Background: Macrophages differentiation and/or polarization have been implicated in many inflammatory diseases.
Results: Matrix metalloproteinase-8 (MMP8) promotes M2-macrophage differentiation and polarization through modulating TGF-β bioactivity.
Conclusion: MMP8 plays a novel role in macrophage differentiation and polarization.
Significance: The findings of this study significantly increased our understanding to biological functions of MMP8, the mechanisms for macrophage differentiation and polarization, and the pathogenesis of pathological conditions in which MMP8 is involved.
Keywords: extracellular matrix protein, inflammation, macrophage, matrix metalloproteinase (MMP), transforming growth factor β (TGF-β), matrix metalloproteinase-8, macrophage polarization
Abstract
Matrix metalloproteinase-8 (MMP8) has been shown to influence various cellular functions. As monocytes and macrophages (Mφ) express MMP8, we investigated if MMP8 played a role in macrophage differentiation and polarization. MMP8 expression was significantly increased during monocyte differentiation into Mφ. Monocyte-derived Mφ from MMP8-deficient mice expressed higher levels of M1-Mφ markers but lower levels of M2-Mφ markers than monocyte-derived Mφ from wild-type mice. Although Mφ from either MMP8-deficient or wild-type mice were inducible by interferon-γ into M1-Mφ, only wild-type Mφ but not MMP8-deficient Mφ could be induced into M2-Mφ by interleukin-4. However, MMP8-deficient Mφ exposed to conditioned culture media of wild-type Mφ developed a M2-Mφ phenotype. Compared with conditioned culture media of wild-type Mφ, conditioned culture media of MMP8-deficient Mφ contained a lower concentration of active transforming growth factor-β (TGF-β), an M2-Mφ inducer. Moreover, evidence also showed that the degradation of the TGF-β sequester, fibromodulin, was modulated by MMP8. The data indicate a previously unknown role of MMP8 in M2-Mφ polarization by cleaving fibromodulin and therefore increasing the bioavailability of the M2-Mφ inducer TGF-β.
Introduction
Macrophages (Mφ)4 play important roles in many inflammatory diseases such as atherosclerosis. Studies have shown that the Mφ phenotype is heterogeneous, plastic, and profoundly affected by local factors within the microenvironment (1–7). Lipopolysaccharides, interleukin-1β (IL-1β), and cytokines secreted by Th1 lymphocytes, such as interferon-γ (IFN-γ), induce a “classic” Mφ phenotype (M1), whereas Th2 lymphocyte cytokines, such as IL-4 and IL-13, promote an “alternative” Mφ phenotype (M2) (1–7). Activated M1-Mφ produce proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-6, and IL-12, whereas M2-Mφ dampen the inflammatory state by producing anti-inflammatory molecules, such as IL-10, IL-1 receptor antagonist, and transforming growth factor (TGF)-β. In response to local factors in the microenvironment, M1-Mφ can change to M2-Mφ, and vice versa. Studies in recent years have revealed a number of signaling pathways, transcriptional networks, and epigenetic mechanisms that influence Mφ differentiation and polarization (8, 9). These findings are important for understanding the pathogenesis of inflammatory diseases and can have potential therapeutic implications.
Matrix metalloproteinase-8 (MMP8), a relatively less studied member of the MMP family, has received increasing attention in recent years. The best known substrates of MMP8 are types I, II, and III collagens. However, a growing number of other proteins have been reported to be also susceptible to cleavage by MMP8, including angiotensin-1 (10, 11), chemokine (C-X-C motif) ligand 5 (CXCL5) (12), CXCL11 (13), and macrophage inflammatory protein-1 (14). Previously it was thought that only neutrophils produce MMP8, reflected by its alias names neutrophil collagenase and polymorphonuclear leukocyte collagenase. However, it is now known that various other types of cells also express this protease. Studies have shown that MMP8 can modulate the behavior and function of several cell types including neutrophils (14, 15), eosinophils (16), smooth muscle cells (17), endothelial cells (18), and stem/progenitor cells (19), underlain by its proteolytic activity on matrix and non-matrix proteins. Furthermore, studies have shown that MMP8 is involved in a number of pathological conditions including atherosclerosis (11, 19), acute hepatitis (20), acute lung injury (14), airway inflammation (16), and cancers (21). Because Mφ play important roles in many pathological conditions, and given that Mφ express MMP8, we investigated in this present study if MMP8 had a role in Mφ differentiation and/or polarization.
Experimental Procedures
Materials
Antibodies against arginase I (rabbit, H-52, sc-20150), arginase II (rabbit, H-64, sc-20151), F4/80 (rat, BM8, sc-52664), CD163 (goat, K18, sc-18796), CD206 (goat, C20, sc-34577), TGF-β (rabbit, sc-146), SMAD3 (mouse, sc-101154), LAP (goat, T-17, sc-34830), fibromodulin (rabbit, H50, sc-33772), and MMP8 (goat, sc-31741, used for double immunostaining with F4/80, 1:50) were purchased from Santa Cruz Biotechnology. Antibodies against MMP8 (rabbit, ab78423, used for Western blot, 1:500 or single immunostaining, 1:100), pSMad3 (phospho-Ser423/Ser425, rabbit, ab51451), and GAPDH (mouse, 6C5, Ab8245) were from Abcam, UK. Antibody against α-tubulin (mouse, T6074) was from Sigma. Cytokines (macrophage colony-stimulating factor (M-CSF), IL-4 and IFN-γ) were from ProSpec Bio, USA. All ELISA kits including TGF-β1, monocyte chemoattractant protein-1 (MCP-1), TNF-α, IFN-γ, IL-6, IL-10, and IL-12 were from Life Technologies. Other materials used in this study were purchased from Sigma unless specifically indicated.
Macrophage Culture and Polarization
Bone Marrow Monocyte-derived Macrophage Culture (BMMφ)
Bone marrow monocytes were isolated from the tibias and femurs of mice 6–12 weeks of age. The bone marrow was triturated using an 18-gauge needle and passed through a 70-μm nylon mesh cell strainer (Becton Dickinson, Franklin Lakes, NJ) to make a single cell suspension in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 2% serum, 10 mm Hepes. Red blood cells in bone marrow cells were lysed with a lysis buffer (8.3 g of NH4Cl, 1.0 g of KHCO3, and 1.8 ml of 5% EDTA in 1000 ml). Bone marrow mononuclear cells were washed three times and cultured in the RPMI-1640 supplemented with 10% serum and 5 ng/ml of M-CSF for 7–14 days to induce BMMφ differentiation and maturation. For each independent experiment, cells isolated from 6–8 WT (wild-type, ApoE−/−/MMP8+/+), MMP8KO (MMP8 knock-out, ApoE−/−/MMP8−/−), or C57BL/6 mice were pooled together, and subjected to different treatments. MMP8 WT and MMP8KO mice were generated in our previous study (11). C57BL/6 mice were purchased from Charles River. All the animal procedures were approved by Queen Mary University of London ethics review board, and performed to conform the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes or the NIH guidelines (Guide for the Care and Use of Laboratory Animals).
Peritoneal Naive Tissue Resident Mφ (pMφ)
pMφ were isolated as described in the previous study (22). Briefly, 4 days before harvesting pMφ, 1.5 ml of 4% (w/v) Brewer thioglycollate medium were injected into the peritoneal cavity of each mouse to induce macrophage infiltration and accumulation into peritoneal cavity. Mice were euthanized, cleaned with 70% ethanol, and mounted on the styrofoam block on its back. 5 ml of ice-cold PBS (with 3% serum) were injected into the peritoneal cavity using a 27-guage needle, and carefully collected using a plastic Pasteur pipette. Cells in the suspension were spun down for direct RNA extraction or re-suspended in the RPMI-1640 supplemented with 10% serum for macrophage culture. For each independent experiment, cells isolated from 6–8 WT or MMP8_KO mice were pooled together, and subjected to different treatments. In some experiments, an equal amount of PBS containing cells and peritoneal cavity fluid (4 ml) were collected from WT or MMP8KO mice and spun down to obtain cell-free supernatant. After mixing with a protease inhibitor mixture, the supernatant were concentrated using centrifugal concentrators (Millipore, UFC901024).
M1- and M2 Macrophage Polarization
Both BMMφ or pMφ were incubated with 10 ng/ml of IFN-γ or IL-4 for 48 h to induce M1- and M2 macrophage polarization, respectively, as described previously (23).
TGF-β and MMP8 Treatments
In separate experiments, BMMφ or pMφ were cultured for 48 h in RPMI culture medium in the presence or absence of 10 ng/ml of TGF-β or 50 ng/ml of MMP8, respectively.
Conditioned Culture Medium Swap
BMMφ or pMφ were differentiated or isolated as described previously, followed by IL-4 polarization for 24 h. The culture medium (3 ml per well for six-well plate) was collected and swapped as indicated in the figures, followed by another 24 h of culture.
Immunoblotting
Cells were harvested and lysed in lysis buffer (50 mm Tris-Cl, pH 7.5, 150 mm NaCl, 1 mm EDTA, pH 8.0) supplemented with protease inhibitors and 0.5% Triton and sonicated to obtain whole cell lysate. 40 μg of protein was separated by SDS-PAGE with 4–20% Tris glycine gel (Invitrogen) and subjected to standard Western blot analysis. In some experiments, the blots were subjected to densitometric analysis with ImageJ software. Relative protein expression level was defined as the ratio of cleaved fragment to their respective full-length of fibromodulin protein expression level, of mature to precursor of TGF-β protein expression levels, of total TGF-β protein expression levels to GAPDH, or pSMAD3 to total SMAD3 expression levels, with that of the respective control sample set as 1.0.
Real-time Quantitative PCR (RT-qPCR)
Real-time quantitative PCR (RT-qPCR) was performed as previously described (24). Briefly, total RNAs were extracted from cells using TRI Reagent (Sigma) according to the manufacturer's instructions. Reverse transcription was performed using an Improm-IITM Reverse Transcription kit (Promega, Madison, WI) with RNase inhibitor (Promega), and Random primers (Promega). The resultant cDNA was diluted to a working concentration of 5 ng/μl and stored at −20 °C. Primers were designed using Primer Express software (Applied Biosystems) and the sequence for each primer was shown in Table 1. Relative mRNA expression level was defined as the ratio of target gene expression level to 18S expression level, respectively, with that of the control sample set as 1.0.
TABLE 1.
Primer sets used in the present study
| Gene names | Forward (5′-3′) | Reverse (5′-3′) | Application |
|---|---|---|---|
| 18S | CCCAGTAAGTGCGGGTCATAA | CCGAGGGCCTCACTAAACC | Real-time RT-PCR (RT-qPCR) |
| Mmp8 | GTCCCAAGTGGACACACACT | TCACTTCAGCCCTTGACAGC | RT-qPCR |
| Cd206 | GTGGAGTGATGGAACCCCAG | CTGTCCGCCCAGTATCCATC | RT-qPCR |
| Arg I | AGCACTGAGGAAAGCTGGTC | CAGACCGTGGGTTCTTCACA | RT-qPCR |
| Arg II | TCTCCTCCACGGGCAAATTC | GCAAGCCAGCTTCTCGAATG | RT-qPCR |
| Cd163 | GCGGATGATCTGGACTTGCT | GTGCCTCTGAATGACCCCTT | RT-qPCR |
| Mcp-1 | CCCCAAGAAGGAATGGGTCC | TGCTTGAGGTGGTTGTGGAA | RT-qPCR |
| Irf5 | TGGATGTGGCATGTAGTAGCC | CTGGGTACTGGCAGCTGTTC | RT-qPCR |
| Tnf-α | AGGCACTCCCCCAAAAGATG | TGAGGGTCTGGGCCATAGAA | RT-qPCR |
| Il-6 | GTGGCTAAGGACCAAGACCA | TAACGCACTAGGTTTGCCGA | RT-qPCR |
| Il-12β | AGTGACATGTGGAATGGCGT | CAGTTCAATGGGCAGGGTCT | RT-qPCR |
| Tgf-β | TGCTAATGGTGGACCGCAA | CACTGCTTCCCGAATGTCTGA | RT-qPCR |
| Irf4 | AATCCCCATTGAGCCAAGCA | CTCGTCGTGGTCAGCTCTTT | RT-qPCR |
| Ppar-γ | CGGGCTGAGAAGTCACGTT | TGCGAGTGGTCTTCCATCAC | RT-qPCR |
| Il-10 | GCTGCCTGCTCTTACTGACT | CTGGGAAGTGGGTGCAGTTA | RT-qPCR |
| Fibromodulin | GTCCACCTACTACGACCCCT | GACAGTCGCATTCTTGGGGA | RT-qPCR |
RAW264.7 Cell Culture and shRNA-mediated Stable Mmp8 Gene Silencing
Murine macrophage cell line, RAW264.7, was purchased from ATCC (ATCC® TIB-71TM) and cultured in DMEM containing 10% fetal bovine serum. Non-target and Mmp8 shRNA lentiviral particles were produced as described in our previous study (19). For lentiviral infection, RAW264.7 cells were plated 24 h prior to infection in 6-well plates at 37 °C. One transducing unit per cell (or 2–3 × 106/well) of Mmp8 shRNA or control virus were added with 10 μg/ml of hexadimethrine bromide (H9268; Sigma). Viral constructs were incubated 24 h with the cells before the media was replaced with complete media containing 2–4 μg/ml of puromycin (P9620, Sigma). For selection of transductants, fresh media containing puromycin was added at 2–3-day intervals for 10–14 days. Stably infected cells were split and frozen for future experiments.
Fibromodulin Knockdown by siRNA and TGF-β Inhibition
A pool of small interfering RNAs (siRNAs) for fibromodulin (MISSION® esiRNA, esiRNA targeting mouse fibromodulin, EMU010381–20UG) and MISSION® siRNA Universal Negative Control number 1 (SIC001–10NMOL) were purchased from Sigma. Bone marrow-differentiated macrophages were cultured in 6-well plates for 7 days, and 6 μl of 10 μm siRNA (final concentration of siRNAs: 60 nm) was introduced into cells with TransIT-X2 Transfection Reagent (Geneflow Limited) according to the protocol provided. 24 h post-transfection, cells were incubated with 10 ng/ml of IL-4 for another 24 h for M2 polarization. Cells were harvested and RT-qPCR analyses were performed. In additional experiments, bone marrow-differentiated macrophages were pre-treated with TGF-β signaling specific inhibitor (SB-431542, 10 μm, Sigma) for 3 h, followed by 10 ng/ml of IL-4 incubation for another 24 h in the presence or absence of 10 μm SB-431542.
Immunofluorescence Staining for Cells
Cells were treated with various conditions as indicated in the figures and fixed, followed by immunohistological analyses with the respective antibody as described before (25, 26). Briefly, cells were blocked with 5% BSA in PBS for 1 h at room temperature in a humid chamber, and incubated with the respective primary antibody or IgG control overnight in the cold room. Followed by incubation with appropriate fluorescent dye-conjugated secondary antibodies, cells were then incubated with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma) for 5 min. Images were assessed with Axioplan 2 imaging microscope with Plan-NEOFLUAR ×20, NA 0.5, objective lenses, AxioCam camera, and Axiovision software (all Carl Zeiss MicroImaging, Inc.) at room temperature, or examined with Zeiss LSM 510 confocal microscope with Plan-NEOFLUAR ×40 objective lenses and Zeiss ZEN microscope software (Carl Zeiss, Germany) at room temperature. All images were processed with Photoshop software (Adobe). The cells positive for M1- or M2-Mφ markers were counted over 200 total Mφ, and the mean fluorescence intensity (MFI) of the cells that were positive for the respective markers were analyzed using Photoshop software by two well trained independent investigators blinded to the treatments, from 20 random selected cells.
ELISA Analyses
The levels of individual cytokine in the conditioned culture medium or concentrated peritoneal cavity fluid (for active TGF-β) were measured using their respective ELISA kit purchased from Life Technologies, according to the manufacturer's instructions.
Statistical Analysis
Data were expressed as mean ± S.E. and analyzed using a two-tailed Student's t test for two-group comparison or one-way analysis of variance followed by Newman-Keuls multiple comparison post-hoc test for comparing different groups. A value of p < 0.05 was considered as statistically significant.
Results
MMP8 Is Significantly Up-regulated during Macrophage Differentiation
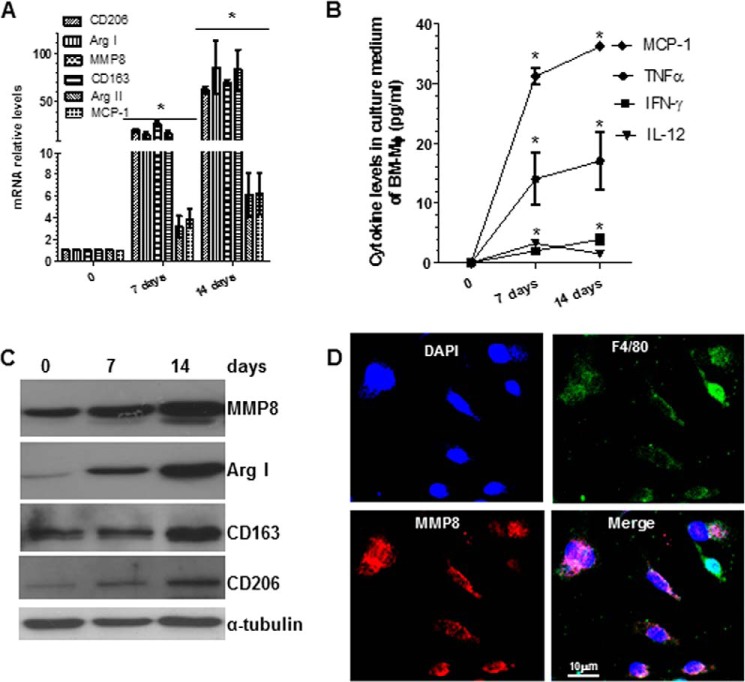
To induce macrophage differentiation and maturation from BMMs, mouse BMMs were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 5 ng/ml of M-CSF for the indicated experimental time points. Total RNA, conditioned culture medium, and proteins were harvested and subjected to RT-qPCR, ELISA, and Western blot analyses, respectively. The RT-qPCR analyses showed significant increases in the expression levels of M2-Mφ markers (arginase (Arg) I, mannose receptor C type 1 (Mrc1)/Cd206, and Cd163), but much lower levels of M1-Mφ markers (Arg II and monocyte chemotactic protein-1 (Mcp-1)), during Mφ differentiation (Fig. 1A). Similarly, ELISA showed significant increases in several inflammatory cytokines (MCP-1, TNF-α, IFN-γ, and IL-12) in conditioned culture media, over the course of Mφ differentiation and maturation (Fig. 1B), and Western blot analyses showed significant induction in three M2-Mφ markers examined in this study (Fig. 1C). Immunofluorescence staining showed that more than 95% of differentiated cells were positive for the Mφ marker F4/80 after 10–14 days (Fig. 1D). These data suggest that M-CSF promotes Mφ differentiation and maturation from BMMs with a predominant M2-Mφ phenotype, as suggested in previous studies (1).
FIGURE 1.
MMP8 is up-regulated during Mφ differentiation. Bone marrow monocytes were induced to differentiate into Mφ by M-CSF. Total RNAs, conditioned culture medium (CM), and cell lysate were harvested at the indicated times, and subjected to RT-qPCR (A), ELISA (B), and Western blot (C) analyses, respectively. M1 (MCP-1 and Arg II) or M2 (CD206, Arg I and CD163)-Mφ genes/proteins were examined along with MMP8. D, immunofluorescence staining analyses of MMP8 expression in the 14 days of differentiated Mφ. The data presented here are an average or representative of three independent experiments. *, p < 0.05 (versus day 0).
Importantly, we also observed that MMP8 expression significantly increased during Mφ differentiation and maturation (Fig. 1, A and C). Double immunostaining demonstrated co-expression of MMP8 with Mφ specific markers F4/80 (Fig. 1D).
MMP8 Deficiency Leads to a M1 Macrophage Phenotype
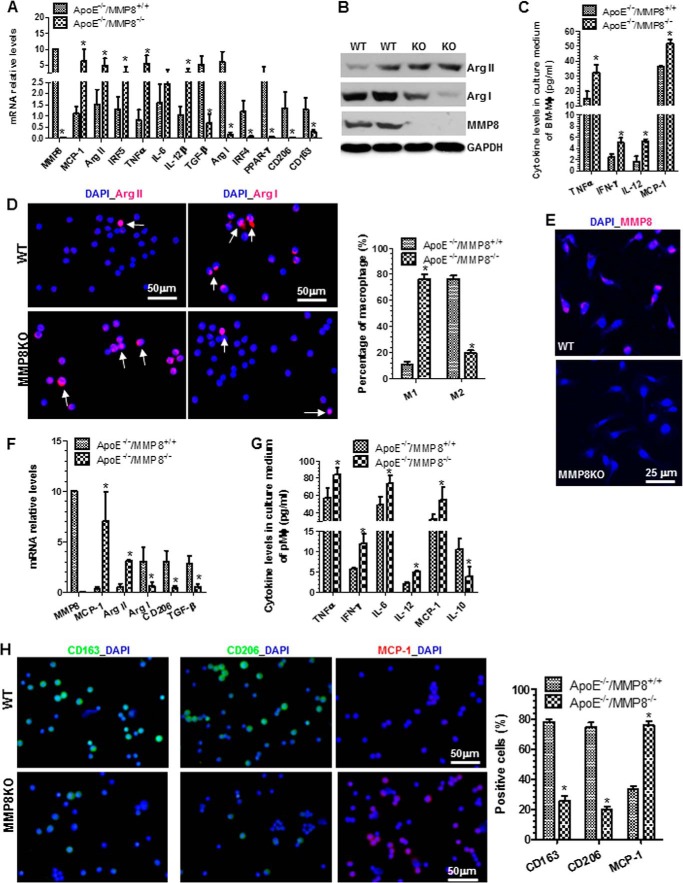
To investigate whether MMP8 plays a role in Mφ differentiation and maturation from BMMs, BMMs isolated from MMP8 knock-out (MMP8KO) mice and WT control littermates were induced to differentiate into Mφ by M-CSF stimulation for 14 days. RT-qPCR analyses showed that MMP8 deficiency significantly increased the expression levels of M1-Mφ genes (Mcp-1, Arg II, Irf5, Tnf-α, Il-6, and Il-12β), but reduced M2-Mφ gene expression (Tgf-β, Arg I, Irf4, peroxisome proliferator-activated receptor-γ, Cd206, and Cd163), in differentiated Mφ (Fig. 2A). Western blot analyses showed corresponding changes in Arg I and Arg II protein levels, and that the MMP8 protein expression was absent in MMP8-deficient Mφ (Fig. 2B). Conditioned culture media of MMP8KO Mφ contained higher levels of inflammatory cytokines including TNF-α, IFN-γ, IL-12, and MCP-1, as demonstrated by ELISA analyses (Fig. 2C). Importantly, at day 14, the percentage of M1-Mφ was significantly higher and the percentage of M2-Mφ was much lower, in Mφ differentiated from MMP8KO BMMs than in Mφ differentiated from WT BMMs, although there was no significant differences in the total numbers of Mφ (F4/80-positive cells, data not shown) (Fig. 2D). Expectedly, whereas MMP8 was clearly expressed in Mφ differentiated from WT BMMs, no apparent signal for MMP8 was detected in Mφ differentiated from MMP8KO BMMs (Fig. 2E). A similar effect of MMP8 deficiency was observed in naive pMφ (peritoneal cavity macrophages) (Fig. 2, F–H). Briefly, compared with WT pMφ, an increased expression level of M1-Mφ markers, but a decreased expression level of M2-Mφ markers, was observed in MMP8-deficient pMφ (Fig. 2, F and G). Similarly, more M1-Mφ (percentage of MCP-1-positive cells) and less M2-Mφ (the percentage of CD163- or CD206-positive cells) were observed in MMP8-deficient pMφ (Fig. 2G).
FIGURE 2.
MMP8 is required for M2-Mφ differentiation. A, RT-qPCR analysis of expression levels of M1- and M2-Mφ genes in the day 14 of differentiated bone marrow Mφ (BMMφ). B, Western blot analyses show that the protein expression levels of Arg I, Arg II, and MMP8 in WT and MMP8KO BMMφ. C, ELISA analysis of the inflammatory cytokine levels in the culture medium conditioned by BMMφ. D, immunofluorescence staining shows that the percentage of M2-Mφ in MMP8KO (ApoE−/−/MMP8−/−) differentiated BMMφ is much lower than that of WT (ApoE−/−/MMP8+/+) BMMφ. Arrows indicate M1-Mφ (cells are positive for Arg II) or M2-Mφ (cells are positive for Arg I). E, immunofluorescence staining of MMP8 in WT and MMP8KO BMMφ. F, RT-qPCR analysis of M1- and M2-Mφ gene expression levels in the naive peritoneal Mφ (pMφ) isolated from the peritoneal cavity of WT and MMP8KO mice. G, ELISA analysis of the inflammatory cytokine levels in the culture medium conditioned by WT and MMP8-deficient pMφ. H, immunofluorescence staining shows that MMP8 deficiency results in a M1-Mφ phenotype in pMφ. The data presented here are representative or an average of three to six independent experiments. *, p < 0.05 (versus WT). Shown in panels D and H are representative images each from three independent experiments, and column charts of the percentage of M1- and M2-Mφ. *, p < 0.05 (versus WT).
MMP8 Is Required for M2-Mφ Polarization
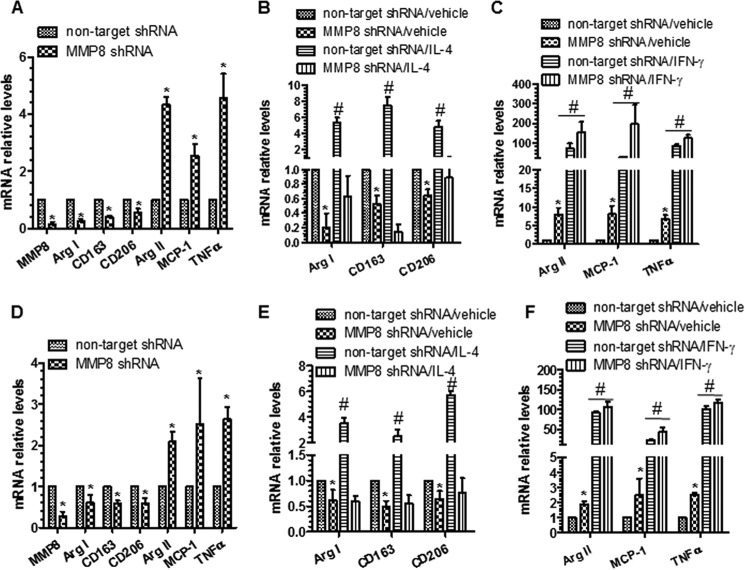
To investigate whether MMP8 is involved in Mφ polarization, BMMφ from MMP8KO and WT mice were incubated with Mφ polarization inducers (10 ng/ml of IFN-γ for M1 priming, and 10 ng/ml of IL-4 for M2, respectively) for 48 h. The M1 priming by IFN-γ increased the expression of M1-Mφ markers, but this effect was significantly greater in MMP8KO BMMφ than in WT BMMφ (Fig. 3, A and B). In contrast, the M2 inducer IL-4 significantly up-regulated the expression of M2-Mφ markers in WT BMMφ, but such an effect was not observed in MMP8KO BMMφ (Fig. 3, C and D), indicating that MMP8 deficiency significantly reduces M2-Mφ polarization. This was confirmed using the naive pMφ (Fig. 3, E–H).
FIGURE 3.
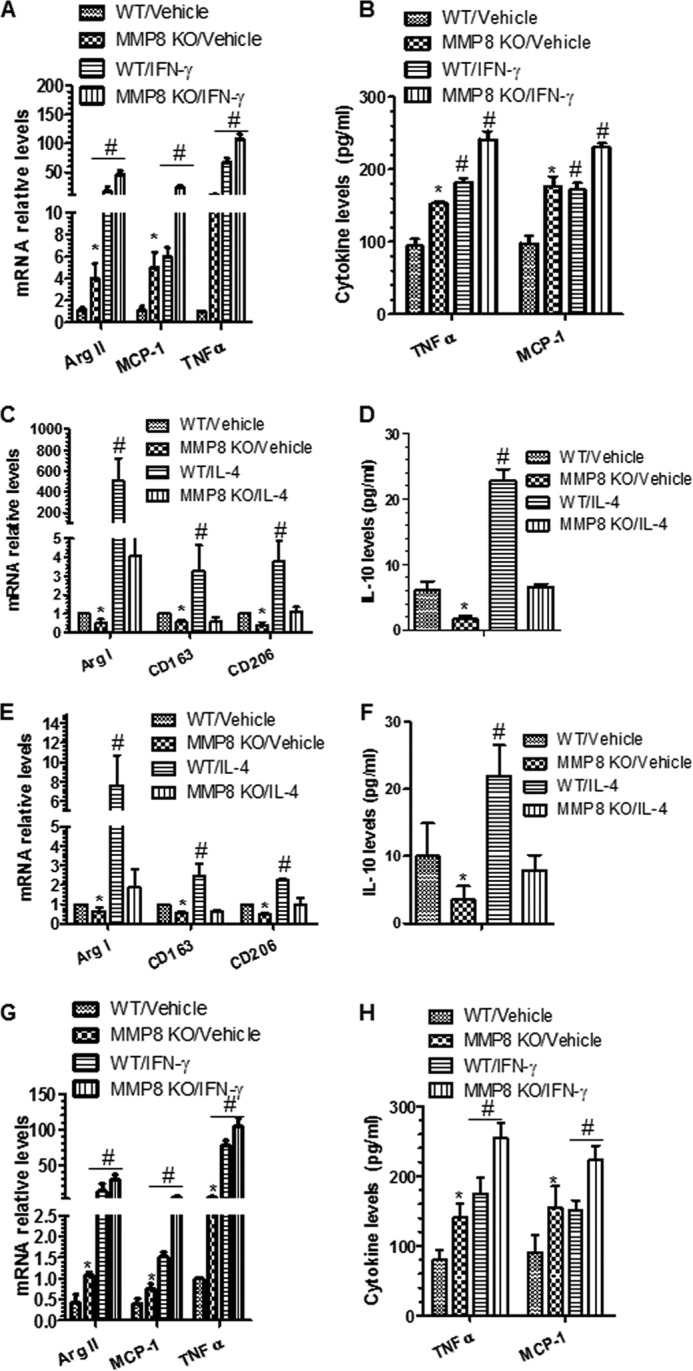
MMP8 is required for M2-Mφ polarization. A and B, IFN-γ significantly up-regulated M1-Mφ gene (A) and protein (B) expression levels in both WT and MMP8-deficient BMMφ. C and D, IL-4 significantly up-regulated M2-Mφ gene (C) and IL-10 protein (D) expression levels in WT, but not in MMP8-deficient BMMφ. E and F, IL-4 significantly increased M2-Mφ gene (E) and IL-10 protein (F) expression levels in WT, but not in MMP8 KO pMφ. G and H, IFN-γ up-regulated M1-Mφ gene (G) and protein (H) expression levels in both WT and MMP8KO pMφ. The data presented here are an average of three to four independent experiments. *, p < 0.05 (MMP8KO versus WT); #, p < 0.05 (Mφ inducers versus control).
It has been reported that ApoE can affect Mφ polarization (27). The MMP8KO and WT mice used in the above experiments were apoE deficient. To investigate if the effect of MMP8 on M2-Mφ polarization is apoE independent, murine macrophage cells, RAW264.7 (apoE wild-type), were infected with either Mmp8-specific shRNA lentivirus and non-target control lentivirus, followed by incubation with either the M1-Mφ (IFN-γ) or the M2-Mφ (IL-4) inducer for 48 h. RT-qPCR analyses showed that MMP8 knockdown significantly down-regulated M2-Mφ genes and up-regulated M1-Mφ genes (Fig. 4A). Furthermore, MMP8 knockdown diminished IL-4 induction of M2-Mφ genes (Arg I, Cd163, and Cd206) in RAW264.7 cells (Fig. 4B) but did not affect IFN-γ-induced expression of M1-Mφ genes (Arg II, Mcp-1, and Tnf-α) (Fig. 4C), suggesting that the effect of MMP8 on Mφ polarization is ApoE independent. To further confirm the functional importance of MMP8 in macrophage polarization in a truly wild-type genetic background, BMMφ from C57BL/6 were incubated with non-target shRNA or Mmp8 shRNA lentivirus and primed to M1- or M2-Mφ as described previously. A similar effect of Mmp8 knockdown was observed in these BMMφ with a truly wild-type genetic background (Fig. 4, D–F), further confirming the functional involvements of MMP8 in M2-Mφ polarization is ApoE independent.
FIGURE 4.
MMP8 plays a similar role in M2-Mφ polarization of Raw264.7 cells and BMMφ differentiated from C57BL/6 bone marrow monocytes. A, RT-qPCR analyses. RAW264.7 cells were infected with non-target or Mmp8 shRNA lentivirus and cultured in the presence of 5 ng/ml of M-CSF for 3 days. Gene expression levels for Mmp8, M1 (Arg II, Mcp-1, and Tnf-α), and M2-Mφ (Arg I, Cd163, and Cd206) genes were analyzed. B, IL-4 significant up-regulated M2-Mφ genes in control Raw264.7 cells, but not in Mmp8 knockdown Raw264.7 cells. C, IFN-γ significant up-regulated M1-Mφ genes. D, RT-qPCR analyses. BMMφ differentiated from C57BL/6 bone marrow monocytes were infected with non-target or Mmp8 shRNA lentivirus and cultured for another 2 days. Gene expression levels for Mmp8, M1, and M2-Mφ genes were analyzed. E, IL-4 significant up-regulated M2-Mφ genes in control BMMφ, but not in Mmp8 knockdown BMMφ. F, IFN-γ significant up-regulated M1-Mφ genes in both control and Mmp8 knockdown BMMφ. The data presented here are an average of three to four independent experiments. *, p < 0.05 (Mmp8 shRNA versus non-target shRNA); #, p < 0.05 (Mφ inducers versus control).
Conditioned Media of Wild-type Mφ Stimulated with IL-4 Induce M2-Mφ Gene Expression in MMP8KO Mφ
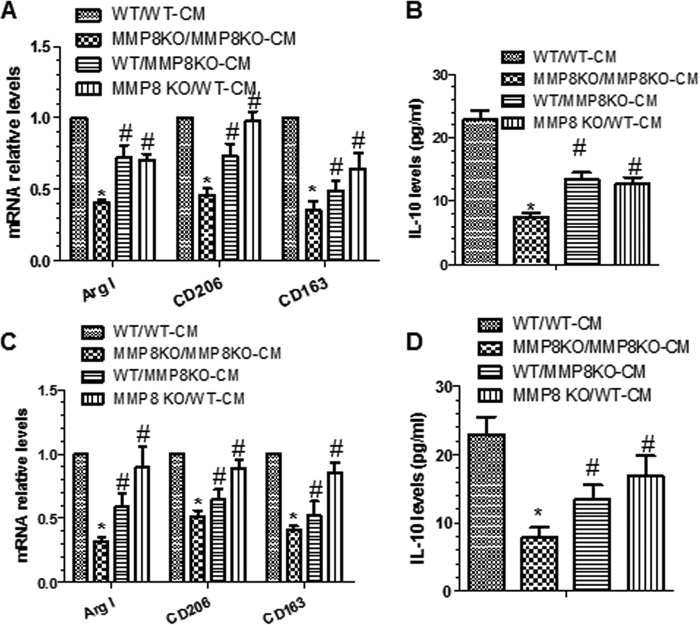
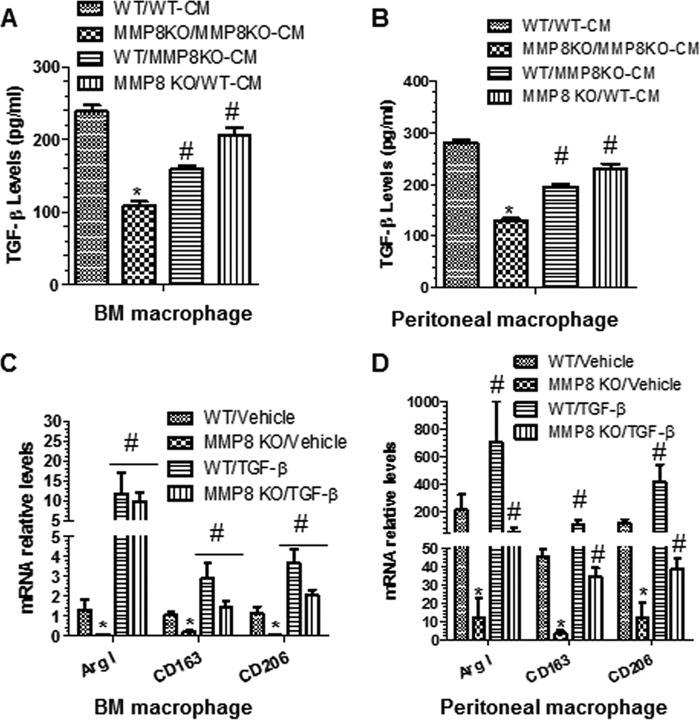
To investigate whether the difference in Mφ polarization between MMP8KO and WT arises from an effect of MMP8 on concentrations of molecules involved Mφ polarization, WT BMMφ was cultured with conditioned medium of MMP8KO BMMφ, and MMP8KO BMMφ was cultured with conditioned medium of WT BMMφ. The experiment showed that culturing WT BMMφ with conditioned medium of MMP8KO BMMφ resulted in a significant decrease in M2-Mφ genes/proteins (Fig. 5, A and B). In contrast, replacing the culture medium for MMP8KO BMMφ with conditioned medium of WT BMMφ almost restored the expression levels of M2-Mφ genes/proteins in MMP8-deficient Mφ (Fig. 5, A and B). An experiment using pMφ showed similar results (Fig. 5, C and D). These data suggest that the effect of MMP8 on M2-Mφ polarization is mediated by certain biological molecule(s) in medium.
FIGURE 5.
Conditioned medium from WT Mφ stimulated with IL-4 rescues M2-Mφ gene expression in MMP8KO Mφ. A and B, monocytes isolated from WT and MMP8KO bone marrow were cultured in the complete medium containing 5 ng/ml of M-CSF for 7 days, followed by IL-4 polarization for 24 h, then subjected to culture medium swapping (WT/MMP8KO-CM indicates that the culture medium for WT Mφ was replaced with the conditioned medium harvested from MMP8KO Mφ, whereas KO/WT-CM indicates that the culture medium for MMP8KO Mφ was replaced with the conditioned medium harvested from WT Mφ; WT/WT-CM and KO/KO-CM indicate that there were no culture medium swapping) and cultured for a further 24 h. Total RNA and culture medium were harvested at the end of medium swapping experiments and subjected to RT-qPCR (A) and ELISA (B) analyses, respectively. C and D, peritoneal macrophages isolated from WT and MMP8KO mice were cultured overnight in the complete medium containing 5 ng/ml of M-CSF, followed by IL-4 polarization for 24 h, then subjected to culture medium swapping as described above and cultured for a further 24 h. Total RNA and culture medium were harvested and subjected to RT-qPCR (C) and ELISA (D) analyses, respectively. The data presented here are an average of five independent experiments. *, p < 0.05 (MMP8KO versus WT); #, p < 0.05 (after versus before medium swapping).
The Effect of MMP8 on M2 Mφ Polarization Is Mediated by TGF-β1
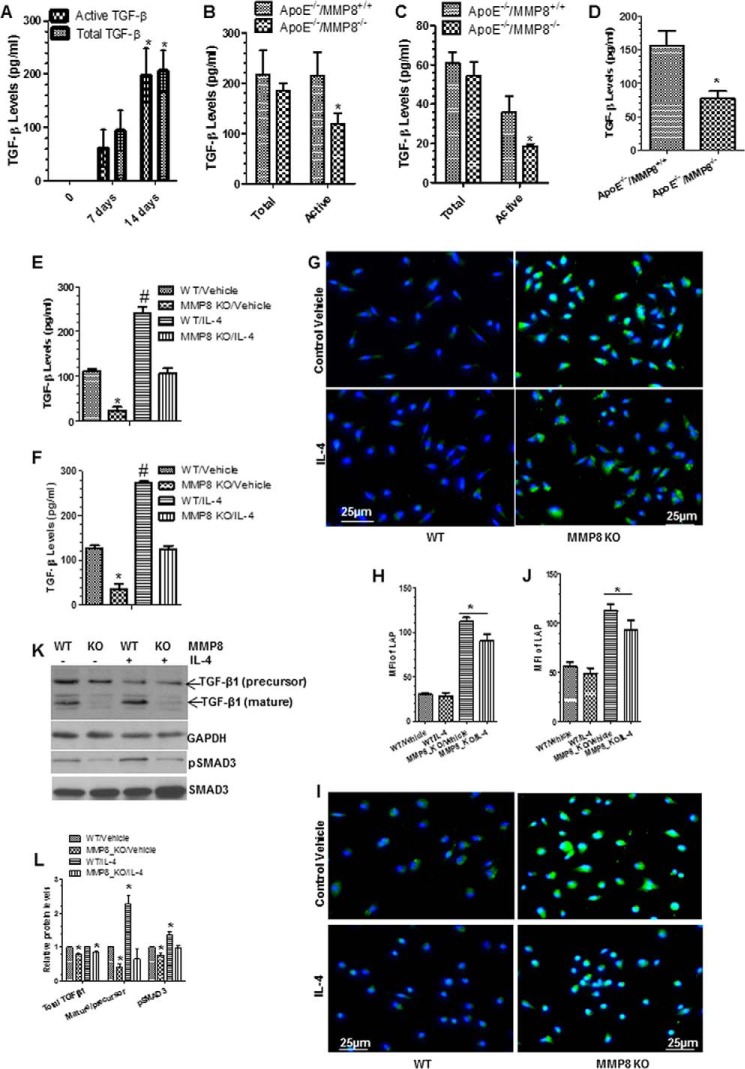
It has been reported that Tgf-β signaling plays a critical role in promoting IL-4-induced alternative Mφ activation (M2-Mφ polarization) (28). We found that TGF-β1 mRNA expression levels were significantly lower in MMP8-deficient BMMφ and pMφ than in WT BMMφ and pMφ (Figs. 2, A and F). To investigate whether the effect of MMP8 on M2 Mφ polarization was mediated by TGF-β1, the concentrations of total and active TGF-β1 in conditioned culture media of BMMφ and pMφ were measured by ELISA. The experiment showed that the levels of total and active TGF-β1 increased during Mφ differentiation (Fig. 6A), and that active TGF-β1 levels, but not total TGF-β1 levels, were lower in conditioned media of MMP8-deficient BMMφ and pMφ than in conditioned media of WT BMMφ and pMφ (Fig. 6, B and C). Importantly, a higher level of active TGF-β1 was also observed in the peritoneal cavity fluid of WT mice induced by Brewer thioglycollate medium injection compared with MMP8KO mice (Fig. 6D).
FIGURE 6.
Bioavailability of TGF-β was mediated by MMP8 during macrophage differentiation and polarization. Cells were cultured and treated as described previously. Conditioned culture medium (CM) were harvested and subjected to ELISA analyses. A, both total and active TGF-β levels were significantly increased during bone marrow macrophage differentiation from monocytes. B and C, active, not total TGF-β levels in MMP8-deficient BMMφ (B) and pMφ (C) were lower than that of WT macrophages. D, active TGF-β levels in the peritoneal cavity fluid of MMP8-deficient mice were lower than that of WT mice. E and F, active TGF-β levels in CM of WT and MMP8-deficient BMMφ (E) and pMφ (F) in response to IL-4 polarization. The data presented here are an average of three to four independent experiments. *, p < 0.05 (day 14 versus 7 or MMP8KO versus WT); #, p < 0.05 (Mφ inducers versus control). G-J, MMP8-deficiency results in higher amount of LAP on BMMφ (G and H) and pMφ (I and J). Cells were fixed and subjected to immunofluorescence staining analyses with antibody against LAP (N terminus of TGF-β1). Shown in the figure are representative images each from three independent experiments, and column charts of mean fluorescence intensity (mean ± S.E., n = 20). *, p < 0.05 (versus controls). K and L, Western analyses of the expression levels of TGF-β and pSMAD3. Proteins were harvested and subjected to Western blot analyses with the antibodies against the C terminus of TGF-β and pSMAD3 (phospho-Ser423/Ser425). Note: the molecular mass for the bands of TGF-β1 (precursor) and TGF-β1 (mature) in panel K are ∼37.5 and ∼12.5 kDa, respectively. GAPDH and total SMAD3 were included as internal controls. Shown in the figure are representative images each from three independent experiments, and column charts of relative protein levels (mean ± S.E., n = 3). *, p < 0.05 (versus WT/vehicle).
TGF-β1 is synthesized as a latent precursor and secreted from cells as a latent complex (an inactive form) containing latent TGF-β-binding protein and TGF-β pro-peptide called latency-associated peptide (LAP) (29, 30). Activation of latent TGF-β involved cleavage of LAP (29, 30). We found that IL-4 priming induced a significant increase in the levels of active TGF-β1 in WT BMMφ and pMφ, but not in MMP8-deficient BMMφ and pMφ (Fig. 6, E and F). Immunostaining with an antibody against LAP showed that MMP8-deficient BMMφ and pMφ expressed higher levels of LAP protein than both WT BMMφ and pMφ (Fig. 6, G–J). Western blot analyses showed a much higher level of TGF-β cleavage (ratio of the mature to precursor of TGF-β) occurred in the WT BMMφ, which was further increased by IL-4. No such induction by IL-4 was observed in MMP8-deficient BMMφ (Fig. 6, K and L). A moderate but significant lower level of total TGF-β was observed in the cell lysate of MMP8-deficient BMMφ (Fig. 6, K and L). Importantly, the protein level of phosphorylated SMAD3 (pSMAD3) was much lower in MMP8-deficient BMMφ than that of WT BMMφ, and IL-4 further increased the pSMAD3 protein level in WT BMMφ, but not in MMP8-deficient BMMφ (Fig. 6, K and L), suggesting that IL-4 induces M2-Mφ polarization through activation of TGF-β signaling, and such signaling is at least partially responsible for MMP8-mediated M2-Mφ polarization.
Furthermore, culturing MMP8-deficient BMMφ and pMφ with conditioned media of WT BMMφ and pMφ for 24 h increased active TGF-β1 levels similar to those in conditioned media of WT BMMφ and pMφ (Fig. 7, A and B). To further determine whether TGF-β1 mediates the effect of MMP8 on M2-Mφ polarization, MMP8KO and WT BMMφ were incubated with a recombinant active TGF-β1, followed by analyses of M2-Mφ genes. The experiment showed that incubation with the recombinant active TGF-β1 significantly increased the expression of M2-Mφ genes in both WT and MMP8KO BMMφ (Fig. 7, C and D). Taken together, the above data suggest that the effect of MMP8 deficiency on M2-Mφ polarization is, at least in part, due to reduced levels of active TGF-β1.
FIGURE 7.
TGF-β activity is responsible for MMP8-mediated M2 macrophage polarization. A and B, the protein levels of active TGF-β in CM of WT and MMP8-deficient BMMφ (A) and pMφ (B) after medium swapping as described in the legend to Fig. 5. C and D, exogenous active TGF-β significantly up-regulated M2-Mφ gene expression levels in both WT and MMP8-deficient BMMφ (C) and pMφ (D). The data presented here are an average of three to five independent experiments. *, p < 0.05 (MMP8KO versus WT); #, p < 0.05 (after versus before medium swapping, or Mφ inducers versus control).
MMP8 Increases TGF-β1 Bioavailability and Regulates M2-Mφ Polarization by Increasing Fibromodulin Cleavage
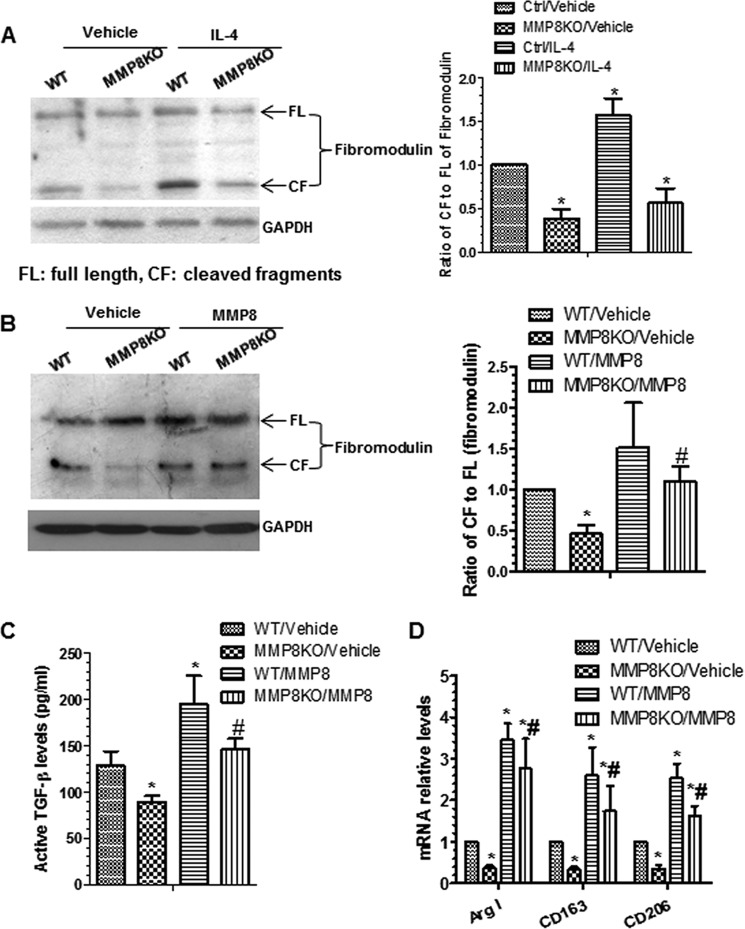
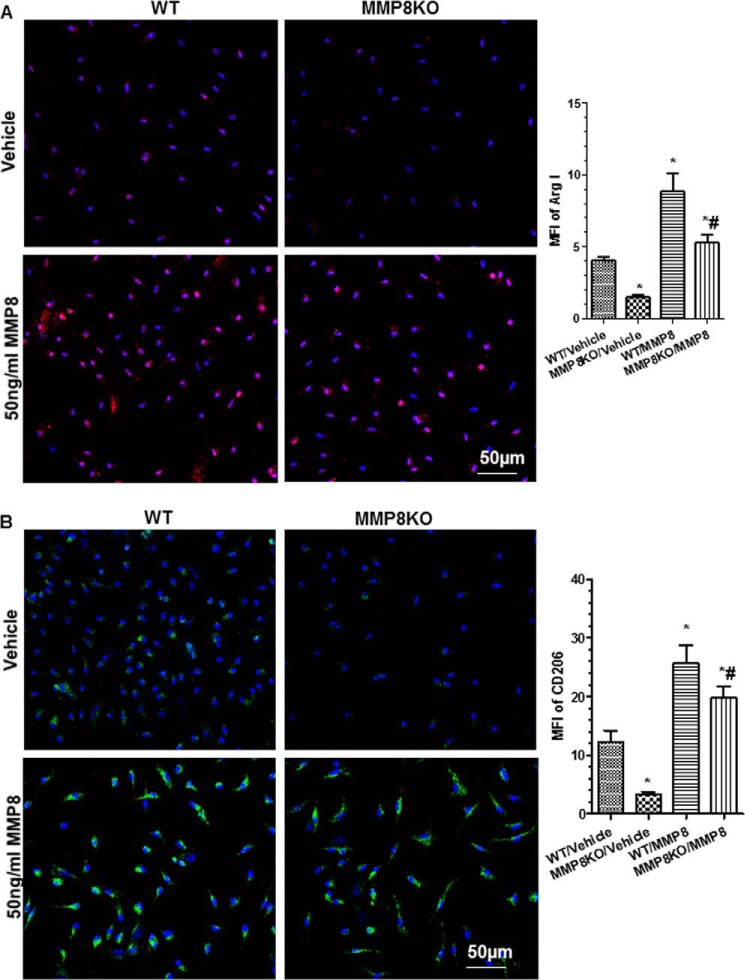
It has been reported that the matrix protein fibromodulin can interact with TGF-β1 and regulate TGF-β1 bioavailability/activity through sequestering TGF-β1 in the extracellular matrix (31). It has been suggested that MMPs can cleave fibromodulin (32). In this study, we found reduced cleavage of fibromodulin (lower ratio of the cleaved fragment to full-length fibromodulin) in MMP8-deficient Mφ (Fig. 8A) and that IL-4 priming induced fibromodulin cleavage in WT BMMφ but not in MMP8-deficient Mφ. Further experiments showed that incubation of Mφ with 50 ng/ml of recombinant activated MMP8 significantly increased fibromodulin cleavage as well as the levels of active TGF-β1, in conditioned culture media of both WT and MMP8-deficient Mφ (Fig. 8, B and C). Furthermore, incubation of Mφ with the recombinant activated MMP8 significantly up-regulated the expression of M2-Mφ genes in both WT and MMP8-deficient Mφ, as demonstrated by RT-qPCR (Fig. 8D) and immunostaining (Fig. 9), suggesting that MMP8 induces M2-Mφ polarization via cleavage of fibromodulin that affects TGF-β1 bioavailability.
FIGURE 8.
MMP8 increases TGF-β1 bioavailability and regulates M2-Mφ polarization by increasing fibromodulin cleavage. A, WT and MMP8KO BMMφ were incubated with IL-4 for 48 h. Proteins were harvested and subjected to Western blot analyses with antibodies against the C terminus of fibromodulin. Shown are representative images each from three independent experiments, and column charts of relative protein levels (mean ± S.E., n = 3). *, p < 0.05 (versus controls). B-D, MMP8 increases fibromodulin cleavage, TGF-β bioavailability, and rescues the M2-Mφ phenotype of MMP8-deficient Mφ. WT and MMP8KO BMMφ were incubated with vehicle or 50 ng/ml of MMP8 for 48 h. Cell lysates, conditioned culture medium, and total RNAs were harvested and subjected to Western blot (B), ELISA (C), and RT-qPCR (D) analyses, respectively. The data presented here are an average of three independent experiments. *, p < 0.05 (treatments versus WT/vehicle); #, p < 0.05 (MMP8 versus vehicle in MMP8KO Mφ).
FIGURE 9.
Exogenous MMP8 restores M2-Mφ polarization (functional properties) of MMP8-deficient Mφ. WT and MMP8 knock-out (MMP8KO) bone marrow (BM) Mφ were incubated with vehicle or 50 ng/ml of MMP8 for 48 h. Cells were fixed and subjected to immunofluorescence staining with antibodies against Arg I (A) and CD206 (B), respectively. Shown in the figure are representative images each from three independent experiments, and column charts of mean fluorescence intensity (MFI; mean ± S.E., n = 20) of Arg I (A) or CD206 (B) on Mφ. *, p < 0.05 (versus controls).
Functional Involvements of Fibromodulin and TGF-β Signaling in IL-4-mediated M2-Mφ Polarization
To further establish the causal link between fibromodulin and M2-Mφ polarization induced by IL-4, fibromodulin knockdown in BMMφ was conducted by using specific Fibromodulin siRNA. As expected, the endogenous expression level of Fibromodulin in BMMφ was successfully knocked down by Fibromodulin-specific siRNAs (Fig. 10A). Consequently, M2-Mφ-specific gene expression levels (Arg I, Cd163, and Cd206) were significantly up-regulated (Fig. 10A, 3rd bar versus 1st bar), such inductions were further increased in the presence of IL-4 (Fig. 10A, 3rd bar versus 4th bar), suggesting a functional role of fibromodulin in M2-Mφ polarization. Moreover, to investigate if TGF-β signaling plays a causal role in IL-4-induced M2-Mφ polarization, the TGF-β signaling specific inhibitor, SB-431542, was used to block activation of the TGF-β signal pathway in the BMMφ. As shown in Fig. 10B, the expression level of phosphorylated SMAD3 (pSMAD3), one of the key downstream effect genes of the TGF-β signal pathway, was significantly up-regulated by IL-4, whereas its induction was almost abolished by incubation with SB-431542. Expectedly, incubation with SB-431542 in BMMφ resulted in a decrease of M2-Mφ gene expressions, and importantly inhibition of the TGF-β signaling pathway almost abrogated M2-Mφ gene expressions induced by IL-4 (Fig. 10C), implying a functional role of TGF-β signaling in IL-4-induced M2-Mφ phenotype.
FIGURE 10.
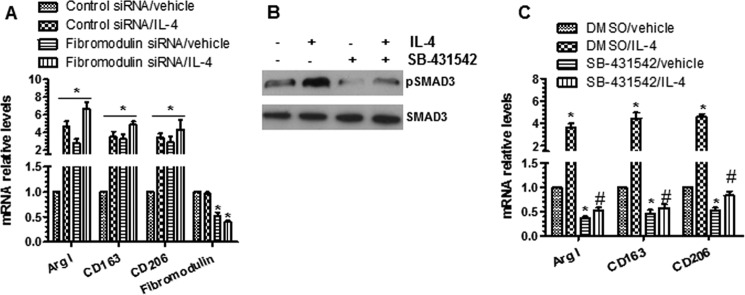
Fibromodulin and TGF-β signaling play a role in IL-4-mediated M2-Mφ polarization. A, knockdown of fibromodulin-promoted M2-Mφ polarization. BMMφ were transfected as control or Fibromodulin-specific siRNAs, followed by IL-4 priming. Total RNAs were harvested and subjected to RT-qPCR analyses. B and C, TGF-β activation is required for IL-4-induced M2-Mφ gene expression. BMMφ were preincubated with 10 μm SB-431542 for 3 h, followed by IL-4 priming. Cell lysate and total RNAs were harvested and subjected to Western blot (B) and RT-qPCR (C) analyses, respectively. The data presented here are an average or representative of three independent experiments. *, p < 0.05 (versus control siRNA/vehicle or DMSO/vehicle); #, p < 0.05 (versus DMSO/IL-4).
Discussion
The key novel finding of this study is that MMP8 deficiency has an effect on Mφ differentiation and polarization, favoring M1 over M2, suggesting a particular role of MMP8 in M2-Mφ differentiation and polarization. This study indicates that this effect is related to fibromodulin cleavage and therefore increased bioavailability of TGF-β1 that induces M2-Mφ. This finding is relevant for the understanding of regulatory mechanisms for Mφ differentiation and polarization, and for the understanding of the pathogenesis of inflammatory conditions in which MMP8 is implicated.
Previous studies have shown that MMP8 has a protective effect against acute hepatitis (20), acute lung injury (14), airway inflammation (16), and cancers (21). These studies have demonstrated that the protective effect of MMP8 in these conditions is related to its influence on the behavior and function of neutrophils (14, 16, 21) or of eosinophils in the case of airway inflammation (16). The finding of the present study raises the possibility that the effect of MMP8 in some of these conditions might also be related to its role in M2-Mφ polarization, which warrants further investigations.
There is also substantial evidence from experimental and clinical studies indicating MMP8 promotes atherosclerosis and a number of atherosclerosis-related conditions including atherosclerotic plaque rupture leading to myocardial infarction, heart failure after myocardial infarction, neointima formation following angioplasty, and abdominal aortic aneurysm. The pathogenesis of atherosclerosis involves a number of cell types, with endothelial cells, macrophages, lymphocytes, and smooth muscle cells having been studied most. The finding of the present study that MMP8 promotes M2-Mφ polarization seems at odds with the finding that MMP8 promotes atherosclerosis, because inflammation plays an important role in atherogenesis and because M2-Mφ are anti-inflammatory. However, atherogenesis also involves a number of other cell types, and previous studies have indicated that MMP8 can promote atherosclerosis partly via its influences on migration and proliferation of stem/progenitor cells (19), smooth muscle cells (17), and endothelial cells (18), as well as leukocyte recruitment (11). Thus, it appears that MMP8 can influence atherosclerosis via multiple mechanisms.
Several other MMPs have been reported to have an effect on Mφ polarization (23, 33). A recent study showed that Mmp28 gene inactivation impaired M2-Mφ polarization and resulted in an aggravated cardiac dysfunction after myocardial infarction in mice (23). Another study showed that loss of MMP28 reduced M2 polarization and protection from bleomycin-induced fibrosis (33). It has also been shown that loss of MMP7 resulted in M1-Mφ polarization within Helicobacter pylori-infected stomachs, and Mφ isolated from MMP7-deficient mice infected with H. pylori expressed significantly higher levels of the M1-Mφ marker IL-1β compared with Mφ isolated from WT mice (34). Furthermore, MMP1, MMP3, and MMP10 have been found to be highly expressed in M1-Mφ, whereas MMP12 has been found to be strongly expressed in M2-Mφ (35). It has also been shown that classical activation of mouse Mφ increased the expression of MMP13, MMP14, and MMP25 but decreased MMP19 and TIMP2, whereas alternative activation with IL-4 increased MMP19 expression (36). Taken together, findings from previous and present studies indicate that various MMPs play important and divergent roles in macrophage polarization. It would be very interesting to study the relationships between these reported MMPs and MMP8 in IL-4-induced M2-Mφ polarization. To this aim, we first examined if the expression levels of these MMPs were regulated by IL-4 in our cell culture system. Our data showed that both Mmp12 and Mmp19 were significantly up-regulated by IL-4 treatment, whereas the expression levels of Mmp7 and Mmp28 were not dramatically changed upon IL-4 treatment (data not shown). Interestingly, the expression levels of Mmp12 and Mmp28 were much lower in MMP8-deficient Mφ than that of WT Mφ, whereas Mmp7 and Mmp19 were mildly increased in MMP8-deficient Mφ compared with that in WT Mφ (data not shown), suggesting a potential role for these MMPs in MMP8-mediated M2-Mφ polarization, which warrants further investigation.
TGF-β signaling has recently been suggested to play a central role in promoting M2-Mφ activation (28). TGF-β1 is synthesized as a latent precursor and secreted from cells as a latent complex. Once secreted from cells, the TGF-β1 latent complex is sequestered in the extracellular matrix through binding to matrix proteins (29, 30). Proteolytic degradation of the LAP or the bound matrix proteins by specific proteolytic enzymes such as MMPs, or mechanical stretching of cell-surface integrins, release active TGF-β and allow it to interact with TGF-β receptors and induce transcription of TGF-β-responsive genes by initiating the canonical Smad-dependent pathway and/or non-canonical pathways involving MAPK and RhoA (37). In the current study, we provided several lines of evidence to support the notion that MMP8 mediated M2-Mφ differentiation and polarization through activating the TGF-β signal pathway. First, our data showed that the gene expression levels (Fig. 2, A and F) and total cellular protein levels (Fig. 6, K and L) of TGF-β were much higher in WT Mφ than that of MMP8KO Mφ. Second, compared with WT, a decreased level of TGF-β, particularly the active form of TGF-β, was observed in the conditioned culture medium of MMP8KO Mφ (Fig. 6, B and C) and in the peritoneal cavity of MMP8KO mice (Fig. 6D). Third, compared with the WT Mφ the level of pSMAD3 was much lower in MMP8-deficient Mφ (Fig. 6, K and L), suggesting that TGF-β signaling is suppressed in MMP8-deficient Mφ. Fourth, exogenous TGF-β could restore the inhibitory effects of Mmp8 gene inactivation on M2-Mφ gene expression (Fig. 7, C and D). Finally, our data also revealed that the TGF-β signalling pathway at least partially was responsible for IL-4-induced M2-Mφ polarization (Fig. 10, B and C). Interestingly, we observed a more significant inhibitory effect of Mmp8 gene inactivation on Tgf-β mRNA expression (Fig. 2, A and F) than the total TGF-β protein level in conditioned culture medium (Fig. 6, B and C), indicating a role for MMP8 in TGF-β post-transcription and/or post-translational regulation, or in modulating TGF-β protein secretion from cells, which remains to be investigated in a future study. Furthermore, it would be interesting to investigate if MMP8 could affect TGF-β dimmer formation. For such a purpose, we have applied different non-reduced and/or non-denatured conditions in our Western blot analyses (e.g. omitting β-mercaptoethanol and/or SDS in loading buffer or running buffer, boiling or not boiling samples), which is prerequisite for detecting the protein dimers in whole cell lysate, but we were unsuccessful to obtain any meaningful data under these conditions. Although we have failed to show the effects of MMP8 deficiency on TGFβ1 dimmer formation, theoretically, the TGFβ1 protein dimer level will be much lower in the MMP8KO macrophage due to the facts that 1) a much lower level of TGFβ1 monomer has been observed in MMP8KO macrophages (Fig. 6K); and 2) there has been no report to suggest that MMP8 plays a role in protein dimmer formation through disulfide bond(s).
Studies have indicated that the matrix protein fibromodulin can interact with TGF-β1 and regulate TGF-β1 bioavailability/activity through sequestering TGF-β1 in the extracellular matrix (31, 38–40). Other studies have shown that MMP2, MMP8, MMP9, and MMP13 can cleave fibromodulin (32). In line with these findings, our present study indicates that MMP8 induces M2-Mφ differentiation and polarization via degrading fibromodulin and increasing the bioavailability of the M2-Mφ inducer TGF-β1. It is noteworthy to mention that one most recent study (41) has suggested an opposite role for MMP8 in regulating TGF-β signaling in breast cancer cells and metastasis progression. Soria-Valles et al. (41) reported that MMP8 inhibited tumor growth and breast cancer cell lung metastasis through supressing TGF-β signaling via cleaving and releasing another matrix protein, decorin. Subsequently, the cleaved decorin by MMP8 can bind to soluble TGF-β and prevent its interaction with TGF-β receptors. Therefore, it would be plausible to speculate that MMP8 may play a distinct role in the TGF-β signaling pathway in different cellular contexts or the regulatory role of MMP8 in the TGF-β signal pathway is likely substrate-dependent. It also would be very interesting to investigate the expression levels and cleavage of decorin by MMP8 in Mφ, however, such investigations are beyond the remit of the current study.
In conclusion, we have identified a novel role of MMP8, i.e. in M2-Mφ differentiation and polarization. The findings of this study are useful for understanding the biological functions of MMP8, the mechanisms for Mφ differentiation and polarization, and the pathogenesis of pathological conditions in which MMP8 is involved.
Author Contributions
G. W. designed and performed the experiments and analyzed the data. C. Z. and Q. C. performed and analyzed RT-qPCR experiments. L. A. L. performed microscopy experiments. A. M. isolated and cultured peritoneal Mφ. S. Y. supervised the study and wrote the manuscript. Q. X. conceived the study, analyzed the data, supervised the study and wrote the manuscript.
Acknowledgments
This work forms part of the research themes contributing to the translational research portfolio of Barts and the London Cardiovascular Biomedical Research Unit, which is supported and funded by the National Institute of Health Research.
This work was supported by British Heart Foundation Grants FS/09/044/28007, PG/11/40/28891, PG/13/45/30326, and PG/15/11/31279. The authors declare that they have no conflicts of interest with the contents of this article.
- Mφ
- macrophages
- MMP8
- matrix metalloproteinase-8
- MCP-1
- monocyte chemoattractant protein-1
- BMMφ
- bone marrow monocyte-derived macrophage
- pMφ
- peritoneal naive macrophage
- qPCR
- quantitative PCR
- LAP
- latency-associated peptide
- CM
- culture medium.
References
- 1. Wolfs I. M., Donners M. M., de Winther M. P. (2011) Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb. Haemost. 106, 763–771 [DOI] [PubMed] [Google Scholar]
- 2. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shimada K. (2009) Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ. J. 73, 994–1001 [DOI] [PubMed] [Google Scholar]
- 4. Williams H. J., Fisher E. A., Greaves D. R. (2012) Macrophage differentiation and function in atherosclerosis: opportunities for therapeutic intervention? J. Innate Immun. 4, 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mantovani A., Garlanda C., Locati M. (2009) Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 29, 1419–1423 [DOI] [PubMed] [Google Scholar]
- 6. Johnson J. L., Newby A. C. (2009) Macrophage heterogeneity in atherosclerotic plaques. Curr. Opin. Lipidol. 20, 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butcher M. J., Galkina E. V. (2012) Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front. Physiol. 3, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas S. K., Mantovani A. (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 11, 889–896 [DOI] [PubMed] [Google Scholar]
- 9. Sica A., Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Lint P., Libert C. (2006) Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 17, 217–223 [DOI] [PubMed] [Google Scholar]
- 11. Laxton R. C., Hu Y., Duchene J., Zhang F., Zhang Z., Leung K. Y., Xiao Q., Scotland R. S., Hodgkinson C. P., Smith K., Willeit J., López-Otín C., Simpson I. A., Kiechl S., Ahluwalia A., Xu Q., Ye S. (2009) A role of matrix metalloproteinase-8 in atherosclerosis. Circ. Res. 105, 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Den Steen P. E., Wuyts A., Husson S. J., Proost P., Van Damme J., Opdenakker G. (2003) Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 270, 3739–3749 [DOI] [PubMed] [Google Scholar]
- 13. Cox J. H., Dean R. A., Roberts C. R., Overall C. M. (2008) Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J. Biol. Chem. 283, 19389–19399 [DOI] [PubMed] [Google Scholar]
- 14. Quintero P. A., Knolle M. D., Cala L. F., Zhuang Y., Owen C. A. (2010) Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1α to reduce acute lung inflammation and injury in mice. J. Immunol. 184, 1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owen C. A., Hu Z., Lopez-Otin C., Shapiro S. D. (2004) Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J. Immunol. 172, 7791–7803 [DOI] [PubMed] [Google Scholar]
- 16. Gueders M. M., Balbin M., Rocks N., Foidart J. M., Gosset P., Louis R., Shapiro S., Lopez-Otin C., Noël A., Cataldo D. D. (2005) Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J. Immunol. 175, 2589–2597 [DOI] [PubMed] [Google Scholar]
- 17. Xiao Q., Zhang F., Grassia G., Hu Y., Zhang Z., Xing Q., Yin X., Maddaluno M., Drung B., Schmidt B., Maffia P., Ialenti A., Mayr M., Xu Q., Ye S. (2014) Matrix metalloproteinase-8 promotes vascular smooth muscle cell proliferation and neointima formation. Arterioscler. Thromb. Vasc. Biol. 34, 90–98 [DOI] [PubMed] [Google Scholar]
- 18. Fang C., Wen G., Zhang L., Lin L., Moore A., Wu S., Ye S., Xiao Q. (2013) An important role of matrix metalloproteinase-8 in angiogenesis in vitro and in vivo. Cardiovasc. Res. 99, 146–155 [DOI] [PubMed] [Google Scholar]
- 19. Xiao Q., Zhang F., Lin L., Fang C., Wen G., Tsai T. N., Pu X., Sims D., Zhang Z., Yin X., Thomaszewski B., Schmidt B., Mayr M., Suzuki K., Xu Q., Ye S. (2013) Functional role of matrix metalloproteinase-8 in stem/progenitor cell migration and their recruitment into atherosclerotic lesions. Circ. Res. 112, 35–47 [DOI] [PubMed] [Google Scholar]
- 20. Van Lint P., Wielockx B., Puimège L., Noël A., López-Otin C., Libert C. (2005) Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J. Immunol. 175, 7642–7649 [DOI] [PubMed] [Google Scholar]
- 21. Balbín M., Fueyo A., Tester A. M., Pendás A. M., Pitiot A. S., Astudillo A., Overall C. M., Shapiro S. D., López-Otín C. (2003) Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 35, 252–257 [DOI] [PubMed] [Google Scholar]
- 22. Ray A., Dittel B. N. (2010) Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 10.3791/1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma Y., Halade G. V., Zhang J., Ramirez T. A., Levin D., Voorhees A., Jin Y. F., Han H. C., Manicone A. M., Lindsey M. L. (2013) Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ. Res. 112, 675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo Z., Wen G., Wang G., Pu X., Ye S., Xu Q., Wang W., Xiao Q. (2013) MicroRNA-200C and -150 play an important role in endothelial cell differentiation and vasculogenesis by targeting transcription repressor ZEB1. Stem Cells 31, 1749–1762 [DOI] [PubMed] [Google Scholar]
- 25. Xiao Q., Luo Z., Pepe A. E., Margariti A., Zeng L., Xu Q. (2009) Embryonic stem cell differentiation into smooth muscle cells is mediated by Nox4-produced H2O2. Am. J. Physiol. Cell Physiol. 296, C711–723 [DOI] [PubMed] [Google Scholar]
- 26. Xiao Q., Zeng L., Zhang Z., Hu Y., Xu Q. (2007) Stem cell-derived Sca-1+ progenitors differentiate into smooth muscle cells, which is mediated by collagen IV-integrin alpha1/beta1/alphav and PDGF receptor pathways. Am. J. Physiol. Cell Physiol. 292, C342–352 [DOI] [PubMed] [Google Scholar]
- 27. Baitsch D., Bock H. H., Engel T., Telgmann R., Müller-Tidow C., Varga G., Bot M., Herz J., Robenek H., von Eckardstein A., Nofer J. R. (2011) Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler. Thromb. Vasc. Biol. 31, 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gong D., Shi W., Yi S. J., Chen H., Groffen J., Heisterkamp N. (2012) TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 13, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Annes J. P., Munger J. S., Rifkin D. B. (2003) Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 [DOI] [PubMed] [Google Scholar]
- 30. Rifkin D. B. (2005) Latent transforming growth factor-β (TGF-β) binding proteins: orchestrators of TGF-β availability. J. Biol. Chem. 280, 7409–7412 [DOI] [PubMed] [Google Scholar]
- 31. Hildebrand A., Romarís M., Rasmussen L. M., Heinegård D., Twardzik D. R., Border W. A., Ruoslahti E. (1994) Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem. J. 302, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heathfield T. F., Onnerfjord P., Dahlberg L., Heinegård D. (2004) Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J. Biol. Chem. 279, 6286–6295 [DOI] [PubMed] [Google Scholar]
- 33. Gharib S. A., Johnston L. K., Huizar I., Birkland T. P., Hanson J., Wang Y., Parks W. C., Manicone A. M. (2014) MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J. Leukoc. Biol. 95, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krakowiak M. S., Noto J. M., Piazuelo M. B., Hardbower D. M., Romero-Gallo J., Delgado A., Chaturvedi R., Correa P., Wilson K. T., Peek R. M., Jr. (2015) Matrix metalloproteinase 7 restrains Helicobacter pylori-induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization. Oncogene 34, 1865–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roch T., Akymenko O., Krüger A., Jung F., Ma N., Lendlein A. (2014) Expression pattern analysis and activity determination of matrix metalloproteinase derived from human macrophage subsets. Clin. Hemorheol. Microcirc. 58, 147–158 [DOI] [PubMed] [Google Scholar]
- 36. Hayes E. M., Tsaousi A., Di Gregoli K., Jenkinson S. R., Bond A. R., Johnson J. L., Bevan L., Thomas A. C., Newby A. C. (2014) Classical and alternative activation and metalloproteinase expression occurs in foam cell macrophages in male and female ApoE null mice in the absence of T and B lymphocytes. Front. Immunol. 5, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horiguchi M., Ota M., Rifkin D. B. (2012) Matrix control of transforming growth factor-β function. J. Biochem. 152, 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soo C., Hu F. Y., Zhang X., Wang Y., Beanes S. R., Lorenz H. P., Hedrick M. H., Mackool R. J., Plaas A., Kim S. J., Longaker M. T., Freymiller E., Ting K. (2000) Differential expression of fibromodulin, a transforming growth factor-β modulator, in fetal skin development and scarless repair. Am. J. Pathol. 157, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Embree M. C., Kilts T. M., Ono M., Inkson C. A., Syed-Picard F., Karsdal M. A., Oldberg A., Bi Y., Young M. F. (2010) Biglycan and fibromodulin have essential roles in regulating chondrogenesis and extracellular matrix turnover in temporomandibular joint osteoarthritis. Am. J. Pathol. 176, 812–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng Z., Lee K. S., Zhang X., Nguyen C., Hsu C., Wang J. Z., Rackohn T. M., Enjamuri D. R., Murphy M., Ting K., Soo C. (March 6, 2014) Fibromodulin-deficiency alters temporospatial expression patterns of transforming growth factor-β ligands and receptors during adult mouse skin wound healing. PLoS ONE 9, e90817, 1–14, 10.1371/journal.pone.0090817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soria-Valles C., Gutiérrez-Fernández A., Guiu M., Mari B., Fueyo A., Gomis R. R., López-Otín C. (2014) The anti-metastatic activity of collagenase-2 in breast cancer cells is mediated by a signaling pathway involving decorin and miR-21. Oncogene 33, 3054–3063 [DOI] [PubMed] [Google Scholar]