. (1993) Transferrin and the transferrin cycle in Belgrade rat reticulocytes. J. Biol. Chem. 268, 14867–14874 [PubMed] [Google Scholar]
- 67. Donovan A., Brownlie A., Zhou Y., Shepard J., Pratt S. J., Moynihan J., Paw B. H., Drejer A., Barut B., Zapata A., Law T. C., Brugnara C., Lux S. E., Pinkus G. S., Pinkus J. L., Kingsley P. D., Palis J., Fleming M. D., Andrews N. C., Zon L. I. (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 [DOI] [PubMed] [Google Scholar]
- 68. Abboud S., Haile D. J. (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 [DOI] [PubMed] [Google Scholar]
- 69. McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F., Hediger M. A., Hentze M. W., Simpson R. J. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 [DOI] [PubMed] [Google Scholar]
- 70. Johnson E. E., Wessling-Resnick M. (2007) Flatiron mice and ferroportin disease. Nutr. Rev. 65, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pietrangelo A. (2004) The ferroportin disease. Blood Cells Mol. Dis. 32, 131–138 [DOI] [PubMed] [Google Scholar]
- 72. Nairz M., Fritsche G., Brunner P., Talasz H., Hantke K., Weiss G. (2008) Interferon-γ limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol. 38, 1923–1936 [DOI] [PubMed] [Google Scholar]
- 73. Nairz M., Theurl I., Ludwiczek S., Theurl M., Mair S. M., Fritsche G., Weiss G. (2007) The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell. Microbiol. 9, 2126–2140 [DOI] [PubMed] [Google Scholar]
- 74. Van Zandt K. E., Sow F. B., Florence W. C., Zwilling B. S., Satoskar A. R., Schlesinger L. S., Lafuse W. P. (2008) The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J. Leukoc. Biol. 84, 689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wessling-Resnick M. (2010) Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 30, 105–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Johnson E. E., Wessling-Resnick M. (2012) Iron metabolism and the innate immune response to infection. Microbes Infect. 14, 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Johnson E. E., Sandgren A., Cherayil B. J., Murray M., Wessling-Resnick M. (2010) Role of ferroportin in macrophage-mediated immunity. Infect. Immun. 78, 5099–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chlosta S., Fishman D. S., Harrington L., Johnson E. E., Knutson M. D., Wessling-Resnick M., Cherayil B. J. (2006) The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect. Immun. 74, 3065–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu M., Kashanchi F., Foster A., Rotimi J., Turner W., Gordeuk V. R., Nekhai S. (2010) Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zohn I. E., De Domenico I., Pollock A., Ward D. M., Goodman J. F., Liang X., Sanchez A. J., Niswander L., Kaplan J. (2007) The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood 109, 4174–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paradkar P. N., De Domenico I., Durchfort N., Zohn I., Kaplan J., Ward D. M. (2008) Iron depletion limits intracellular bacterial growth in macrophages. Blood 112, 866–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ben-Othman R., Flannery A. R., Miguel D. C., Ward D. M., Kaplan J., Andrews N. W. (2014) Leishmania-mediated inhibition of iron export promotes parasite replication in macrophages. PLoS Pathog. 10, e1003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Madejczyk M. S., Ballatori N. (2012) The iron transporter ferroportin can also function as a manganese exporter. Biochim. Biophys. Acta 1818, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yin Z., Jiang H., Lee E. S., Ni M., Erikson K. M., Milatovic D., Bowman A. B., Aschner M. (2010) Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J. Neurochem. 112, 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim J., Buckett P. D., Wessling-Resnick M. (2013) Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One 8, e64944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brasse-Lagnel C., Karim Z., Letteron P., Bekri S., Bado A., Beaumont C. (2011) Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 140, 1261–1271.e1 [DOI] [PubMed] [Google Scholar]
- 87. Liuzzi J. P., Aydemir F., Nam H., Knutson M. D., Cousins R. J. (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 103, 13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pinilla-Tenas J. J., Sparkman B. K., Shawki A., Illing A. C., Mitchell C. J., Zhao N., Liuzzi J. P., Cousins R. J., Knutson M. D., Mackenzie B. (2011) Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 301, C862–C871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao N., Gao J., Enns C. A., Knutson M. D. (2010) ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 285, 32141–32150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Taylor K. M., Morgan H. E., Johnson A., Nicholson R. I. (2005) Structure-function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 579, 427–432 [DOI] [PubMed] [Google Scholar]
- 91. Jenkitkasemwong S., Wang C. Y., Mackenzie B., Knutson M. D. (2012) Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gao J., Zhao N., Knutson M. D., Enns C. A. (2008) The hereditary hemochromatosis protein, HFE, inhibits iron uptake via down-regulation of Zip14 in HepG2 cells. J. Biol. Chem. 283, 21462–21468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Min K. S., Takano M., Amako K., Ueda H., Tanaka K. (2013) Involvement of the essential metal transporter Zip14 in hepatic Cd accumulation during inflammation. Toxicol. Lett. 218, 91–96 [DOI] [PubMed] [Google Scholar]
- 94. Tuschl K., Clayton P. T., Gospe S. M., Jr., Gulab S., Ibrahim S., Singhi P., Aulakh R., Ribeiro R. T., Barsottini O. G., Zaki M. S., Del Rosario M. L., Dyack S., Price V., Rideout A., Gordon K., Wevers R. A., Chong W. K., Mills P. B. (2012) Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 90, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Quadri M., Federico A., Zhao T., Breedveld G. J., Battisti C., Delnooz C., Severijnen L. A., Di Toro Mammarella L., Mignarri A., Monti L., Sanna A., Lu P., Punzo F., Cossu G., Willemsen R., Rasi F., Oostra B. A., van de Warrenburg B. P., Bonifati V. (2012) Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fritsche G., Nairz M., Theurl I., Mair S., Bellmann-Weiler R., Barton H. C., Weiss G. (2007) Modulation of macrophage iron transport by Nramp1 (Slc11a1). Immunobiology 212, 751–757 [DOI] [PubMed] [Google Scholar]
- 97. Wang L., Johnson E. E., Shi H. N., Walker W. A., Wessling-Resnick M., Cherayil B. J. (2008) Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 181, 2723–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gomez M. A., Li S., Tremblay M. L., Olivier M. (2007) NRAMP-1 expression modulates protein-tyrosine phosphatase activity in macrophages: impact on host cell signaling and functions. J. Biol. Chem. 282, 36190–36198 [DOI] [PubMed] [Google Scholar]
- 99. Biggs T. E., Baker S. T., Botham M. S., Dhital A., Barton C. H., Perry V. H. (2001) Nramp1 modulates iron homoeostasis in vivo and in vitro: evidence for a role in cellular iron release involving de-acidification of intracellular vesicles. Eur. J. Immunol. 31, 2060–2070 [DOI] [PubMed] [Google Scholar]
- 100. Blackwell J. M., Searle S., Mohamed H., White J. K. (2003) Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol. Lett. 85, 197–203 [DOI] [PubMed] [Google Scholar]