Background: Promoter hypermethylation affects the regulation of transcription factors for target genes.
Results: SP1 activates FOXF2 transcription, but this activation is prevented through FOXF2 promoter methylation.
Conclusion: FOXF2 transcription is regulated through the combined effects of DNA methylation and SP1 transcriptional regulation.
Significance: Herein, we describe a new regulatory mechanism for the subtype-specific expression of FOXF2 in breast cancer.
Keywords: breast cancer, DNA methylation, transcription factor, transcriptional regulation, transcription target gene
Abstract
FOXF2 (forkhead box F2) is a mesenchyme-specific transcription factor that plays a critical role in tissue homeostasis through the maintenance of epithelial polarity. In a previous study, we demonstrated that FOXF2 is specifically expressed in basal-like breast cancer (BLBC) cells and functions as an epithelial-mesenchymal transition suppressor. FOXF2 deficiency enhances the metastatic ability of BLBC cells through activation of the epithelial-mesenchymal transition program, but reduces cell proliferation. In this study, we demonstrate that CpG island methylation of the FOXF2 proximal promoter region is involved in the regulatory mechanism of the subtype-specific expression of FOXF2 in breast cancer cells. DNMT1, DNMT3A, and DNMT3B commonly or individually contributed to this DNA methylation in different breast cancer cells. SP1 regulated the transcriptional activity of FOXF2 through direct binding to the proximal promoter region, whereas this binding was abrogated through DNA methylation. FOXF2 mediated the SP1-regulated suppression of progression and promotion of proliferation of non-methylated BLBC cells. Thus, we conclude that the subtype-specific expression and function of FOXF2 in breast cancer cells are regulated through the combined effects of DNA methylation and SP1 transcriptional regulation.
Introduction
FOXF2, a member of the FOX transcription factor superfamily, is expressed in the mesenchymal cells adjacent to epithelial cells and has pleiotropic regulatory functions in tissue-specific gene expression patterns during embryogenesis and tissue development (1), extracellular matrix synthesis (2), and epithelial-mesenchymal interactions (3). Recent reports have indicated that FOXF2 functions as a tumor suppressor in prostate cancer (4, 5) and breast cancer (6). We also have provided clinical evidence that FOXF2 underexpression is correlated with early-onset metastasis and poor prognosis in patients with histological grade II and triple-negative breast cancer (7). Further experimental evidence shows that FOXF2 is specifically expressed in basal-like breast cancer (BLBC)2 cells and functions as an epithelial-mesenchymal transition suppressor; FOXF2 deficiency enhances the metastatic ability of BLBC cells through activation of the epithelial-mesenchymal transition program by up-regulating TWIST1 transcription. In contrast to its metastasis-suppressing properties in BLBC cells, FOXF2 also has a tumor growth-promoting property (8). Nevertheless, the regulatory mechanism of the subtype-specific expression and dual functions of FOXF2 in breast cancer cells remains unknown.
DNA methylation is a common epigenetic modification that results in the heritable regulation of gene expression without any change in DNA sequence. Promoter hypermethylation is associated with transcriptional suppression through prevention of the binding of positive transcription factors to their recognition elements (9). In addition, the cell type-specific regulation of gene expression likely depends on methylation within the CpG context (10). Accumulating evidence demonstrates that abnormal DNA methylation is involved in cancer progression in various human cancers (11–13). Recently, Dunwell et al. (14) screened out FOXF2 as a candidate frequently methylated gene in childhood acute lymphoblastic leukemia samples and cell lines as well as in multiple epithelial cancer cell lines. This result implies that methylation of the FOXF2 gene might be a frequent event in human cancers. Thus, we speculated that DNA methylation contributes to the silencing of FOXF2 expression, which is involved in cancer development and progression. Currently, whether and how DNA methylation affects the transcription and function of FOXF2 in cancer cells remain unknown.
In this study, we identified a CpG island in the proximal promoter region of FOXF2 and showed that the transcription of FOXF2 in breast cancer is associated with hypermethylation of the proximal promoter region of this gene. The DNA methylation of FOXF2 is regulated by specific DNA methyltransferases (DNMTs) in different breast cancer cells. Furthermore, we demonstrate that FOXF2 is transcriptionally activated by SP1, whereas methylation of the CpG island in the proximal promoter region of FOXF2 leads to abrogation of SP1 binding. In addition, we found that FOXF2 mediates the SP1-regulated suppression of progression and promotion of proliferation of non-methylated BLBC cells.
Experimental Procedures
Cell Culture
The breast cancer cell lines MCF-7, MDA-MB-453, and MDA-MB-231 and the human mammary epithelial cell line MCF-10A were obtained from American Type Culture Collection (Manassas, VA). All cell lines were cultured as described previously (15).
Tissue Specimens
A total of 20 primary breast cancer tissue specimens were obtained from patients diagnosed with invasive ductal carcinoma who underwent breast surgery in the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). The protocol and use of the specimens in this study were approved by the Institutional Review Board, and written consent was obtained from all participants.
Drug Treatments
To determine the methylation regulation of FOXF2 expression, the MCF-7, MDA-MB-453, MDA-MB-231, and MCF-10A cell lines were treated with the DNA-demethylating agent 5-aza-2′-deoxycytidine (AZA; Sigma-Aldrich). For dose-response experiments, the cultured cells were treated with AZA at 0.5, 1.0, 1.5, 2.0, and 2.5 μm for 4 days. The drug-containing medium was changed every 24 h. After the drug treatments, the cells were washed with PBS and harvested to measure gene expression. To determine the functional relevance of the transcription factor SP1 to FOXF2 expression, MDA-MB-231 and MCF-10A cells were treated with mithramycin A (Sigma-Aldrich), a selective inhibitor of SP1-mediated transcriptional activation, at 50, 100, and 200 nm for 24 h, and the cells were subsequently harvested to measure gene and protein expression.
Plasmid Construction, siRNA, and Transfection
Human full-length FOXF2 and SP1 cDNAs were each subcloned into the pcDNA3.1 vector. siRNAs targeting the human SP1 (siSP1), FOXF2 (siFOXF2), DNMT1, DNMT3A, and DNMT3B genes and a non-targeting control siRNA (siControl) were purchased from RiboBio Co., Ltd. (Guangzhou, China). For gain or loss of gene expression in breast cancer cells, the gene expression plasmid and vector control or the targeting siRNA and siControl were transiently transfected into cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Chromatin Immunoprecipitation
The ChIP assay was performed using a ChIP assay kit (Millipore). Briefly, the cells were fixed with 1% formaldehyde at 37 °C for 10 min, washed twice with ice-cold PBS containing protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 1 μg/ml pepstatin A), scraped, pelleted, and resuspended in ChIP lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris, and protease inhibitor, pH 8.1). The cell lysate was subjected to sonication and then incubated overnight with anti-SP1 (Millipore), anti-DNMT1, anti-DNMT3A, or anti-DNMT3B (Santa Cruz Biotechnology) antibody, followed by incubation with a 50% slurry of protein A-agarose/salmon sperm DNA for 3 h at 4 °C. Normal rabbit IgG (Millipore) was used as a negative control. After a series of washes, the bound DNA-protein complexes were eluted, and the cross-linking was reversed. The proximal promoter region of FOXF2 in the resulting DNA fragments was PCR-amplified. The ChIP-PCR products were revealed by electrophoresis on 2% agarose gel.
Dual-Luciferase Reporter Assay
To obtain luciferase reporter constructs containing the FOXF2 proximal promoter region with a deletion of the SP1 regulatory element ranging from −960 to +128, −503 to +128, −247 to +128, or −64 to +128 relative to the transcription start site (TSS), the sequences were amplified from the genomic DNA of MCF-10A cells and subsequently inserted into the pGL3-Basic vector (Promega), a promoterless luciferase expression vector, between the XhoI and HindIII restriction sites (designated as pGL3-P1, pGL3-P2, pGL3-P3, and pGL3-P4, respectively). Three site-directed mutant constructs (pGL3-MT1, pGL3-MT2, and pGL3-MT1/2) based on the pGL3-P1 structure were generated using a Fast mutagenesis system (TransGen Biotech Co., Ltd., Beijing, China). For analysis of promoter activation, the cells were plated at a density of 2 × 105 cells/well in 24-well plates. siSP1 or siControl was cotransfected with FOXF2 promoter constructs and the internal control pRL-TK. Firefly and Renilla luciferase activities were measured 48 h post-transfection using a Dual-Luciferase reporter assay system (Promega). Relative promoter activation is represented as the ratio of firefly to Renilla luciferase activity.
RNA Extraction and Quantitative RT-PCR (qRT-PCR)
Total RNA isolation from cultured cells, RT, quantitative PCR, and quantification of target gene expression were performed as described previously (7). GAPDH mRNA expression was used as an internal control for normalization of target gene expression.
DNA Extraction and Bisulfite Sequencing PCR
Genomic DNA was isolated from cultured cells or frozen tissue specimens using a genomic DNA isolation kit (Thermo Scientific). The DNA (2 mg) was converted using an EpiTect bisulfite kit (Qiagen). The bisulfite-converted genomic DNA fragments of the FOXF2 proximal promoter region ranging from −655 to +114 relative to the TSS were PCR-amplified. The PCR products were resolved on a 1% agarose gel, gel-purified using a QIAquick gel extraction kit (Qiagen), and cloned using a pGEM-T easy vector system (Promega). Ten colonies from each ligation were randomly selected and sequenced using an ABI 3730xl genetic analyzer (Applied Biosystems). The methylation status of each CpG site was determined by assessing the presence of T (non-methylated) versus C (methylated).
Influence of CpG Island Methylation Status on FOXF2 Promoter Activity
To investigate the influence of CpG island methylation on FOXF2 promoter activity, the CpG island of FOXF2 (−655 to +128) was amplified from the genomic DNA of MCF-10A cells. The amplified promoter fragments were gel-purified as described above and cloned into pGL3-Basic. The enriched fragments were removed from pGL3-Basic and methylated in vitro using SssI, HhaI, and HpaII methylases (New England Biolabs) or no enzyme (mock) according to the manufacturer's instructions. The methylation efficiency was confirmed by restriction enzyme digestion using McrBC, HhaI, and HpaII (New England Biolabs). Furthermore, the methylated or mock-methylated fragments were religated into pGL3-Basic to assess differentially methylated promoter activity.
Cell Proliferation, Migration, and Invasion Assays
Cell proliferation was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded at a density of 2 × 103 cells/well onto 96-well plates. On days 1, 2, 3, 4, and 5, the cells were incubated with 10 μl of MTT solution (5 mg/ml in PBS) at 37 °C for 4 h. After removal of the medium, 150 μl of dimethyl sulfoxide was added to each well, and the absorbance was measured at 570 nm. Cell viability was calculated as the value relative to control cultures. The invasion and migration of breast cells in vitro were assessed using Matrigel-coated and uncoated Transwell inserts (BD Biosciences), respectively, as described previously (15). The number of invading or migrating cells was counted in five predetermined fields for each membrane using a microscope at ×400 magnification.
Immunoblotting
Immunoblotting was performed as described previously (15). The primary antibodies used were anti-SP1, anti-FOXF2 (Abnova, Taipei, Taiwan), anti-DNMT1, anti-DNMT3A, anti-DNMT3B, and anti-β-actin (Sigma-Aldrich).
Gene Expression Profiling Data Set and Data Analysis
The gene expression profiling data set from 427 breast cancer tissues with Gene Expression Omnibus accession number GSE25066 (16) was used to analyze the relationship between combined SP1 (probe set 214732_at) and FOXF2 (probe set 206377_at) mRNA expression levels and the distant metastasis-free survival (DMFS) of the patient. Among the 427 breast cancer patients, 189 cases were classified into the basal-like subtype, and 238 cases were classified into the luminal subtype based on the PAM50 signature; 110 cases developed distant metastasis within a 5-year follow-up.
Statistical Analysis
Data from the in vitro experiments are presented as the mean ± S.D. Student's t test and the rank sum test were used to compare the differences between the experimental and control groups, as well as the difference in the methylation rates of breast cancer tissues between the high and low FOXF2 mRNA-expressing groups. The receiver operating characteristic curves were generated based on the SP1 or FOXF2 mRNA levels of samples and the corresponding DMFS status of the patients. The optimal cutoff value selected based on the receiver operating characteristic curve was used to group patients with different SP1 and FOXF2 expression levels. Kaplan-Meier survival analysis was used to compare the DMFS status in patients with different SP1 and FOXF2 expression levels. Statistical significance was defined as p < 0.05.
Results
Expression of FOXF2 in Breast Cancer Cells Is Inversely Correlated with CpG Island Methylation of Its Proximal Promoter Region
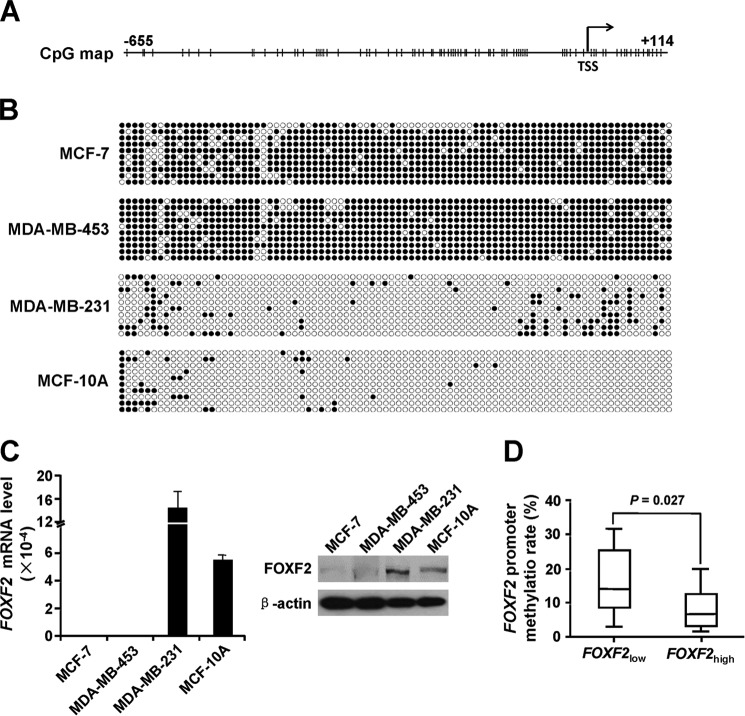
Genomic DNA sequence analysis revealed that the proximal promoter region of FOXF2 from −655 to +114 relative to the TSS contained a long-range CpG island (Fig. 1A). To determine whether DNA methylation of the CpG island silences the expression of FOXF2, the DNA methylation status and FOXF2 expression levels in the breast cancer cell lines MCF-7, MDA-MB-453, MDA-MB-231, and MCF-10A were examined by bisulfite sequencing, qRT-PCR, and immunoblotting. The results revealed that the proximal promoter region of FOXF2 was highly methylated in the non-basal-like subtype cells MCF-7 and MDA-MB-453, but non-methylated in the basal-like subtype cells MDA-MB-231 and MCF-10A (Fig. 1B). FOXF2 mRNA and protein were consistently expressed at high levels in MDA-MB-231 and MCF-10A cells, but at low levels in MCF-7 and MDA-MB-453 cells (Fig. 1C). Furthermore, we determined the DNA methylation status and mRNA expression level of FOXF2 in 20 breast cancer tissues. The results showed that the methylation rate of the high FOXF2 mRNA-expressing group (n = 10, median = 6.6%) was significantly lower than that of the low FOXF2 mRNA-expressing group (n = 10, median = 14.0%; p = 0.027) (Fig. 1D), indicating that FOXF2 expression is inversely correlated with the methylation status of the CpG island in the proximal promoter region of this gene in breast cancer cells.
FIGURE 1.
FOXF2 expression is inversely correlated with CpG island methylation in the proximal promoter region of this gene in breast cancer cells. A, diagram of the CpG island of the FOXF2 proximal promoter ranging from −655 to +114 relative to the TSS. The arrow displays the NCBI-predicted TSS, and the vertical bars indicate CpG sites. B, the DNA methylation status of the CpG island in the indicated breast cancer cell lines was analyzed by bisulfite sequencing. ●, methylated cytosine residues; ○, non-methylated cytosine residues. C, FOXF2 mRNA and protein levels in the indicated cell lines were measured by qRT-PCR and immunoblotting. D, the methylation rate of the CpG island of the FOXF2 proximal promoter in low (FOXF2low; n = 10) and high (FOXF2high; n = 10) FOXF2 mRNA-expressing breast cancer tissues was determined by bisulfite sequencing and is shown as a box plot.
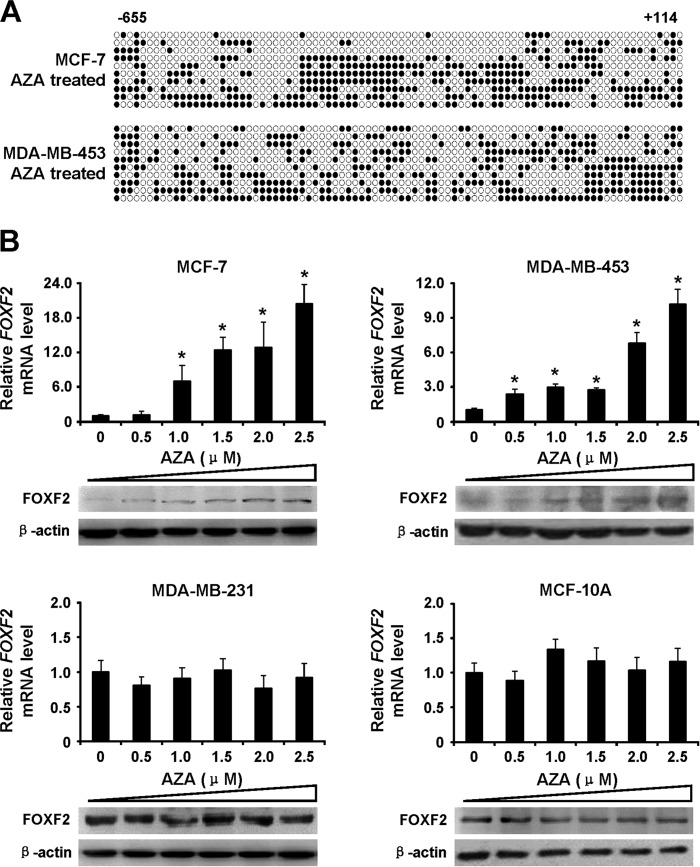
FOXF2 Expression Is Induced by AZA Treatment in Methylated Breast Cancer Cells
To investigate the effect of DNA methylation on FOXF2 expression, the four breast cancer cell lines were treated with the DNMT inhibitor AZA at of 0, 0.5, 1.0, 1.5, 2.0, and 2.5 μm, and the FOXF2 expression levels were subsequently measured by qRT-PCR and immunoblotting. The demethylation effect of AZA on the CpG island of FOXF2 in the highly methylated MCF-7 and MDA-MB-453 cells was confirmed by bisulfite sequencing (Fig. 2A). AZA elicited the dose-dependent induction of FOXF2 expression in the highly methylated non-basal-like cells MCF-7 and MDA-MB-453, but did not affect FOXF2 expression at both the mRNA and protein levels in the non-methylated basal-like cells MDA-MB-231 and MCF-10A (Fig. 2B). These results demonstrate that methylation of the FOXF2 proximal promoter region leads to suppression of FOXF2 expression in non-basal-like breast cancer cell lines.
FIGURE 2.
FOXF2 expression is induced by AZA treatment in methylated breast cancer cells. A, the DNA methylation status of the FOXF2 proximal promoter was analyzed by bisulfite sequencing in AZA-treated cells. B, FOXF2 mRNA and protein levels were determined by qRT-PCR and immunoblotting in the indicated cells treated with varying concentrations of AZA. Data are presented as the mean ± S.D. of three independent experiments. *, p < 0.05.
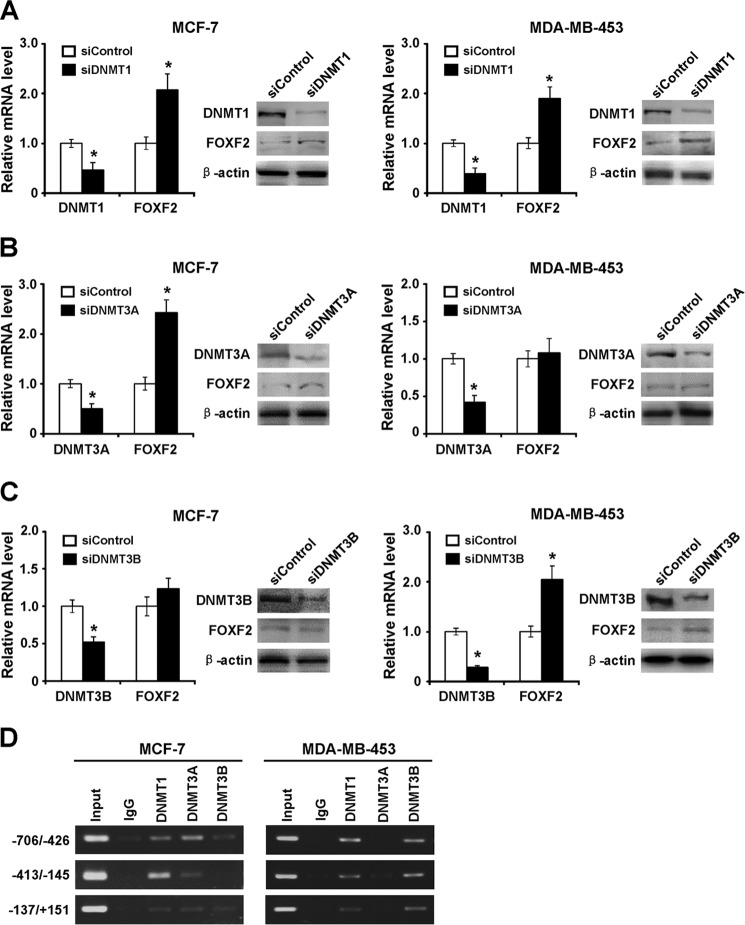
Specific DNMTs Contribute to Silencing of FOXF2 Expression
To further determine which DNMTs contribute to FOXF2 promoter methylation, siRNAs targeting DNMTs, including DNMT1, DNMT3A, and DNMT3B siRNAs, were transiently transfected into MCF-7 and MDA-MB-453 cells (Fig. 3, A–C). We then tested the effect of the depletion of each specific DNMT on FOXF2 expression. The results showed that FOXF2 expression was induced by DNMT1 or DNMT3A (but not DNMT3B) depletion in MCF-7 cells. We also observed that FOXF2 expression was restored by DNMT1 or DNMT3B (but not DNMT3A) depletion in MDA-MB-453 cells (Fig. 3, A–C). To further validate the role of DNMT-modulated repression of FOXF2 transcription, ChIP assays were performed using anti-DNMT1, anti-DNMT3A, and anti-DNMT3B antibodies in MCF-7 and MDA-MB-453 cells to examine the binding of DNMTs to the methylated promoter spanning the entire CpG island. The results revealed that in MCF-7 cells, DNMT1 and DNMT3A bound to the methylated promoter, whereas DNMT3B showed less binding to this region, and in MDA-MB-453 cells, DNMT1 and DNMT3B (but not DNMT3A) bound to this region (Fig. 3D). These results demonstrate that the common (DNMT1) and individual (DNMT3A or DNMT3B) DNMTs are recruited on the CpG island of the FOXF2 promoter and that methylation of the promoter affects transcription of this gene in different breast cancer cells.
FIGURE 3.
Specific DNMTs contribute to the silencing of FOXF2 expression. MCF-7 and MDA-MB-453 cells with a methylated FOXF2 proximal promoter region were transiently transfected with DNMT1 siRNA (siDNMT1; A), DNMT3A siRNA (siDNMT3A; B), and DNMT3B siRNA (siDNMT3B; C), and the DNMT1, DNMT3A, DNMT3B, and FOXF2 mRNA and protein expression levels in the indicated cells were detected by qRT-PCR and immunoblotting. Data are presented as the mean ± S.D. of three independent experiments. *, p < 0.05. D, ChIP-PCR assays demonstrated the enrichment of FOXF2 proximal promoter fragments using anti-DNMT1, anti-DNMT3A, and anti-DNMT3B antibodies in MCF-7 and MDA-MB-453 cells.
DNA Methylation Represses FOXF2 Promoter Activity
To examine whether DNA methylation directly represses FOXF2 promoter activity, we cloned the FOXF2 proximal promoter region from −655 to +128, containing 79 CpG sites, into a luciferase reporter construct. The cloned inserts were then methylated using SssI, HhaI, and HpaII methylases. SssI methylates all 5′-CpG-3′ sites (79 CpG sites), HhaI methylates only the CpG within the sequence 5′-GCGC-3′ (10 CpG sites), and HpaII methylates only the CpG within the sequence 5′-CCGG-3′ (11 CpG sites). Proper methylation of the inserts was confirmed by digestion with the restriction enzymes McrBC (methylation-specific restriction enzyme), HhaI, and HpaII (methylation-sensitive restriction enzyme) (Fig. 4A). The promoter activity of the differentially methylated FOXF2 proximal promoter region with SssI, HhaI, or HpaII methylase was assessed by transfection of luciferase reporter constructs into MDA-MB-231 cells. The results showed that FOXF2 promoter activity was repressed after methylation with the SssI, HpaII, or HhaI methylase. SssI, which methylates all CpG sites, showed the greatest repression of FOXF2 promoter activity, whereas both HhaI and HpaII, which methylate a portion of CpG sites, showed less repression (Fig. 4B). These results demonstrate that DNA methylation represses FOXF2 promoter activity.
FIGURE 4.
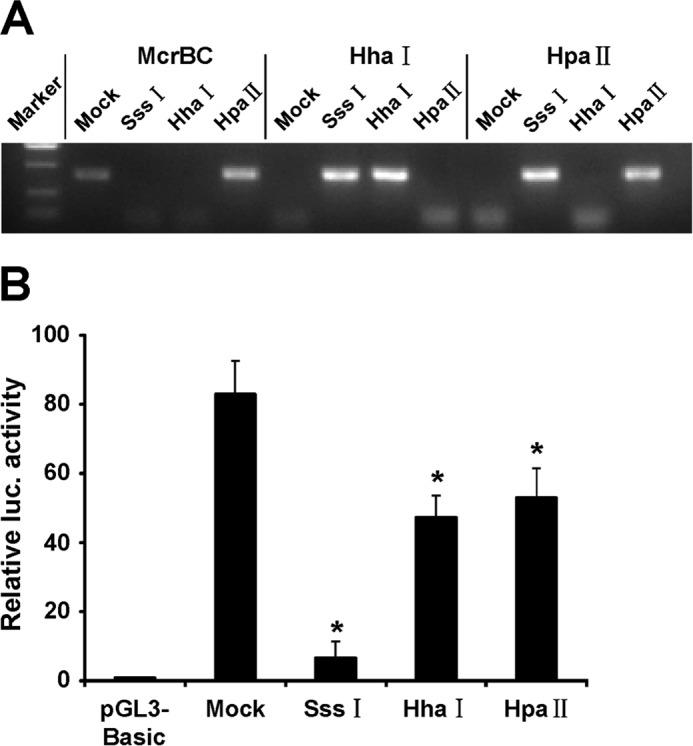
DNA methylation represses FOXF2 promoter activity. A, following in vitro methylation with SssI, HhaI, or HpaII methylase, the proximal promoter region (−655/+128) of FOXF2 was digested with McrBC, HhaI, or HpaII to confirm the methylation status. B, the activity of the FOXF2 proximal promoter differentially methylated with SssI, HhaI, or HpaII methylase were assessed using a Dual-Luciferase reporter assay in MDA-MB-231 cells transfected with luciferase reporter constructs. Data are presented as the mean ± S.D. of three independent experiments. *, p < 0.05. luc., luciferase.
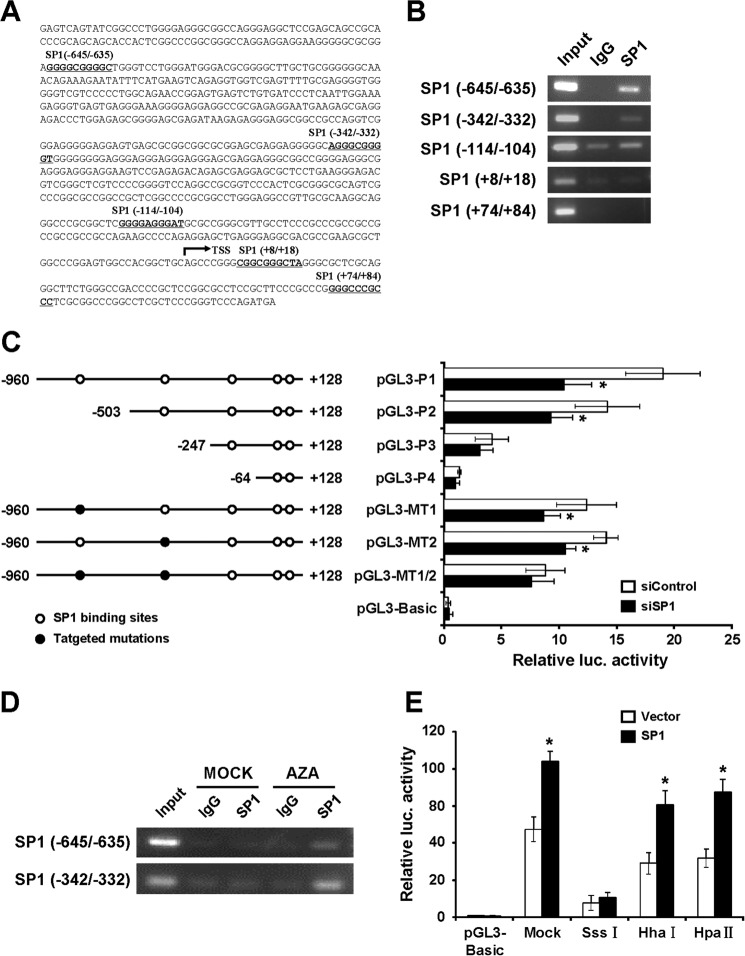
SP1 Binding to the FOXF2 Proximal Promoter Region Is Abrogated by DNA Methylation
To investigate whether the DNA methylation of the FOXF2 proximal promoter region affects the transcriptional regulation of FOXF2 expression, we performed an online search for the prediction of transcription factor-binding sites in the FOXF2 proximal promoter region using the Web platform MAPPER (Multi-genome Analysis of Positions and Patterns of Elements of Regulation) and found five putative binding sites for transcription factor SP1 located at −645 to −635, −342 to −332, −114 to −104, +8 to +18, and +74 to +84 (Fig. 5A). To confirm the binding of SP1 to the predicted sites in vivo, a ChIP assay was performed using an anti-SP1 antibody in MDA-MB-231 cells with a non-methylated FOXF2 proximal promoter region. The anti-SP1 antibody-enriched DNA sequences were amplified by PCR for the five regions containing the putative SP1-binding sites. The results showed that SP1 clearly bound to the FOXF2 promoter at −645 to −635 and −342 to −332, whereas no binding was observed at the other predicted sites (Fig. 5B).
FIGURE 5.
SP1 binding to the FOXF2 proximal promoter region is abrogated by DNA methylation. A, location of the predicted SP1-binding sites in the FOXF2 proximal promoter region. The sequences depicted in boldface and underlined denote predicted binding sites for SP1, and the TSS is labeled. B, ChIP assay demonstrated the direct binding of SP1 to the FOXF2 promoter in MDA-MB-231 cells. The ChIP-enriched DNA fragments of the FOXF2 promoter using IgG and an anti-SP1 antibody were amplified by PCR. Total input (5%) was used as a positive control. C, sequential deletion and substitution mutation analyses identified SP1-responsive regions in the FOXF2 proximal promoter region. Serially truncated and mutated FOXF2 promoter constructs were cotransfected with siSP1 or siControl into MDA-MB-231 cells, and the relative luciferase (luc.) activities were determined. Data are presented as the mean ± S.D. of twice-repeated experiments, each in duplicate. *, p < 0.05. D, ChIP analysis using an anti-SP1 antibody was performed in MCF-7 cells with or without AZA treatment. E, effect of SP1 overexpression on differentially methylated promoter-driven luciferase activity. MDA-MB-231 cells were cotransfected with SP1 expression plasmids or the vector control, along with differentially methylated FOXF2 promoter constructs and the internal control pRL-TK. Data are presented as the mean ± S.D. of twice-repeated experiments, each in duplicate. *, p < 0.05.
To identify which binding site is functionally required for SP1-regulated FOXF2 promoter activation, we generated sequential deletions of these sites and performed a luciferase reporter assay in the presence of siControl or siSP1. pGL3-P1, which contains the five putative SP1-binding sites, showed maximum promoter activity, but exhibited a prominent reduction in promoter activity in cells transfected with siSP1. Deletion of the region containing only the −645/−635 site (pGL3-P2) also caused a significant decrease in FOXF2 promoter activity upon SP1 depletion. The deletion constructs pGL3-P3 and pGL3-P4 in siSP1-transfected cells showed similar promoter activity as control cells expressing endogenous SP1 (Fig. 5C). To determine whether the two identified SP1-binding sites play a role in the transcriptional activation of FOXF2, substitution mutations of the sites were generated individually (pGL3-MT1 and pGL3-MT2) and in combination (pGL3-MT1/2). A significant reduction in FOXF2 promoter activity was observed when the −645/−635 and −342/−332 sites were individually or jointly mutated. Consistent with the data obtained with the deletion mutants, the abrogation of individual sites resulted in a small decrease in FOXF2 promoter activation, and no effect on the double SP1-binding site-mutated promoter was observed in SP1-depleted cells (Fig. 5C). Thus, these results demonstrate that both the −645/−635 and −342/−332 sites are essential for SP1-regulated FOXF2 promoter activity.
To further validate the effect of FOXF2 promoter methylation on the promoter binding of SP1, MCF-7 cells with the methylated FOXF2 proximal promoter region were treated with 2 μm AZA for 4 days and then subjected to ChIP assays using an anti-SP1 antibody. The results showed weak SP1 binding to the FOXF2 promoter in MCF-7 cells without treatment, but markedly enhanced binding in cells treated with AZA (Fig. 5D). Furthermore, Dual-Luciferase reporter assays were performed in MDA-MB-231 cells after cotransfection with differentially methylated FOXF2 promoter constructs and SP1 expression plasmids or vector controls. The SP1-binding sites could be methylated by SssI methylase, but not by HhaI or HpaII methylase. The results revealed that exogenous SP1 expression significantly enhanced the activity of FOXF2 promoter after treatment with HhaI, HpaII, or no methylase compared with the vector control, whereas SP1 barely activated the SssI-methylated FOXF2 promoter (Fig. 5E). Collectively, these results demonstrate that DNA methylation abrogates SP1 binding to the FOXF2 promoter.
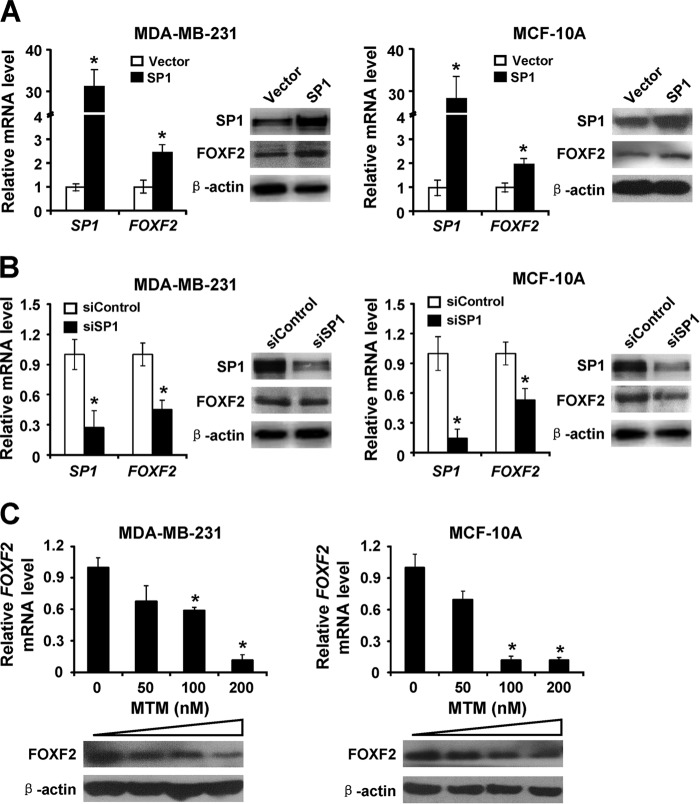
SP1 Up-regulates FOXF2 Expression in Breast Cancer Cells with a Non-methylated FOXF2 Proximal Promoter Region
To investigate the role of SP1 in the regulation of FOXF2 expression in breast cancer cells without FOXF2 proximal promoter region methylation, an SP1 expression plasmid or SP1 siRNA was transiently transfected into MDA-MB-231 and MCF-10A cells, respectively. We observed that SP1 overexpression significantly increased FOXF2 mRNA and protein expression (Fig. 6A), whereas SP1 knockdown decreased FOXF2 expression in MDA-MB-231 and MCF-10A cells (Fig. 6B). In addition, mithramycin A was used to inhibit the SP1-regulated transcriptional activation of FOXF2 in these cells, and the results confirmed that expression of FOXF2 mRNA and protein was reduced with increasing mithramycin A concentrations (Fig. 6C). Taken together, these results indicate that SP1 transcriptionally up-regulates FOXF2 expression by binding to its proximal promoter region with non-methylation.
FIGURE 6.
SP1 induces FOXF2 expression in breast cancer cells without FOXF2 proximal promoter region methylation. Following transfection of the SP1 expression plasmid (A) or siSP1 (B) into MDA-MB-231 and MCF-10A cells, SP1 and FOXF2 mRNA and protein levels were determined by qRT-PCR and immunoblotting. C, FOXF2 mRNA and protein expression levels in MDA-MB-231 and MCF-10A cells untreated or treated with varying concentrations of mithramycin A (MTM) were measured by qRT-PCR and immunoblotting. Data are presented as the mean ± S.D. of three independent experiments. *, p < 0.05.
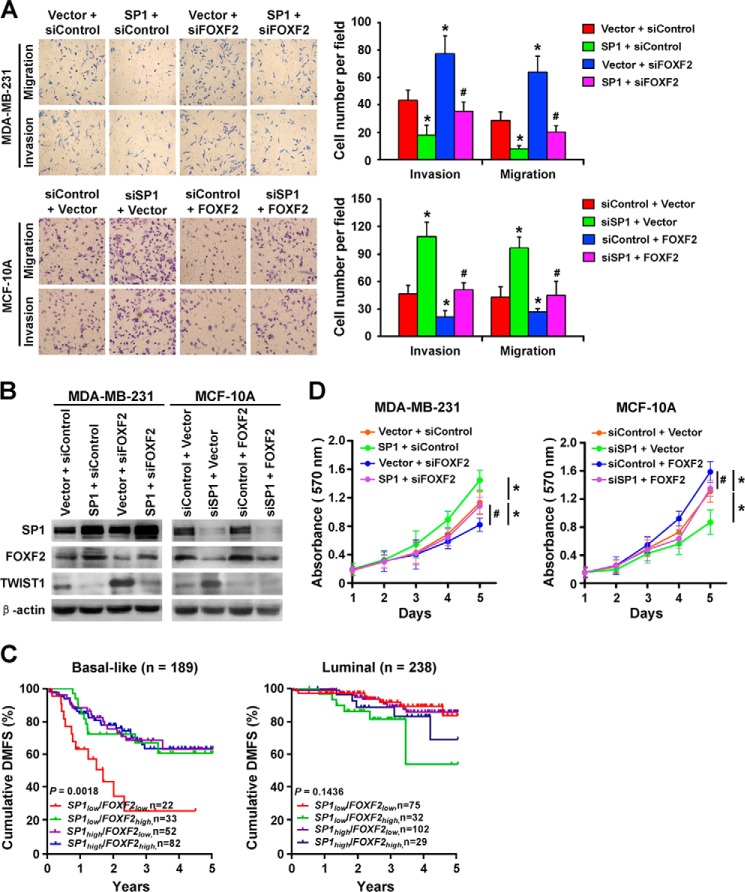
FOXF2 Mediates SP1-regulated Suppression of Progression and Promotion of Proliferation of Non-methylated Breast Cancer Cells
SP1 has been implicated as a suppressor of human cancer progression (17). On the basis of a previous report on the role of FOXF2 in the suppression of metastasis in breast cancer, we speculated that FOXF2 mediates the SP1-suppressed aggressive phenotype in breast cancer cells. Thus, the SP1 expression plasmid was cotransfected with siFOXF2 or siControl into MDA-MB-231 cells, and cell migration and invasion capacities were assessed using in vitro Transwell assays. The results showed that SP1 overexpression attenuated both cell migration and invasion abilities, and these effects were reversed by siFOXF2 transfection (Fig. 7A). In addition, TWIST1, a FOXF2 target gene (8), was reduced by exogenous SP1 expression, which was recovered by transfection with siFOXF2 (Fig. 7B). Conversely, when MCF-10A cells were cotransfected with siSP1 and the FOXF2 expression plasmid or vector control, the increased cell migration and invasion by SP1 depletion were reversed by FOXF2 transfection (Fig. 7A). SP1 depletion up-regulated TWIST1 expression, and exogenous FOXF2 expression inhibited this effect in MCF-10A cells (Fig. 7B).
FIGURE 7.
FOXF2 mediates the SP1-regulated suppression of progression and promotion of proliferation in non-methylated breast cancer cells. A, cell invasion and migration abilities were assessed using Matrigel-coated and uncoated Transwell assays, respectively, in MDA-MB-231 and MCF-10A cells treated as indicated. B, SP1, FOXF2, and TWIST1 expression in MDA-MB-231 and MCF-10A cells treated as indicated was detected by immunoblotting. C, analysis of DMFS in patients with different SP1 and FOXF2 expression statuses in basal-like (n = 189) and luminal (n = 238) breast cancer subtypes. SP1 and FOXF2 mRNA levels were mined from the gene expression profiling data set of 427 breast cancer tissues with Gene Expression Omnibus accession number GSE25066. SP1low and SP1high, low and high SP1 mRNA expressing groups; FOXF2low and FOXF2high, low and high FOXF2 mRNA expressing groups. D, cell proliferation abilities were assessed by MTT assays in MDA-MB-231 and MCF-10A cells treated as indicated. Data are presented as the mean ± S.D. of three independent experiments. *, p < 0.05 versus MDA-MB-231 or MCF-10A cells cotransfected with siRNA and vector control (siControl + Vector); #, p < 0.05 versus MDA-MB-231 cells cotransfected with siFOXF2 and vector control (Vector + siFOXF2) or MCF-10A cells cotransfected with siControl and FOXF2 expression plasmid (siControl + FOXF2).
To provide further clinical evidence for the SP1/FOXF2-regulated function in the progression of different breast cancer subtypes, we mined the SP1 and FOXF2 mRNA expression data from the gene expression profiling data set of 427 breast cancer tissues (Gene Expression Omnibus accession number GSE25066) (16) and analyzed the DMFS in patients with different SP1 and FOXF2 expression statuses in the basal-like and luminal subtypes. The patients were grouped into the high SP1/high FOXF2 mRNA-expressing group (n = 111), low SP1/high FOXF2 mRNA-expressing group (n = 65), high SP1/low FOXF2 mRNA-expressing group (n = 154), and low SP1/low FOXF2 mRNA-expressing group (n = 97) using the optimal cutoff values for SP1 and FOXF2 mRNA expression. For the basal-like subtype (n = 189), the patients in the low SP1/low FOXF2 mRNA-expressing group had the lowest DMFS compared with those in the other three groups. For the luminal subtype (n = 238), there was no significantly different DMFS among the four groups (Fig. 7C). This result suggests that the low SP1/low FOXF2 mRNA expression is involved in BLBC metastasis, but not in luminal breast cancer metastasis.
In addition to the role of SP1 in suppressing the progression of human cancer cells, SP1 is also considered to be a promoter of cell proliferation (18). Consistent with the dual roles of SP1, we have found that FOXF2 has such dual functions in BLBC cells: the suppression of progression and the promotion of proliferation (8). Thus, we performed MTT assays in these cells to assess the role of FOXF2 in mediating SP1-regulated cell proliferation. The results revealed that SP1 overexpression enhanced the proliferation ability of MDA-MB-231 cells and that this effect was reversed by siFOXF2 transfection (Fig. 7D). Conversely, the attenuated proliferation ability of MCF-10A cells upon SP1 depletion was restored by FOXF2 transfection (Fig. 7D). Taken together, these results suggest that FOXF2 is essential for the SP1-regulated suppression of progression and promotion of proliferation of breast cancer cells without the methylation of the FOXF2 proximal promoter region.
Discussion
FOXF2 is a mesenchymal transcription factor that controls epithelial cell proliferation and survival (2). Although FOXF2 exhibits stromal cell-specific expression, it was found to be uniquely expressed in some androgen-independent prostate cancer xenografts (19). In a previous study, we observed that FOXF2 was specifically expressed in basal-like breast cell lines, and we identified FOXF2 as an epithelial-mesenchymal transition-suppressing and mesenchymal differentiation-promoting factor in BLBC (8). However, the regulatory mechanism underlying the cell type-specific expression of FOXF2 remains unknown. The results of the present study reveal an inverse correlation between the CpG island methylation of the FOXF2 proximal promoter region and the FOXF2 expression in human breast cancer. Importantly, CpG island methylation of the FOXF2 proximal promoter region occurred in the non-basal-like breast cancer cell lines MCF-7 and MDA-MB-453, but not in the basal-like breast cell lines MDA-MB-231 and MCF-10A. These results might partially explain the regulatory mechanism of the subtype-specific expression of FOXF2 in breast cancer cells.
DNMTs comprise a conserved family of enzymes that play a special role in chromatin remodeling and regulation of gene expression. DNMTs catalyze the addition of a methyl group to the cytosine residues of CpG dinucleotides, resulting in the modification of chromatin and/or DNA methylation. Mammalian DNMTs, including DNMT1, DNMT3A, and DNMT3B, are responsible for DNA methylation pattern acquisition during embryogenesis and somatic tissue development (20). DNMT1 is a maintenance methyltransferase, and DNMT3A and DNMT3B are de novo methyltransferases. Increasing evidence suggests a complex regulatory network of recruited DNMTs at specific genomic regions to establish common or individual DNA methylation patterns that depend on the cellular internal environment and regulation of extracellular signals (21, 22). The results of this study indicate that FOXF2 transcription is communally and/or individually modulated by DNMT1, DNMT3A, and DNMT3B, which are specifically recruited to the FOXF2 promoter region in different non-basal-like breast cancer cells. What regulates DNMTs to methylate the FOXF2 promoter and inhibits its expression in a cell type-specific manner should be further investigated.
SP1 is a zinc-finger protein that belongs to the SP family of transcription factors. The canonical sequence of the SP1-binding site is 5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′ in GpC-rich promoter regions (23). The binding of SP1 to target genes can be interrupted by DNA methylation, resulting in the silencing of gene expression. A number of genes with GC-rich promoter regions, such as CADM1 (24), KEAP1 (25), and NDRG2 (26), were found to be regulated by the combined effects of SP1 and DNA methylation. In this study, we identified FOXF2 as a novel transcriptional target of SP1 and demonstrated that the CpG island methylation of the FOXF2 proximal promoter region abrogated SP1 binding, leading to the silencing of FOXF2 expression.
As a ubiquitous transcriptional activator, SP1 has been implicated in various biological processes, including cell proliferation and progression. However, the roles of SP1 in human cancer remain elusive. SP1 has been implicated as either a promoter (27–30) or repressor (31–33) of cell proliferation and progression. Indeed, the complex biological roles of SP1 might be context-dependent and regulated by interaction with cofactors (31). Hsu et al. (17) reported that SP1 was negatively correlated with tumor progression in lung adenocarcinoma, but was required for lung tumor growth in transgenic mice bearing Kras-induced lung tumors. The dual functions of SP1 in lung cancer are consistent with our proposed functions of FOXF2 in breast cancer. Because FOXF2 is a transcriptional target of SP1, we speculated that SP1 might play critical roles in breast cancer proliferation and progression through activation of FOXF2 transcription. Indeed, we demonstrated that FOXF2 mediates the function of SP1 in suppressing migration and invasion and promoting proliferation of BLBC cells. In a previous study, we identified FOXF2 as a novel epithelial-mesenchymal transition-suppressing transcription factor that negatively regulates the transcription of TWIST1 (8). In the present study, we further demonstrated that the SP1-regulated suppression of migration and invasion partially depends on the FOXF2/TWIST1-mediated control of the aggressive properties of BLBC cells.
In conclusion, we have described a new regulatory mechanism for the subtype-specific expression and function of FOXF2 in breast cancer cells. DNA methylation silences FOXF2 expression in breast cancer cells through abrogation of SP1 binding to the proximal promoter region, affecting the SP1-regulated dual functions of FOXF2 in breast cancer cells: suppression of progression and promotion of proliferation.
Author Contributions
Y.-M. F. conceived and coordinated the study and wrote the paper. H.-P. T. designed, performed, and analyzed experiments and wrote the paper. S.-M. L. and H.-J. H. contributed to the in vitro experiments. S.-M. L. and P.-Z. K. constructed vectors for expression of SP1 and FOXF2. R. H. analyzed the online clinical data shown in Fig. 7C. Q.-S. W. and X.-Q. L. provided technical assistance. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by National Natural Science Foundation of China Grants 81272357 and 81472680 and Major Program of Applied Basic Research Projects of Tianjin Grant 13JCZDJC30100. The authors declare that they have no conflicts of interest with the contents of this article.
- BLBC
- basal-like breast cancer
- DNMT
- DNA methyltransferase
- AZA
- 5-aza-2′-deoxycytidine
- siSP1
- SP1 siRNA
- siFOXF2
- FOXF2 siRNA
- siControl
- non-targeting control siRNA
- TSS
- transcription start site
- qRT-PCR
- quantitative RT-PCR
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMFS
- distant metastasis-free survival.
References
- 1. Wang T., Tamakoshi T., Uezato T., Shu F., Kanzaki-Kato N., Fu Y., Koseki H., Yoshida N., Sugiyama T., Miura N. (2003) Forkhead transcription factor Foxf2 (LUN)-deficient mice exhibit abnormal development of secondary palate. Dev. Biol. 259, 83–94 [DOI] [PubMed] [Google Scholar]
- 2. Ormestad M., Astorga J., Landgren H., Wang T., Johansson B. R., Miura N., Carlsson P. (2006) Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 133, 833–843 [DOI] [PubMed] [Google Scholar]
- 3. Aitola M., Carlsson P., Mahlapuu M., Enerbäck S., Pelto-Huikko M. (2000) Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal interactions. Dev. Dyn. 218, 136–149 [DOI] [PubMed] [Google Scholar]
- 4. van der Heul-Nieuwenhuijsen L., Hendriksen P. J., van der Kwast T. H., Jenster G. (2006) Gene expression profiling of the human prostate zones. BJU Int. 98, 886–897 [DOI] [PubMed] [Google Scholar]
- 5. Hirata H., Ueno K., Shahryari V., Deng G., Tanaka Y., Tabatabai Z. L., Hinoda Y., Dahiya R. (2013) MicroRNA-182–5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS ONE 8, e55502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi W., Gerster K., Alajez N. M., Tsang J., Waldron L., Pintilie M., Hui A. B., Sykes J., P'ng C., Miller N., McCready D., Fyles A., Liu F. F. (2011) MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 71, 2926–2937 [DOI] [PubMed] [Google Scholar]
- 7. Kong P.-Z., Yang F., Li L., Li X.-Q., Feng Y.-M. (2013) Decreased FOXF2 mRNA expression indicates early-onset metastasis and poor prognosis for breast cancer patients with histological grade II tumor. PLoS ONE 8, e61591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q.-S., Kong P.-Z., Li X.-Q., Yang F., Feng Y.-M. (2015) FOXF2 deficiency promotes epithelial-mesenchymal transition and metastasis of basal-like breast cancer. Breast Cancer Res. 17, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones P. A. (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 [DOI] [PubMed] [Google Scholar]
- 10. Medvedeva Y. A., Khamis A. M., Kulakovskiy I. V., Ba-Alawi W., Bhuyan M. S., Kawaji H., Lassmann T., Harbers M., Forrest A. R., Bajic V. B. (2014) Effects of cytosine methylation on transcription factor binding sites. BMC Genomics 15, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valastyan S., Weinberg R. A. (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C. Y., Tsai Y. P., Wu M. Z., Teng S. C., Wu K. J. (2012) Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet. 28, 454–463 [DOI] [PubMed] [Google Scholar]
- 13. Huang Y., Rao A. (2014) Connections between TET proteins and aberrant DNA modification in cancer. Trends Genet. 30, 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunwell T., Hesson L., Rauch T. A., Wang L., Clark R. E., Dallol A., Gentle D., Catchpoole D., Maher E. R., Pfeifer G. P., Latif F. (2010) A genome-wide screen identifies frequently methylated genes in haematological and epithelial cancers. Mol. Cancer 9, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu Y., Xiao C. H., Tan L. D., Wang Q.-S., Li X.-Q., Feng Y.-M. (2014) Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 110, 724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatzis C., Pusztai L., Valero V., Booser D. J., Esserman L., Lluch A., Vidaurre T., Holmes F., Souchon E., Wang H., Martin M., Cotrina J., Gomez H., Hubbard R., Chacón J. I., Ferrer-Lozano J., Dyer R., Buxton M., Gong Y., Wu Y., Ibrahim N., Andreopoulou E., Ueno N. T., Hunt K., Yang W., Nazario A., DeMichele A., O'Shaughnessy J., Hortobagyi G. N., Symmans W. F. (2011) A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 305, 1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu T. I., Wang M. C., Chen S. Y., Yeh Y. M., Su W. C., Chang W. C., Hung J. J. (2012) Sp1 expression regulates lung tumor progression. Oncogene 31, 3973–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Black A. R., Black J. D., Azizkhan-Clifford J. (2001) Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell. Physiol. 188, 143–160 [DOI] [PubMed] [Google Scholar]
- 19. van der Heul-Nieuwenhuijsen L., Dits N. F., Jenster G. (2009) Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 103, 1574–1580 [DOI] [PubMed] [Google Scholar]
- 20. Turek-Plewa J., Jagodziński P. P. (2005) The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell. Mol. Biol. Lett. 10, 631–647 [PubMed] [Google Scholar]
- 21. Denis H., Ndlovu M. N., Fuks F. (2011) Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 12, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang H. C., Cho C. Y., Hung W. C. (2006) Silencing of the metastasis suppressor RECK by RAS oncogene is mediated by DNA methyltransferase 3b-induced promoter methylation. Cancer Res. 66, 8413–8420 [DOI] [PubMed] [Google Scholar]
- 23. Narayan V. A., Kriwacki R. W., Caradonna J. P. (1997) Structures of zinc finger domains from transcription factor Sp1. Insights into sequence-specific protein-DNA recognition. J. Biol. Chem. 272, 7801–7809 [DOI] [PubMed] [Google Scholar]
- 24. Reamon-Buettner S. M., Borlak J. (2008) Epigenetic silencing of cell adhesion molecule 1 in different cancer progenitor cells of transgenic c-Myc and c-Raf mouse lung tumors. Cancer Res. 68, 7587–7596 [DOI] [PubMed] [Google Scholar]
- 25. Guo D., Wu B., Yan J., Li X., Sun H., Zhou D. (2012) A possible gene silencing mechanism: hypermethylation of the Keap1 promoter abrogates binding of the transcription factor Sp1 in lung cancer cells. Biochem. Biophys. Res. Commun. 428, 80–85 [DOI] [PubMed] [Google Scholar]
- 26. Shen L., Qu X., Ma Y., Zheng J., Chu D., Liu B., Li X., Wang M., Xu C., Liu N., Yao L., Zhang J. (2014) Tumor suppressor NDRG2 tips the balance of oncogenic TGF-β via EMT inhibition in colorectal cancer. Oncogenesis 3, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jungert K., Buck A., von Wichert G., Adler G., König A., Buchholz M., Gress T. M., Ellenrieder V. (2007) Sp1 is required for transforming growth factor-β-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 67, 1563–1570 [DOI] [PubMed] [Google Scholar]
- 28. Lu S., Archer M. C. (2010) Sp1 coordinately regulates de novo lipogenesis and proliferation in cancer cells. Int. J. Cancer 126, 416–425 [DOI] [PubMed] [Google Scholar]
- 29. Verras M., Lee J., Xue H., Li T. H., Wang Y., Sun Z. (2007) The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 67, 967–975 [DOI] [PubMed] [Google Scholar]
- 30. Yue L., Li L., Liu F., Hu N., Zhang W., Bai X., Li Y., Zhang Y., Fu L., Zhang X., Ye L. (2013) The oncoprotein HBXIP activates transcriptional coregulatory protein LMO4 via Sp1 to promote proliferation of breast cancer cells. Carcinogenesis 34, 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolesnikoff N., Attema J. L., Roslan S., Bert A. G., Schwarz Q. P., Gregory P. A., Goodall G. J. (2014) Specificity Protein 1 (Sp1) maintains basal epithelial expression of the miR-200 family. Implications for epithelial-mesenchymal transition. J. Biol. Chem. 289, 11194–11205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun L., Li H., Chen J., Dehennaut V., Zhao Y., Yang Y., Iwasaki Y., Kahn-Perles B., Leprince D., Chen Q., Shen A., Xu Y. (2013) A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis. J. Natl. Cancer Inst. 105, 887–898 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y. N., Lee W. W., Wang C. Y., Chao T. H., Chen Y., Chen J. H. (2005) Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene 24, 8277–8290 [DOI] [PubMed] [Google Scholar]