Background: Obesogenic effects of diets provided after undernutrition are controversial.
Results: Undernourished rats transferred to high-lipid diets show hyperphagia, dyslipidemia, ectopic lipids, adipose inflammation, and signs of browning.
Conclusion: Chronic food restriction increases metabolic risks of subsequent nutritional recovery, without inducing obesity.
Significance: The present study may help to understand some deleterious consequences undergone by undernourished people submitted to nutritional rehabilitation.
Keywords: adipose tissue, hypothalamus, insulin, leptin, nutrition, undernutrition
Abstract
Human studies have suggested that early undernutrition increases the risk of obesity, thereby explaining the increase in overweight among individuals from developing countries who have been undernourished as children. However, this conclusion is controversial, given that other studies do not concur. This study sought to determine whether rehabilitation after undernutrition increases the risk of obesity and metabolic disorders. We employed a published experimental food-restriction model. Wistar female rats subjected to severe food restriction since fetal stage and controls were transferred to a moderately high-fat diet (cafeteria) provided at 70 days of life to 6.5 months. Another group of undernourished rats were rehabilitated with chow. The energy intake of undernourished animals transferred to cafeteria formula exceeded that of the controls under this regime and was probably driven by hypothalamic disorders in insulin and leptin signal transduction. The cafeteria diet resulted in greater relative increases in both fat and lean body mass in the undernourished rats when compared with controls, enabling the former group to completely catch up in length and body mass index. White adipose tissues of undernourished rats transferred to the high-lipid regime developed a browning which, probably, contributed to avoid the obesigenic effect observed in controls. Nevertheless, the restricted group rehabilitated with cafeteria formula had greater accretion of visceral than subcutaneous fat, showed increased signs of macrophage infiltration and inflammation in visceral pad, dyslipidemia, and ectopic fat accumulation. The data indicate that early long-term undernutrition is associated with increased susceptibility to the harmful effects of nutritional rehabilitation, without causing obesity.
Introduction
Adverse environmental factors during growth stages can affect vulnerable immature tissues, leading to increased morbidity later in life. Nourishment has a major influence on development. Early diet imbalances such as overnutrition and undernutrition are common worldwide, and their consequences are a major public health concern, given that they are associated with an increased risk of various diseases in adulthood, as shown by data from epidemiological, clinical, and experimental studies (1, 2). Paradoxically, one of the possible sequelae of early undernutrition is obesity. Ravelli et al. (3) reported that poor maternal nutrition during pregnancy, resulting from the historic Dutch Hunger Winter, could have resulted in an increased tendency toward obesity. Other historic famines have lead authors to similar conclusions (4). Statistical data suggest a high obesity risk among population groups with the highest rates of past poverty, which usually have experienced previous undernutrition (5). A correlation between low birth weight and an increased risk of obesity has also been documented among children subjected to poor nutrition who were subsequently integrated into feeding programs or transitioned into affluent conditions (6). However, it is an issue still open because some authors have found no association between low birth weight and obesity (7). In fact, there are no systematic reports showing an inverse association between birth weight and subsequent risk of obesity (8). Most studies in this area have focused on obesity itself. However, fat distribution plays a key role in the development of metabolic disorders: visceral fat is harmful, rather than the obesity itself (9). Thus, individuals with normal BMI and visceral adiposity have higher susceptibility to cardiovascular diseases (10), and women with low BMI and high visceral fat show increased risk of mortality (11).
A large number of people, who were poorly nourished as children, are now experiencing a shift toward a Western pattern diet, which is hypercaloric. To investigate whether the harmful effects of these diets are aggravated by a pre-condition of early undernutrition, animal models have been used, generally rodents; however, most experimental studies have used highly fat-enriched formulas, which are obesogenic even without prior undernutrition (12). On the other hand, human undernutrition is frequently not limited to the early stages of development but becomes a lasting condition (13). However, the metabolic risks associated with long-term undernutrition have not been extensively investigated.
We developed a rat model of early, severe, and permanent food restriction and studied the impact on glucose homeostasis in females (14, 15). The undernourished animals were exposed for 6.5 months to standard chow or to a cafeteria diet containing ∼25% calories from fats, a formula that is less rich in lipids than those usually labeled as high-fat diets. The control group was given a cafeteria diet during the same period. We evaluated the effects of these diets on adiposity distribution, ectopic triglycerides, serum insulin and leptin, hypothalamic responses to these hormones, and whole insulin sensitivity. The aim was to investigate whether precocity and severity of lasting undernutrition facilitates obesity and/or some associated metabolic alterations.
Our main results show that undernutrition established a state of hyperphagia with the moderately high-lipid diet, probably driven by hypothalamic insulin and leptin resistance. This diet was slightly obesigenic for the control rats but not for those undernourished, despite the fact that their gain in body fat was proportionally higher than that of the controls. Perhaps, the protection against obesity is associated with a striking increase of UCP-1 observed in visceral and subcutaneous white adipose tissue, indicating a browning of these fat pads. However, these rats had higher triglyceride deposits in the liver and muscle, showing signs of steatosis. Also, the cafeteria formula increased tumor necrosis factor α (TNF-α) content in visceral fat in the undernourished rats. Finally, both the control and undernourished rats developed insulin resistance when they were subjected to any of the ad libitum diets. Our results favor the view that severe early undernutrition leads to harmful metabolic alterations, although not inducing obesity.
Experimental Procedures
Animals
All studies were conducted under an approved protocol (BFU 2011-25420) according to the principles and procedures outlined in the Committee for Animal Experimentation of UCM (Madrid, Spain). Wistar rats were bred in our laboratory with controlled conditions of temperature and lighting (light cycle from 0700 to 1900). Females were caged with males, and mating was confirmed by the presence of spermatozoa in vaginal smears. From the 14th day of pregnancy and during suckling, rats were subjected to a pattern of severe undernutrition previously described in detail (16). Control rats were bred in parallel. From weaning to 70 days of life, females of each litter were selected and housed in pairs. Control rats (C)2 were fed a standard rat chow ad libitum. Their food intake was calculated daily by weighing the remaining chow. Undernourished rats (U) were provided every day with 35% of the amount of chow eaten by controls. These groups were studied at 70 days (C70d and U70d). From that age up to 9 months, five groups were established as follows: C was randomly segregated into two groups: C9m, given the chow, and C-Caf9m, transferred to cafeteria diet, both ad libitum. Another three groups were formed from U animals: U9m kept on the standard chow restricted to 35%, U-Std9m switched to lab chow, and U-Caf9m, transferred to cafeteria formula, both supplied ad libitum. Spillage was negligible. All animals had free access to water. 16-h fasting rats were killed by decapitation and trunk blood was collected. Plasma and serum were stored at −80 °C until analyzed. Similarly, adult rats were decapitated and blood was harvested from the trunk. The skull was opened, the brain was removed, and the hypothalami was rapidly dissected and frozen in liquid nitrogen. For immunohistochemical studies the brains were processed differently, as indicated below. On the other hand, pieces of liver, skeletal muscle (quadriceps), and visceral adipose tissue were also collected and placed in liquid nitrogen. All tissue samples were stored at −80 °C until use.
Diets
Commercial A04 pelleted chow from SAFE (Scientific Animal Food and Engineering, PANLAB, Spain) contains by dry weight: 16% protein, 50% carbohydrate (starch and sucrose), 3% lipid, 4% cellulose, 5% vitamin and mineral mixture, and 12% water, with a caloric density of 2.9 Kcal/g. Cafeteria diet was manually prepared by adding a series of complementary ingredients to the commercial chow. Specifically: 330 g of full-cream milk, 150 g of full-cream milk powder, 100 g of cupcakes, and 90 g of sugar were mixed with 330 g of triturated standard chow, obtaining a homogenate used to prepare pellets, in the same size as the commercial chow, which were stored at 2–4 °C. In terms of macronutrients, this diet corresponded to: 15% protein, 45% starch, 25% sucrose, and 13% lipids, by dry weights. The caloric density was 4.6 Kcal/g, calculated from the caloric equivalences of macronutrients.
Analytic Procedures
Serum leptin was measured using a conventional sandwich ELISA (Millipore Corporation). Plasma insulin was determined by RIA, using rat insulin as standard (Linco Research, MO). Serum glucose was analyzed by a glucose oxidase method (BioSystems Reagents and Instruments, Barcelona, Spain). Serum triglycerides and cholesterol were determined colorimetrically by diagnostic kits (BioSystems). Hepatic and muscle lipids were extracted using a method previously described (17). After purification, the lipids were resuspended in isopropyl alcohol and cholesterol and triglycerides were analyzed as indicated above. Uric acid was assessed in hepatic homogenates by an uricase/peroxidase method (BioSystems).
Intracerebroventricular Hormone Treatments
Intracerebroventricular leptin and insulin administrations were performed as previously described (18). Briefly, rats were anesthetized with i.p. sodium pentobarbital and placed in a stereotaxic frame (David Koppf, Tujunga, CA). An opening in the skull was made with a dental drill. Then rats were injected with leptin (25 mg/kg body weight) (A. F. Parlow National Hormone and Peptide Program, Torrance, CA), insulin (10 IU/kg body weight) (Actrapid, Novo, Copenhagen), or an equal volume of vehicle saline. Fifteen minutes after injections, rats were euthanized by decapitation. The hypothalamus was dissected and frozen in liquid nitrogen.
Euglycemic Insulin Clamp Studies
These studies were performed in rats anesthetized with pentobarbital, as detailed previously (19). Insulin (Actrapid, Novo, Copenhagen) was infused through a saphenous vein at a constant rate (5 IU/h/Kg). 30% glucose was infused through the other saphenous vein at a variable rate. After 30 min of starting the clamp, plasma glucose, and insulin remained constant without further adjusting the infusion rate. At this steady state, the overall glucose utilization reaches a constant value, which was monitored for an additional 30 min. Glucose disposal was determined from the rate of infusion normalized to body weight (mg of glucose/min/kg).
Immunoprecipitation
Lysates containing 500 μg of proteins were immunoprecipitated overnight at 4 °C in the presence of 2 μg of anti-insulin receptor β subunit, followed by the addition of protein A-agarose (Roche Diagnostics). After mixing for 2 h the pellets were collected by centrifugation (1000 × g, 20 s) and the supernatants were discarded. Then the pellets were washed and used in Western blot analysis with antiphosphotyrosine.
Western Blot Analysis
The samples were subjected to SDS-PAGE on 6.5–10% polyacrylamide gels. Proteins were electrotransferred to polyvinylidine difluoride filters (PVDF), then blocked with 5% nonfat dry milk or 3% bovine serum albumin (for antiphosphotyrosine antibody) and incubated with primary antibody. Protein bands were visualized using ECL Western blotting detection kit (Amersham Biosciences). The following antibodies were purchased: anti-IRS-1, anti-IRS-2, anti-PTP1B, anti-JAK2, and anti-phospho-JAK2 (Chemicon/Millipore, Temecula, CA); anti-insulin receptor β subunit and antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY); anti-phospho-GSK3 α/β, anti-Akt, anti-phospho-Akt, anti-STAT3, anti-phospho STAT3, and anti-SOCS3 (Cell Signaling Technology, Beverly, MA); anti-GSK3 α/β, anti-POMC, anti-ObRb, anti-phospho-ObRb, anti-TNF-α, and antiphosphoinsulin receptor (Santa Cruz Biotechnology); and anti-β-actin (Sigma). β-Actin was used as loading control.
Quantitative RT-PCR Analysis
Total RNA was isolated using the TRIzol Reagent Kit (Invitrogen) following the manufacturer's instructions and reverse transcribed by a High Capacity cDNA Reverse Transcription kit (Applied Biosystems). For quantitative PCR analysis, mRNA expressions were determined using TaqMan gene expression assays (Applied Biosystems), for Rps18s (Rn01428913_gH), pro-opiomelanocortin (POMC) (Rn00595020_m1), neuropeptide Y (NPY) (Rn01410145_m1), glutathione peroxidase-1 (Rn00577994_g1), glutathione reductase (Rn01482159_m1), MCP-1 (Rn0058055_m1), and UCP-1 (Rn00562126_m1). The ΔΔCt method was used to calculate the relative expression levels of mRNA between experimental conditions and control groups using Rps18s as the internal control (20).
Magnetic Resonance Imaging
Body fat was measured using a Bruker BioSpin (BioSpec: 47/40, Ettlingen, Germany) at Research Assisting Centre of Nuclear Magnetic Resonance (UCM, Madrid). Rats were anesthetized using isoflurane for the duration of the study. All images were acquired as longitudinal slices individually assessed using Image J Launcher 1.46r software. This analysis provides a direct measure of the total volume of adipose pads in every animal. From magnetic resonance images, it is possible to differentiate well between visceral and subcutaneous adipose tissues. Fat masses were calculated assuming density as 0.9 g/ml and equal tissue-hydration degree in all subjects and in the two fat depots (21).
Body Mass Index and Lean Body Mass
The body mass index (BMI) was calculated from body weight and the body length (nose-to-anus length) using the formula: BMI = body weight (Kg)/lenght2 (m2). Lean body mass (LBM) was estimated by subtracting the fat from body weight.
Statistical Analysis
All data are reported as mean ± S.E. The difference between two mean values is assessed with t test. For multiple comparisons, significance was evaluated by analysis of variance, followed by the protected least significance test.
Results
Calorie Intakes of Undernourished Rats Submitted to Cafeteria Diet Was Increased
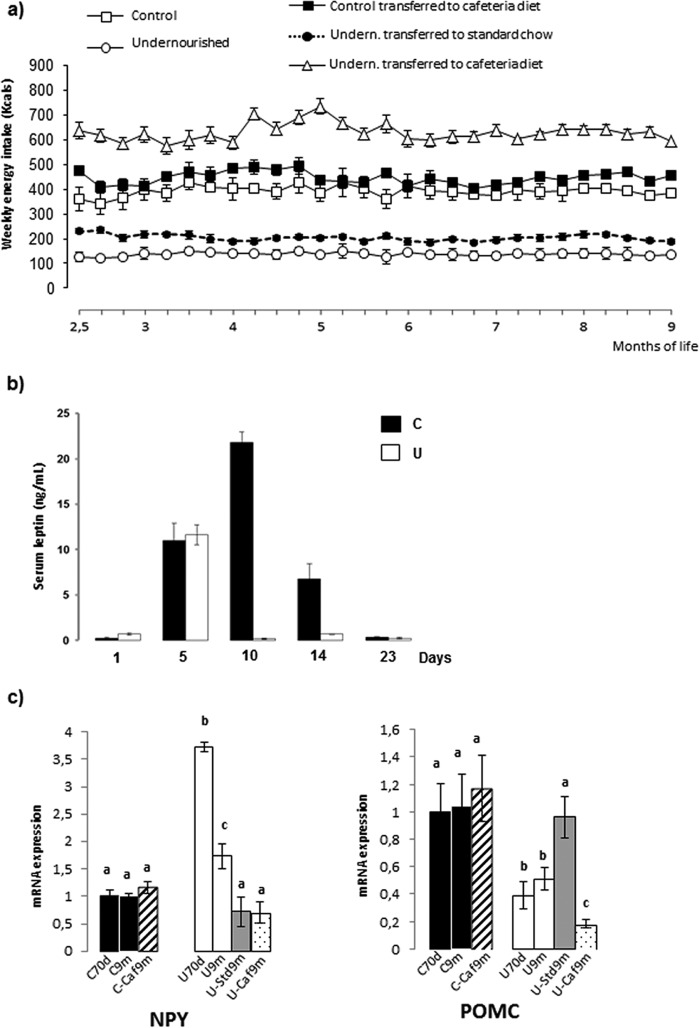
To evaluate to what extent early undernutrition induced overfeeding when food was available ad libitum, we measured the mean cumulative weekly calorie intakes during the period from 2.5 to 9 months of life, results are shown in Fig. 1a. We found no major differences between control groups, regardless of the type of diet. As expected, calorie intakes of undernourished rats increased when they were switched to standard chow but remained below control values. In contrast, calorie intakes of these restricted animals were greatly above all other groups after its transfer to cafeteria diet.
FIGURE 1.
Average quantity of energy consumed weekly by rats submitted to the different schedule diets as described under “Experimental Procedures” from 2.5 to 9 months of age. Each point represents the mean ± S.E. for 10–12 animals (a). Serum leptin concentration during suckling of control (C) and undernourished (U) rats is shown. Values are the mean ± S.E. for 6–7 rats (b). Levels of NPY mRNA and POMC mRNA in hypothalamus of control (C) and undernourished (U) rats at 70 days (70d) and 9 months (9m) of age, as well as those of animals rehabilitated with either lab chow (Std) or cafeteria formula (Caf) (c). The relative levels of mRNA are expressed as indicated under “Experimental Procedures.” Densitometric assessment of bars is expressed as arbitrary units. Each bar represents mean ± S.E. for 6–7 rats. Values with a different letter differ significantly, p < 0.05.
Anthropometric Indices of Restricted Rats Submitted to Cafeteria Formula Are Normalized
To determine whether ad libitum diets were able to achieve the recovery of restricted rats we evaluated several anthropometric parameters. As shown in Table 1, undernourished 70-day-old rats weighed nearly 50% less than controls and their length was reduced by 20%. Both parameters remained under control values in the permanently restricted rats at 9 months of age. Rats fed with cafeteria diet weighed more than their matched lab chow animals. The percent of weight gained after exposure to this diet was much higher in the restricted rats than in controls: 138% (U-Caf9m) and 44% (C-Caf9m). The percentage of weight gained by U-Std9m rats was 113%, also much higher than that of controls (C9m, 29%). Between 70 days and 9 months body length was enhanced by 20% in control rats, regardless of the type of diet. During this period, the length increased by 30% in the permanently food-restricted rats (U9m), by 40% in those refed with the lab chow (U-Std9m), and by 48% in those transferred to cafeteria diet (U-Caf9m), whose final length was equivalent to controls. Abdominal and thoracic diameters of 9-month-old control rats on the cafeteria diet were about 10% higher than those of rats on chow. No significant differences in these parameters were observed between U9m and U-Std9m animals. However, the cafeteria diet induced a 26% increase in the abdominal diameter of restricted rats, thus matching the control value. There were no differences in the BMI between the two groups of 70-day-old animals. This parameter remained unchanged until 9 months in the controls fed the standard chow and increased in those transferred to the cafeteria diet. BMI underwent with age a nearly 25% drop in undernourished rats, which did not happen if they were transferred to lab chow or cafeteria formula. The LBM increased between 70 days and 9 months in control rats: 32% under chow and 46% under the cafeteria diet. It was lower in undernourished but increased by 50% over this period, despite maintaining food restriction. Importantly, the LBM increase markedly in U rats transferred to ad libitum regimes: 140 and 123% with cafeteria diet and lab chow, respectively. This parameter was normalized in both cases.
TABLE 1.
Anthropometric characteristics and serum profiles of different groups of rats
Values are the mean ± S.E. for 6–7 determinations. Values in the same row with different superscript letters are significantly different (p < 0.05).
| 70 days | 9 months |
70 days |
9 months |
||||
|---|---|---|---|---|---|---|---|
| C70d | C9m | C-Caf9m | U70d | U9m | U-Std9m | U-Caf9m | |
| Body weight (g) | 188 ± 2a | 243 ± 5b | 271 ± 5c | 97 ± 4d | 143 ± 4e | 207 ± 6f | 231 ± 7b |
| Length nose-anus (cm) | 19.6 ± 0.2a | 23.5 ± 0.3b | 23.6 ± 0.2b | 15.7 ± 0.2c | 20.6 ± 0.2d | 21.9 ± 0.4e | 23.2 ± 0.2b |
| Abdominal diameter (cm) | 70.4 ± 1.6a | 70.8 ± 2.9a | 80.0 ± 1.7b | 38.0 ± 1.0c | 56.9 ± 1.4d | 53 ± 0.4e | 71.9 ± 1.8a |
| Toracic diameter (mm) | 35.8 ± 1.3a | 34.3 ± 0.7a | 37.3 ± 0.3b | 19.4 ± 0.7c | 33.2 ± 0.6a | 36.5 ± 0.4a | 35.3 ± 0.5a |
| BMI (kg/m2) | 4.5 ± 0.1a | 4.4 ± 0.1a | 4.9 ± 0.1b | 4.7 ± 0.2a | 3.4 ± 0.2c | 4.5 ± 0.1a | 4.3 ± 0.1a |
| LBM (g) | 161 ± 3a | 212 ± 4b | 235 ± 6c | 87 ± 4d | 131 ± 6e | 194 ± 5b | 210 ± 7b |
| Serum glucose (mg/100 of ml) | 80 ± 3a | 89 ± 6a | 95 ± 3a | 81 ± 3a | 81 ± 8a | 93 ± 8a | 81 ± 3a |
| Serum insulin (ng/ml) | 2.0 ± 0.3a | 2.8 ± 0.6a | 2.0 ± 0.6a | 0.5 ± 0.1b | 2.2 ± 0.4a | 0.8 ± 0.1b,c | 0.9 ± 0.1c |
| Serum triglycerides (mg/100 of ml) | 45 ± 4a | 50 ± 4a | 53 ± 2a | 30 ± 2b | 34 ± 4b | 33 ± 2b | 59 ± 2c |
| Serum cholesterol (mg/100 of ml) | 62 ± 2a | 63 ± 3a | 62 ± 3a | 71 ± 3b | 56 ± 5a | 72 ± 6b,c | 78 ± 2c |
High-lipid Diet Increases Serum Triglycerides and Cholesterol without Altering Glycemia
We wanted to know if undernutrition following overfeeding entailed changes in the levels of some blood metabolites and hormones. There were no significant differences in glycemia of different groups (Table 1). Serum levels of triglycerides and cholesterol were unaffected, or less affected, by age or type of diet in control rats (Table 1). Triglycerides were decreased and cholesterol increased in 70-day-old restricted rats, compared with controls. At 9 months, cholesterol was increased in those animals that, after undernutrition, were transferred to chow or cafeteria diet. Concerning triglycerides, they were markedly increased in undernourished animals rehabilitated with the cafeteria formula.
Undernutrition Modifies Insulinemia and Reduces the Postnatal Surge of Leptin
Serum insulin was markedly reduced in 70-day-old restricted rats but increased at 9 months to reach the values of age-matched controls. At that UCaf9m and UStd9m had higher insulinemia than U70d animals, although lower than U9m and all control groups (Table 1). Serum leptin was analyzed at different stages during suckling (Fig. 1b). At 1 day of life we found similar levels in control and restricted pups. It was followed by an equivalent rise at 5 days. Leptin continued to increase transiently in controls, reaching a peak at 10 days, contrasting with the profile of the restricted pups at 10 and 14 days, when leptinemia was extremely low as compared with controls. At weaning there were similar low levels in both populations.
Cafeteria Formula Does Not Induce Obesity in the Previously Undernourished Rats
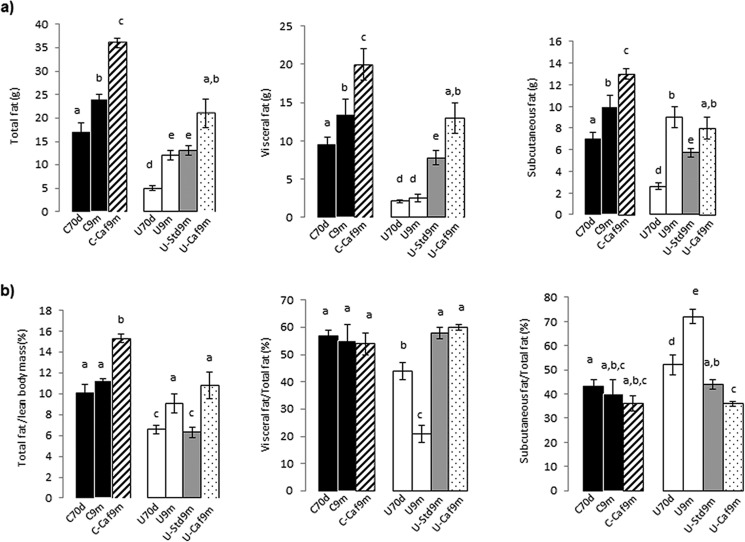
It is still a matter of discussion whether overfeeding after early growth retardation increases the risk of obesity. To provide information regarding this issue in a condition of severe retardation, we evaluated by NMR the amount of white adipose tissue. The whole body fat mass was about 3-fold lower in 70-day-old undernourished rats than in controls: 5.9 ± 0.5 versus 17.0 ± 2.0 g (p < 0.001), due to marked reductions in both visceral and subcutaneous fat depots (Fig. 2a). When rats were maintained until 9 months on standard chow provided either limitedly (U9m) or ad libitum (C9m, U-Std9m), the total fat mass increased. It was the same in control animals subjected to cafeteria diet (C-Caf9m) but they accumulated more fat mass. Two remarkable results were the following: (a) undernourished rats transferred to a high-lipid diet experienced the largest relative increase considering the previous content: 3.5- versus 2.3-fold in controls and (b) total fat measured in rats rehabilitated with lab chow was similar to that present in the permanently restricted animals. With the cafeteria formula, the increment in specific visceral fat was much higher in relative terms in undernourished than in control rats: 6- (U-Caf9m) versus 2-fold (C-Caf9m). In undernourished rats rehabilitated with the standard chow (U-Std9m) the increase in this parameter was ∼4-fold, also higher than in controls (Fig. 2a). Regarding subcutaneous fat, we found an increase between 70 days and 9 months in control rats, higher in the group under the cafeteria formula. An age-related increase of this fat pad was also found in the restricted group, but more pronounced (Fig. 2a).
FIGURE 2.
Body fat content and fat distribution between visceral and subcutaneous pads, expressed as total mass (a). Total fat expressed per unit of lean body mass, visceral, and subcutaneous fat expressed as percentage of total body fat (b). The different groups of rats are the same indicated in legend to Fig. 1c. Values are the mean ± S.E. for 6–7 rats. Values with a different letter differ significantly, p < 0.05.
Fat accumulated per unit of LBM remained unchanged with age in control rats, but increased by 50% under the cafeteria diet (C-Caf9m). This ratio was lower in 70-day-old food-restricted rats but increased with age despite maintaining restriction. This parameter did not increase when these animals were transferred to lab chow ad libitum but was notably increased (by 64%) when they were rehabilitated with cafeteria formula (Fig. 2b).
The amounts of each specific adipose depot relative to total fat content are shown in Fig. 2b. These proportions experienced no significant changes in the control groups. At 70 days and, especially, 9 months the proportions of visceral fat were lower in restricted rats than in controls, but they underwent marked increases after rehabilitation, irrespective of the type of diet. Conversely, the subcutaneous fat relative to total fat was significantly elevated in undernourished animals of both ages and decreased to control values with nutritional rehabilitations.
Ectopic Triglycerides Are Increased in Undernourished Rats Switched to Cafeteria Formula
The increase of triglycerides within cells of non-adipose tissues induces a loss of insulin sensitivity, so we explored the existence of ectopic lipid deposits in liver and muscle. No significant differences in triglycerides deposited in liver were found between control groups of rats but undernutrition decreased them by 50% (Table 2). Transfer of restricted animals to ad libitum feeding increased these lipids: about twice with lab chow and three times with cafeteria diet. Hepatic cholesterol decreased by 25% with age in all control rats. This lipid decreased in undernourished rats, rising after rehabilitation with the cafeteria diet but not with standard chow. Muscle triglycerides were slightly enhanced in restricted rats, an unexpected result, and transfer to ad libitum diets implied further increases, most notably with the high lipid diet.
TABLE 2.
Liver and muscle deposits of triglycerides and cholesterol
Values are the mean ± S.E. for 6–7 determinations. Values in the same row with different superscript letters are significantly different (p < 0.05).
| 70 days | 9 months |
70 days |
9 months |
||||
|---|---|---|---|---|---|---|---|
| C70d | C9m | C-Caf9m | U70d | U9m | U-Std9m | U-Caf9m | |
| Liver triglycerides (μg/mg of protein) | 10.8 ± 1.1a | 10.9 ± 1.5a | 12.2 ± 1.2a | 5.9 ± 0.4b | 5.2 ± 0.7b | 13.4 ± 2.1a | 18.7 ± 1.9c |
| Liver cholesterol (μg/mg of protein) | 4.3 ± 0.1a | 3.2 ± 1.2b | 3.6 ± 0.2b | 3.2 ± 0.1b | 3.0 ± 0.2b | 3.0 ± 0.2b | 3.9 ± 0.2a |
| Muscle triglycerides (μg/mg of protein) | 3.9 ± 0.2a | 5.8 ± 0.6b | 5.3 ± 1.0b | 6.6 ± 1.5b | 7.2 ± 1.4b | 9.7 ± 0.7c | 20.1 ± 3.9d |
| Muscle cholesterol (μg/mg of protein) | 1.2 ± 0.1a | 1.6 ± 0.2a | 1.1 ± 0.09a | 1.7 ± 0.2a | 1.6 ± 0.1a | 1.0 ± 0.2a | 1.1 ± 0.09a |
POMC Is Reduced and NPY Is Increased in Hypothalamus of Undernourished Rats
Trying to explain the cause of hyperphagia experienced by undernourished rats, we analyzed two essential factors for hypothalamic regulation of food intake: NPY, orexigenic, and POMC, anorexigenic. No changes were found in their expressions in control groups (Fig. 1c). Food restriction significantly increased NPY, whereas reducing POMC. The switch to the ad libitum feeding regime resulted in a significant decline in NPY expression to control values, regardless of the type of diet. In contrast, POMC expression was stimulated by the transfer to the standard laboratory diet and depressed by the cafeteria formula.
Undernutrition Induces Hypothalamic Leptin and Insulin Resistances
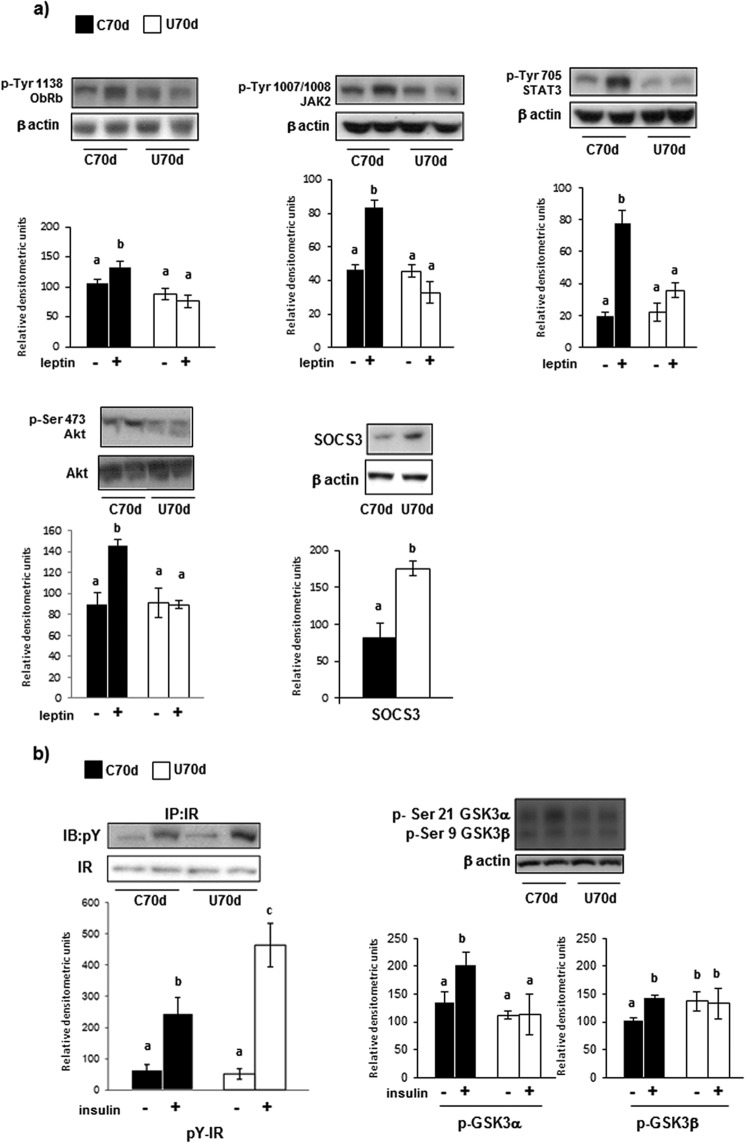
Leptin and insulin are afferent signals to the hypothalamus, exerting overlapping effects that result in a reduction of appetite. So we analyzed their main signaling proteins in the hypothalamus of 70-day-old rats, checking possible changes to help understand the hyperphagia of undernourishment. The abundances of leptin receptor (ObRb), JAK2, and STAT-3 were not affected by undernutrition (results not shown). Central administration of leptin increased the phosphorylation of ObRb, JAK2, and STAT3 in control but not in restricted rats. Because the PI3K/Akt pathway is also involved in leptin action, we analyzed the effect of intracerebroventricular leptin administration on p-Akt, showing a marked increase in control rats without changes in undernourishment. The amount of SOCS3 was higher in these animals than in their controls (Fig. 3a). With respect to insulin signaling, there were no differences between the two groups of 70-day-old rats regarding the receptor (βIR), IRS-1/2, Akt, and GSK3 (results not shown). After intracerebroventricular administration of insulin, the receptor phosphorylation increased in both groups of animals, more markedly in the restricted. Analysis of p-GSK3 showed that insulin stimulated the phosphorylation of two isoforms in control rats, a response not elicited in the restricted (Fig. 3b).
FIGURE 3.
Proteins involved in leptin (a) and insulin (b) signaling quantified in hypothalamus of control (C) and undernourished (U) 70-day-old rats, analyzed by Western immunoblotting. The results of densitometry, expressed as arbitrary units and given as bars, are the mean ± S.E. of 6–7 separate determinations. The blots shown are representative. Data were normalized to level of β-actin. Leptin (25 mg/kg body weight), insulin (10 IU/kg body weight), or vehicle were injected into the third cerebral ventricle, as indicated under “Experimental Procedures.” Values with a different letter differ significantly, p < 0.05.
Undernutrition followed by High-lipid Diet Increases the Markers of Inflammation and Browning in White Adipose Tissue
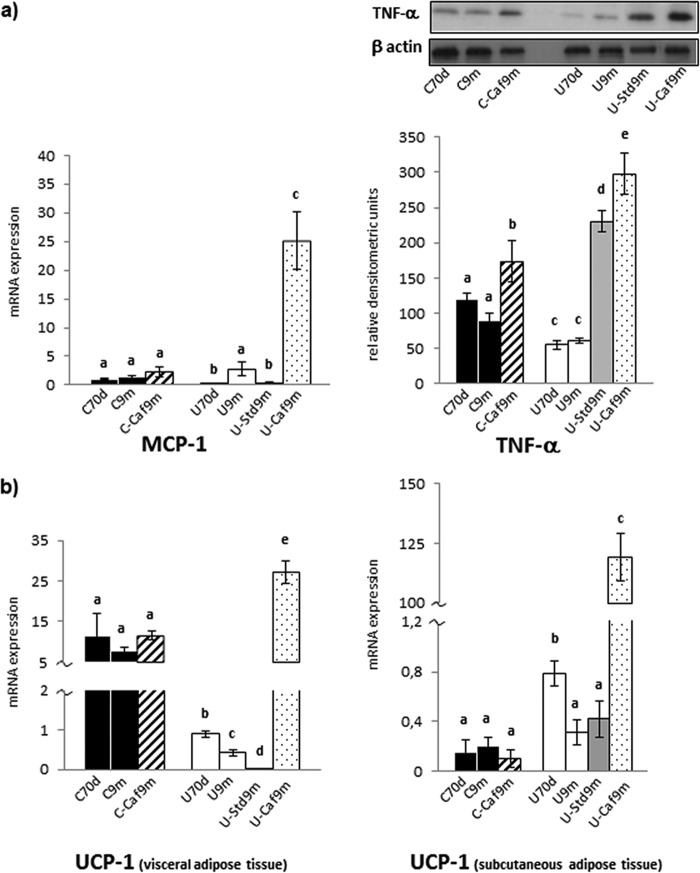
As has been seen, restricted rats submitted to the high-lipid diet did not develop obesity but showed typical alterations of this condition. Obesity results in an inflammatory state in which macrophage accumulation in adipose tissue plays a crucial role. mRNA levels of MCP-1, which contributes to macrophage infiltration, were similarly low in visceral adipose tissue of all control groups. The most striking result occurred in restricted rats transferred to the cafeteria formula, in which MCP-1 mRNA was increased by 10-fold (Fig. 4a). The content of the inflammatory factor TNF-α was assessed in the same tissue. It was depressed in undernourished animals. Hypercaloric feeding induced an increase of this protein in control rats, but was much higher in the undernourished rats under either of the two ad libitum diets (Fig. 4a).
FIGURE 4.
MCP-1 mRNA expression, TNF-α protein level (a) and UCP-1 mRNA expression in visceral white adipose tissue and in subcutaneous adipose tissue (b) of control (C), undernourished (U), and rehabilitated rats, as indicated in the legend of Fig. 1c. The relative levels of mRNA are expressed as indicated under “Experimental Procedures.” Each bar represents mean ± S.E. for 6–7 rats. Representative blots shown correspond to TNF-α and β-actin, analyzed to normalization. Values with a different letter differ significantly, p < 0.05.
Brown-like adipocytes in white adipose tissue (referred to as brite) can carry out thermogenesis (22), a mechanism that may reduce obesity. To test the possibility of browning, we analyzed the uncoupling protein UCP-1 levels in both visceral and subcutaneous white adipose tissues, showing no alterations among control groups. Food restriction markedly decreased UCP-1 expression in visceral fat, which was barely detectable in rats rehabilitated with laboratory chow. In contrast, this expression experienced a sharp increase, nearly 60 times, in animals transferred to the cafeteria formula (Fig. 4b). Unlike what was observed in visceral pad, UCP-1 expression in subcutaneous fat was elevated in 70-day-old undernourished rats as compared with controls and it decreased to control values at 9 months, both in restricted rats and in those switched to standard chow. The most striking result was the sharp rise in UCP-1 expression in UCaf-9m: nearly 280 times (Fig. 4b).
Hypercaloric Diet Affects the Hepatic Redox Status More Markedly after Undernutrition
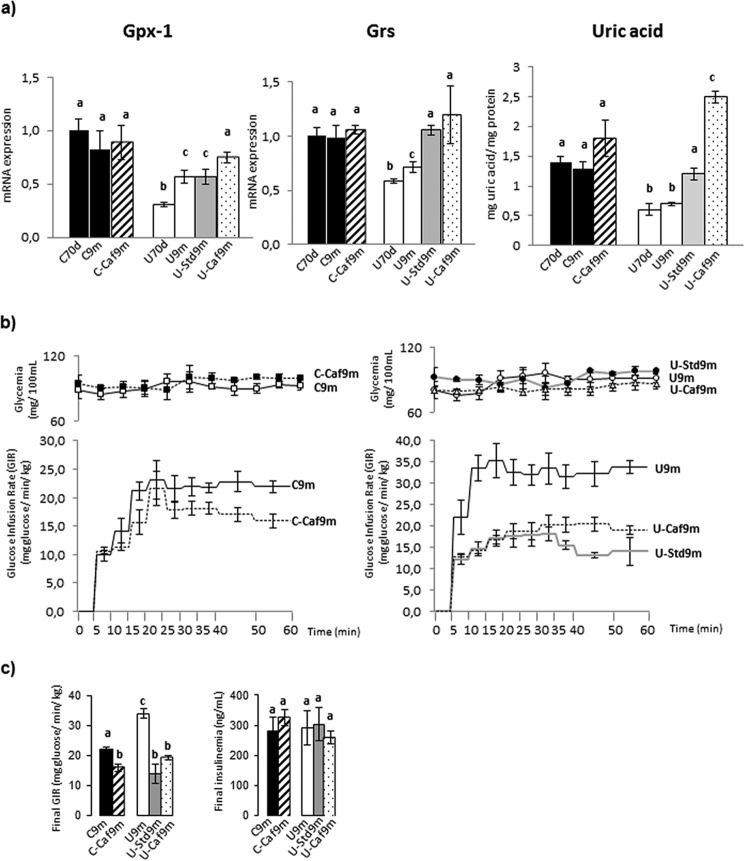
We analyzed three factors closely related to oxidative stress in liver: the antioxidant enzymes glutathione peroxidase-1 (GPx-1) and glutathione reductase (GRs) and also uric acid (Fig. 5a). Both enzyme expressions and uric acid remained unchanged among control groups. Undernutrition induced significant decreases in all of them. They tended to rise following the transfer of restricted rats to diets ad libitum, except GPx-1, which was not altered in the group rehabilitated with laboratory chow. It should be noted the sharp increase in uric acid in Ucaf9m rats.
FIGURE 5.
GPx-1 mRNA, GRs mRNA, and uric acid content in liver of C, U, and rehabilitated rats, as indicated in legend of Fig. 1c. The relative levels of mRNA are expressed as indicated under “Experimental Procedures” (a). Glucose infusion rate, glycemia during the euglycemic-hyperinsulinemic clamps (b), final glucose infusion rate and final steady-state insulinemia in 9-month-old control (C), undernourished (U), and rehabilitated rats, are as indicated in the legend of Fig. 1c (b). Data, given as bars, are the mean ± S.E. of 6 to 7 independent determinations in each case. Values with a different letter differ significantly, p < 0.05.
Undernutrition Increases Insulin Sensitivity, Which Is Reduced by the High-lipid Diet
Nutritional conditions strongly affect the tissue responsiveness to insulin. Therefore, it was important to know if insulin hypersensitivity resulting from undernutrition was reversed after submission to ad libitum diets. To this end, we measured the overall insulin sensitivity by clamp experiments. Fig. 5, b and c, depict the results from clamps performed on 9-month-old rats. Blood glucose concentrations were maintained within the physiological range in all groups (Fig. 5b). The glucose infusion rate was then 55% greater in the restricted rats (U9m) than in controls (C9m). The change of diet regime was associated with significant declines in all cases but the reduction was proportionally greater in animals previously undernourished than in controls (Fig. 5, b and c). Steady-state hyperinsulinemia was at the same level in all groups of animals (Fig. 5c).
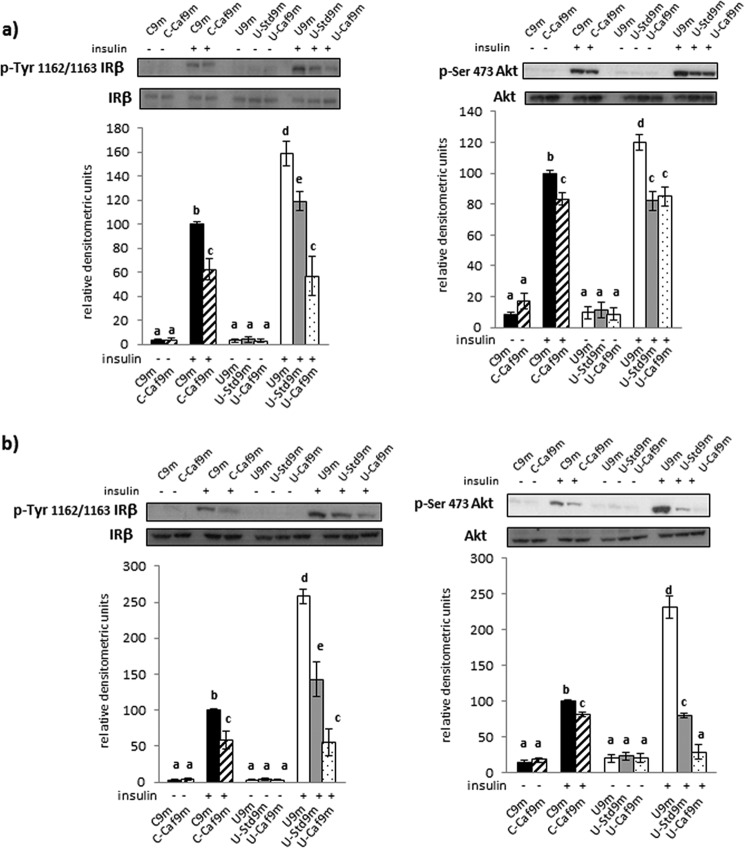
As a first approach to find specific tissues involved in the changes of whole body insulin sensitivity, we examined two steps of the insulin-signaling pathway, i.e. receptor and Akt phosphorylations, in liver and muscle of all 9-month-old populations. We did not find alterations in the total content of these proteins. Phosphorylation of the hepatic insulin receptor was increased in all groups following insulin stimulation but more markedly in undernourished rats. Transfer to the cafeteria diet reduced this response in controls. In the case of undernourished rats, the intensity of phosphorylation was also reduced after switching to ad libitum feeding regimes, especially with the cafeteria formula (Fig. 6a). Comparable results were observed regarding Akt phosphorylation, although in this case we did not find differences in the level of phospho-Akt between the groups subjected to either chow or the cafeteria diet (Fig. 6a). As expected, insulin receptor phosphorylation increased in the skeletal muscle of control and undernourished rats following insulin. Again, this response was markedly higher in the restricted animals. The high-lipid diet reduced the proportion of such responses in all groups of animals, but most notably in the undernourished (Fig. 6b). With regard to Akt phosphorylation, the changes observed after insulin treatment were roughly parallel to those of receptor phosphorylation (Fig. 6b).
FIGURE 6.
Phosphoinsulin receptor and phospho-Akt protein level in liver (a) and skeletal muscle (b) of C, U, and rehabilitated rats, as indicated in the legend of Fig. 1c. Each bar represents mean ± S.E. for 6–7 rats. Representative blots shown correspond to phospho-IR, and phospho-Akt. Total insulin receptor and total Akt were analyzed to normalization. Values with a different letter differ significantly, p < 0.05.
Discussion
Hyperphagia
Most hyperlipidic diets in experimental models of obesity contain a high proportion of calories from fat, 40–60%, to quickly achieve an obese insulin-resistant phenotype (23). This proportion was lower in the cafeteria formula used in this study, ∼25%. High-lipid diets tend to induce hyperphagia, partially due to its concomitant richness in sucrose. The control rats under our cafeteria formula reduced the intake in weight terms, which, nevertheless, was similar in terms of calories to that of controls fed with standard chow a not uncommon result. In the undernourished rats, the consequences of rehabilitation differed: calorie intake with the lab chow increased marginally, whereas the high-fat formula induced hyperphagia. It is known that early undernutrition induces a specific preference for high-fat foods (24), although the determinants of such behavior are not fully known. Nevertheless, as discussed below, some hypothalamic changes as well as leptin and insulin resistance could partially explain these disorders. In addition, the rate of gastric emptying is higher in early undernourished rats transferred to high-fat diets, which might lessen the feeling of satiety (25).
Central Changes
The hypothalamic changes seen in NPY and POMC expression fit well with hyperphagia of undernourished rats. Particularly, disorders in the central anorexigenic pathways are essential for the programming of long-lasting energy balance disorders associated with early undernutrition (26), so the fact that POMC expression remained low in restricted rats rehabilitated with cafeteria diet, suggests an important role in their hyperphagia. The arcuate nucleus contains receptors for both leptin and insulin. They activate and inhibit POMC and NPY neurons, respectively, resulting in reduced food intake. Leptin binding to its receptor (ObRb) stimulates the JAK/STAT and IRS/PI3K pathways. Our data show a defective leptin signaling in both pathways of undernourished rats that might be responsible for its hyperphagia. This does not rule out that other mechanisms, such as blood-brain barrier disorders, could account for this hyperphagia. Indeed, Yura et al. (27) reported impaired transport of leptin across the brain-blood barrier in adult mice with fetal undernutrition. We also found a sharp increase in SOCS3, a suppressor of cytokine signaling, in undernourished rats. García et al. (28) have shown that the offspring of food-restricted dams also present high hypothalamic SOCS3 expression.
Insulin binding to its receptor (IR) activates PI3K through IRS1/2 phosphorylation, resulting in Akt activation. GSK3, a substrate of Akt, is an important transducer signal for the anorectic effects of insulin. The increase in phosphorylated IR, following hormone administration, was much higher in undernourished animals than in controls, as has been observed in adult rats with fetal undernutrition (29). However, insulin did not increase phosphorylated GSK3, indicating hormone resistance. The same results have been reported for undernourished fetuses (30). The insulin and leptin central resistances together with their low plasma levels, discussed later, help explain the increased energy intake of the undernourished animals.
Leptin Surge
Plasma leptin undergoes a transient increase (the leptin surge) in suckling rodents, showing a peak around the 10th day of life (31). It participates in the growth of neuronal circuitry involved in energy homeostasis. We found that the leptin surge was much shorter in the undernourished rats and did not peak at 10 days. Comparable results have been reported in other models of early nutrition imbalances (32, 33). These deficiencies could reflect decreased milk or leptin production, or impaired absorption. Leptin is essential for the connections between NPY neurons and the paraventral nucleus, as well as for POMC projections toward other nuclei (34). Yura et al. (27) have reported these disorders in prenatally undernourished mice, which experienced premature leptin surge; these animals developed an obesity-prone phenotype. Offspring of obese rats displayed a prolonged leptin surge and subsequently exhibited hyperphagia (35). On this basis, we suggest that the impaired leptin surge seen in the offspring of food-restricted rats leads to the improper development of hypothalamic functions involved in appetite control, leading to the excessive intake of high-fat food.
Adiposity
The results of our study are consistent with the fact that fat mass increases through middle age in both humans and rodents (36). In the control rats, both visceral and subcutaneous adipose tissues contributed to the increase, whereas only subcutaneous fat significantly developed with age in the undernourished. Exposure to the cafeteria diet resulted in fat gain in both rat groups. However, obesity only occurred in the control animals, whose body weight and BMI significantly increased. Another marked difference between the control and food-restricted rats was that the relative gain in both types of fat was much larger in the latter group, showing that undernutrition can program a rapid replenishment of fat stores. It should be noted that this condition does not amplify the obesogenic effect of the cafeteria formula despite the relatively larger accretion of fat. This is due to the fact that LBM increases in undernourished rats. It enables them to completely catch up on the parameters of length and BMI and keeps the fat/LBM ratio low. The present study was conducted in female rats so it is not possible to draw conclusions on the consequences in males.
The control rats' LBM increased throughout the study period, especially when they became obese after transferring to the cafeteria diet. A comparable phenomenon has been seen in humans, whose LBM tends to rise with obesity (37). After switching to ad libitum diets, the undernourished rats' LBM increased more markedly, which explains why their body lengths reached similar (with chow) or identical (with cafeteria formula) values to that of the age-matched controls, despite the severity and duration of food restriction. In view of the undernourished animals' high capacity to grow, the specific effect of the nutritional transfer on adiposity can be better estimated by analyzing the changes in the body fat/LBM ratio between 70 days and 9 months. This ratio increased in all rat groups subjected to the cafeteria formula but the increase was proportionally greater in the undernourished, thus confirming their notably higher capacity for fat accumulation under hyperlipidic regimes. In contrast, when lab chow was available ad libitum, the animals' body fat/LBM ratio did not change, that is, the rats did not gain fat per unit of lean mass. The proportion of visceral and subcutaneous fat pads relative to total fat mass remained unchanged in the control rats transferred to the cafeteria diet. In contrast, this formula, and the chow, had significant effects on these parameters in the undernourished. Both diets increased the proportion of visceral fat. Thus, undernutrition seems to predispose to a change in fat distribution when the circumstances allow for fat accretion, a fact that may involve an increase of metabolic risks. We therefore decided to explore whether adipose tissue from the restricted-rehabilitated rats had unhealthy characteristics, focusing on visceral fat, which is the most important with regard to various diseases (38).
Inflammation and Macrophages
The dramatic increase in MCP-1 expression specifically seen in the visceral adipose tissue of undernourished rats subjected to the high-lipid diet suggests a high capacity for accumulating macrophages. Adipose tissue macrophages are classified as proinflammatory (M1) or anti-inflammatory (M2). A switch from the M2 to M1 phenotype occurs after migration to obese adipose tissue. M1 macrophages synthesize large amounts of proinflammatory mediators, including the majority of TNF-α present in adipose tissue. Undernutrition was associated with low TNF-α level. They increased in all rehabilitated rats but more markedly in the undernourished transferred to the high-fat diet, which is consistent with their higher MCP-1 expression. The rise in TNF-α in the other groups was unexpected, considering their low MCP-1 mRNA levels; however, adipocytes can also synthesize TNF-α (39). Perhaps an increase in TNF-α production by fat cells occurred in these groups. TNF-α plays a prominent role in the low-grade inflammation associated with obesity and insulin resistance. Therefore, our study demonstrated that early chronic undernutrition worsens the inflammatory profile associated with fat mass expansion in response to a high-lipid diet. Even a balanced diet seems to aggravate this profile for rats exposed to early chronic food restriction.
Browning
The distinct dietary regimes had no effect on UCP-1 expression in white adipose tissues from the control rats. This expression was markedly depressed in the visceral fat of the food-restricted rats, as seen in early undernourished mice (40). This decrease probably limits energy expenditure thereby favoring fat deposition, adaptations appropriate for food restriction. The transfer of these rats to high-lipid diets significantly increased visceral UCP-1 expression, which probably indicates larger amounts or activity of brite adipocytes, an adaptation that could constrain a rapid gain in fat mass despite their hyperphagia. Surprisingly, UCP-1 expression declined to nearly undetectable levels after rehabilitation with chow, perhaps to minimize the fat catabolism for heat generation in white adipose tissue, which is inadequate when the priority is to catch up. Contrary to what was found in the visceral pad, subcutaneous UCP-1 expression was enhanced in 70-day-old undernourished rats, an unexpected result not explained at present. A most remarkable result was the increase in the subcutaneous UCP-1 of rats transferred to the high-lipid diet, which was much higher than that shown in visceral pad. It is known that subcutaneous white adipose tissue is more prone to browning than visceral, because of its high PRDM16 expression, which is a major transcriptional regulator of brown-like fat cell differentiation (41). Increased brite adipocyte levels and hyperexpression of ectopic UCP-1 confer resistance to diet-induced obesity (42, 43), as probably occurs in the undernourished rats.
Dyslipidemia, Ectopic Lipids, and Oxidative Stress
The cafeteria formula did not modify either triglyceridemia or cholesterolemia in the control rats, in contrast to other regimes richer in fat (44). When the undernourished were transferred to lab chow, their reduced triglyceride levels persisted. However, a transfer to the cafeteria diet raised serum triglyceride and cholesterol levels, causing hyperlipidemia. Therefore, a moderately hyperlipidic diet following chronic undernutrition promotes a biochemical blood profile associated with cardiovascular risk.
Hypercaloric feeding increases ectopic lipid levels. The moderately high-lipid cafeteria formula did not change the hepatic content of triglycerides in the control rats, but this diet and the lab chow induced significant increases in the previously food-restricted animals. Earlier studies support the concept that uric acid is a biomarker of fatty liver (45, 46). Thus, the observed increase of this metabolite in liver of undernourished-rehabilitated rats would be in agreement with the possible development of fatty liver, as also suggested by the above mentioned increase in triglycerides. Regarding muscle, the restricted rats also showed a slight increase in triglyceride levels, which might have resulted from reduced oxidation as an adaptation to the limited dietary lipid availability. Muscle triglyceride levels notably increased when these animals were given ad libitum food, especially the high-lipid diet. This seems consistent with a further reduction in lipid catabolism. In fact, high-fat diets decrease muscle oxidative capacity, inducing a fiber transition from type I to II (47), with fewer mitochondria.
It is known that undernutritrion increases insulin sensitivity (see below), whereas ectopic lipids lead to insulin resistance. Consistently, we have found that the elevated effects of insulin on its receptor and Akt activations in liver and skeletal muscle of undernourished rats were markedly reduced after rehabilitation with the cafeteria diet, in coincidence with the ectopic lipid deposition in these tissues.
Oxidative stress is involved in liver disorders and can be produced by increasing pro-oxidant systems and/or reducing antioxidant enzymes. GPx and GRs were not affected by the high-lipid diet in the controls. However, both activities were decreased in the undernourished rats, which could be an adaptation to hypothetically reduced reactive oxygen species generation associated with caloric restriction (48). With the transfer to the ad libitum regime, the rats' GRs expression increased with both diets, as did GPx expression with the cafeteria formula. A stimulated liver catabolism resulting from the greater availability of nutrients could result in greater reactive oxygen species generation and a consequent increase in antioxidant defenses, which did not happen in the control rats. These results suggest that hypercaloric diets affect the hepatic redox state more strongly when provided after undernutrition.
Insulin Sensitivity
Our clamp studies corroborate that undernutrition increases insulin sensitivity (16). Exposure to the high-fat diet decreased it in both the control and restricted-diet rats, confirming that these diets promote insulin resistance (49). The new finding here is that insulin responsiveness is reduced after chronic food restriction even with a balanced diet, suggesting that undernutrition predisposes animals to this disorder. The results regarding effects on liver and muscle insulin signaling corroborate this suggestion.
It is noteworthy that the controls transferred to the cafeteria formula remain normoglycemic and normoinsulinemic despite their insulin resistance. At present, the mechanisms leading to this unexpected situation are unknown.
In 9-month-old undernourished rats, insulinemia has been normalized, which could be explained by a regenerative capacity of β-cells previously reported in undernourished adult rats (50). After rehabilitation, these rats became hypoinsulinemic, regardless of dietary regime. Perhaps alterations in liver responsiveness to counterregulatory hormones could be an explanation. In fact, it has been shown that early restricted rats have liver glucagon resistance (14). The possibility that a similar situation develops in restricted-rehabilitated rats remain to be investigated.
Conclusion
Population studies have suggested an association between early undernutrition and increased risk for obesity, although this is controversial. In the present study, based on a rat model of severe food restriction, the rehabilitation of these animals with either a moderately high-lipid or a balanced diet did not lead to overt obesity. However, the previous undernutrition worsened the deleterious effects associated with rehabilitation, especially with the high-fat diet. The undernourished rats experienced hypothalamic disorders favoring hyperphagia. After transferring these rats to a hypercaloric diet, the rapid replenishment of fat stores and weight recovery were accompanied by dyslipidemia and ectopic lipid deposition, along with signs of steatosis. In addition, these animals increased the proportion of visceral fat, which presented a more proinflammatory profile. It can be concluded that adult rats subjected to severe undernutrition at an early age have a greater susceptibility to present metabolic alterations after nutritional rehabilitation, even if they do not achieve obesity parameters.
Author Contributions
E. L. and F. E. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. F. E. was the study supervisor and the corresponding author. F. E., E. L., and C. A. were responsible for the study concept and design. Data was acquired by E. L., E. F., M. G., J. T., T. F., M. R., C. A., and F. E. and was analyzed and interpreted by all authors. Intracerebroventricular injections were performed by M. G., T. F., and M. R. The manuscript was drafted by E. L. and F. E. and all authors revised it critically for important intellectual content. All authors gave final approval to the version of the manuscript to be published.
Acknowledgments
We thank Dr. M. P. Ramos (Universidad San Pablo CEU, Madrid) who kindly provided the formulation of the experimental diet. We also acknowledge Dr. A. F. Parlow, from the National Hormone & Peptide Program (Harbor-UCLA Medical Center, Torrance, CA), for facilitating the leptin.
This work was supported Grant BFU 2011–25420 from the Ministerio de Economía y Competitividad (MINECO), Grant MOIR S2010/BMD-2423 from Comunidad de Madrid, CIBER de Diabetes y Enfermedades Metabólicas (CIBERDEM, ISCIII), and Grant CCG97-UCM/SAL-30041 from UCM-Comunidad de Madrid. The authors declare that they have no conflicts of interest with the contents of this article.
- C
- control
- U
- undernourished
- C70D
- control 70 days
- U70D
- undernourished 70 days
- C9m
- control 9 months given chow
- C-Caf9m
- control 9 months given cafeteria diet
- U9m
- undernourished 9 months kept on the standard chow restricted to 35%
- U-Std9m
- undernourished 9 months switched to lab chow
- U-Caf9m
- undernourished 9 months transferred to cafeteria formula
- POMC
- pro-opiomelanocortin
- NPY
- neuropeptide Y
- BMI
- body mass index
- LBM
- lean body mass
- GPx-1
- glutathione peroxidase-1
- GRs
- glutathione reductase.
References
- 1. Koletzko B., Chourdakis M., Grote V., Hellmuth C., Prell C., Rzehak P., Uhl O., Weber M. (2014) Regulation of early human growth: impact on long-term health. Ann. Nutr. Metab. 65, 101–109 [DOI] [PubMed] [Google Scholar]
- 2. Vickers M. H., Sloboda D. M. (2010) Prenatal nutritional influences on obesity risk in offspring. Nutr. Diet. Suppl. 2, 137–149 [Google Scholar]
- 3. Ravelli A. C., van Der Meulen J. H., Osmond C., Barker D. J., Bleker O. P. (1999) Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 70, 811–816 [DOI] [PubMed] [Google Scholar]
- 4. de Rooij S. R., Roseboom T. J., Painter R. C. (2014) Famines in the last 100 years: implications for diabetes. Curr. Diab. Rep. 14, 536. [DOI] [PubMed] [Google Scholar]
- 5. Drewnowski A., Specter S. E. (2004) Poverty and obesity: the role of energy density and energy costs. Am. J. Clin. Nutr. 79, 6–16 [DOI] [PubMed] [Google Scholar]
- 6. Misra A., Khurana L. (2008) Obesity and the metabolic syndrome in developing countries. J. Clin. Endocrinol. Metab. 93, S9-S30 [DOI] [PubMed] [Google Scholar]
- 7. Yu Z. B., Han S. P., Zhu G. Z., Zhu C., Wang X. J., Cao X. G., Guo X. R. (2011) Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes. Rev. 12, 525–542 [DOI] [PubMed] [Google Scholar]
- 8. Plagemann A., Harder T., Schellong K., Schulz S., Stupin J. H. (2012) Early postnatal life as a critical time window for determination of long-term metabolic health. Best Pract. Res. Clin. Endocrinol. Metab. 26, 641–653 [DOI] [PubMed] [Google Scholar]
- 9. Würtz P., Wang Q., Kangas A. J., Richmond R. C., Skarp J., Tiainen M., Tynkkynen T., Soininen P., Havulinna A. S., Kaakinen M., Viikari J. S., Savolainen M. J., Kähönen M., Lehtimäki T., Männistö S., Blankenberg S., Zeller T., Laitinen J., Pouta A., Mäntyselkä P., Vanhala M., Elliott P., Pietiläinen K. H., Ripatti S., Salomaa V., Raitakari O. T., Järvelin M. R., Smith G. D., Ala-Korpela M. (2014) Metabolic signatures of adiposity in young adults: mendelian randomization analysis and effects of weight change. PLoS Med. 11, e1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomura K., Eto M., Kojima T., Ogawa S., Iijima K., Nakamura T., Araki A., Akishita M., Ouchi Y. (2010) Visceral fat accumulation and metabolic risk factor clustering in older adults. J. Am. Geriatr. Soc. 58, 1658–1663 [DOI] [PubMed] [Google Scholar]
- 11. Zhang X., Shu X. O., Yang G., Li H., Cai H., Gao Y. T., Zheng W. (2007) Abdominal adiposity and mortality in Chinese women. Arch. Intern Med. 167, 886–892 [DOI] [PubMed] [Google Scholar]
- 12. Buettner R., Schölmerich J., Bollheimer L. C. (2007) High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity 15, 798–808 [DOI] [PubMed] [Google Scholar]
- 13. Bergeron G., Castleman T. (2012) Program responses to acute and chronic malnutrition: divergences and convergences. Adv. Nutr. 3, 242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lizarraga-Mollinedo E., Fernández-Millán E., Martín Jde. T., Martínez-Honduvilla C., Escrivá F., Alvarez C. (2012) Early undernutrition induces glucagon resistance and insulin hypersensitivity in the liver of suckling rats. Am. J. Physiol. Endocrinol. Metab. 302, E1070-E1077 [DOI] [PubMed] [Google Scholar]
- 15. Gavete M. L., Agote M., Martin M. A., Alvarez C., Escriva F. (2002) Effects of chronic undernutrition on glucose uptake and glucose transporter proteins in rat heart. Endocrinology 143, 4295–4303 [DOI] [PubMed] [Google Scholar]
- 16. Escrivá F., Rodríguez C., Cacho J., Alvarez C., Portha B., Pascual-Leone A. M. (1992) Glucose utilization and insulin action in adult rats submitted to prolonged food restriction. Am. J. Physiol. 263, E1-E7 [DOI] [PubMed] [Google Scholar]
- 17. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 18. Fernández-Galaz C., Fernández-Agulló T., Pérez C., Peralta S., Arribas C., Andrés A., Carrascosa J. M., Ros M. (2002) Long-term food restriction prevents aging-associated central leptin resistance in Wistar rats. Diabetologia 45, 997–1003 [DOI] [PubMed] [Google Scholar]
- 19. Escriva F., Agote M., Rubio E., Molero J. C., Pascual-Leone A. M., Andres A., Satrustegui J., Carrascosa J. M. (1997) In vivo insulin-dependent glucose uptake of specific tissues is decreased during aging of mature Wistar rats. Endocrinology 138, 49–54 [DOI] [PubMed] [Google Scholar]
- 20. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−[Delta][Delta] CT) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 21. Thomas E. L., Bell J. D. (2003) Influence of undersampling on magnetic resonance imaging measurements of intra-abdominal adipose tissue. Int. J. Obes. Relat. Metab. Disord. 27, 211–218 [DOI] [PubMed] [Google Scholar]
- 22. Shabalina I. G., Petrovic N., de Jong J. M., Kalinovich A. V., Cannon B., Nedergaard J. (2013) UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 5, 1196–1203 [DOI] [PubMed] [Google Scholar]
- 23. Lai M., Chandrasekera P. C., Barnard N. D. (2014) You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diab. 4, 10.1038/nutd.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bellinger L., Lilley C., Langley-Evans S. C. (2004) Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br. J. Nutr. 92, 513–520 [DOI] [PubMed] [Google Scholar]
- 25. De Toro-Martín J., Fernández-Millán E., Lizárraga-Mollinedo E., López-Oliva E., Serradas P., Escrivá F., Alvarez C. (2014) Predominant role of GIP in the development of a metabolic syndrome-like phenotype in female Wistar rats submitted to forced catch-up growth. Endocrinology 155, 3769–3780 [DOI] [PubMed] [Google Scholar]
- 26. Wattez J. S., Delahaye F., Lukaszewski M. A., Risold P. Y., Eberlé D., Vieau D., Breton C. (2013) Perinatal nutrition programs the hypothalamic melanocortin system in offspring. Horm. Metab. Res. 45, 980–990 [DOI] [PubMed] [Google Scholar]
- 27. Yura S., Itoh H., Sagawa N., Yamamoto H., Masuzaki H., Nakao K., Kawamura M., Takemura M., Kakui K., Ogawa Y., Fujii S. (2005) Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 1, 371–378 [DOI] [PubMed] [Google Scholar]
- 28. García A. P., Palou M., Priego T., Sánchez J., Palou A., Picó C. (2010) Moderate caloric restriction during gestation results in lower arcuate nucleus NPY- and αMSH-neurons and impairs hypothalamic response to fed/fasting conditions in weaned rats. Diabetes Obes. Metab. 12, 403–413 [DOI] [PubMed] [Google Scholar]
- 29. Sardinha F. L., Telles M. M., Albuquerque K. T., Oyama L. M., Guimarães P. A., Santos O. F., Ribeiro E. B. (2006) Gender difference in the effect of intrauterine malnutrition on the central anorexigenic action of insulin in adult rats. Nutrition 22, 1152–1161 [DOI] [PubMed] [Google Scholar]
- 30. Liu X., Qi Y., Gao H., Jiao Y., Gu H., Miao J., Yuan Z. (2013) Maternal protein restriction induces alterations in insulin signaling and ATP sensitive potassium channel protein in hypothalami of intrauterine growth restriction fetal rats. J. Clin. Biochem. Nutr. 52, 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahima R. S., Prabakaran D., Flier J. S. (1998) Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Invest. 101, 1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vickers M. H., Breier B. H., Cutfield W. S., Hofman P. L., Gluckman P. D. (2000) Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 279, E83-E87 [DOI] [PubMed] [Google Scholar]
- 33. Delahaye F., Breton C., Risold P. Y., Enache M., Dutriez-Casteloot I., Laborie C., Lesage J., Vieau D. (2008) Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 149, 470–475 [DOI] [PubMed] [Google Scholar]
- 34. Bouret S. G., Draper S. J., Simerly R. B. (2004) Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 [DOI] [PubMed] [Google Scholar]
- 35. Kirk S. L., Samuelsson A. M., Argenton M., Dhonye H., Kalamatianos T., Poston L., Taylor P. D., Coen C. W. (2009) Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4, e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tchkonia T., Morbeck D. E., Von Zglinicki T., Van Deursen J., Lustgarten J., Scrable H., Khosla S., Jensen M. D., Kirkland J. L. (2010) Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forbes G. B. (1987) Lean body mass-body fat interrelationships in humans. Nutr. Rev. 45, 225–231 [DOI] [PubMed] [Google Scholar]
- 38. Rocha V. Z., Libby P. (2009) Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6, 399–409 [DOI] [PubMed] [Google Scholar]
- 39. Cawthorn W. P., Sethi J. K. (2008) TNF-α and adipocyte biology. FEBS Lett. 582, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kozak L. P., Koza R. A., Anunciado-Koza R., Mendoza T., Newman S. (2012) Inherent plasticity of brown adipogenesis in white fat of mice allows for recovery from effects of postnatal malnutrition. PLoS One 2, e30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonet M. L., Oliver P., Palou A. (2013) Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 1831, 969–985 [DOI] [PubMed] [Google Scholar]
- 42. Schulz T. J., Huang P., Huang T. L., Xue R., McDougall L. E., Townsend K. L., Cypess A. M., Mishina Y., Gussoni E., Tseng Y. H. (2013) Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leonardsson G., Steel J. H., Christian M., Pocock V., Milligan S., Bell J., So P. W., Medina-Gomez G., Vidal-Puig A., White R., Parker M. G. (2004) Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc. Natl. Acad. Sci. U.S.A. 101, 8437–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sumiyoshi M., Sakanaka M., Kimura Y. (2006) Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J. Nutr. 136, 582–587 [DOI] [PubMed] [Google Scholar]
- 45. Li Y., Xu C., Yu C., Xu L., Miao M. (2009) Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J. Hepatol. 50, 1029–1034 [DOI] [PubMed] [Google Scholar]
- 46. Sánchez-Lozada L. G., Mu W., Roncal C., Sautin Y. Y., Abdelmalek M., Reungjui S., Le M., Nakagawa T., Lan H. Y., Yu X., Johnson R. J. (2010) Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur. J. Nutr. 49, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Couturier A., Ringseis R., Mooren F. C., Krüger K., Most E., Eder K. (2013) Carnitine supplementation to obese Zucker rats prevents obesity-induced type II to type I muscle fiber transition and favors an oxidative phenotype of skeletal muscle. Nutr. Metab. (Lond). 10, 10.1186/1743-7075-10-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barja G. (2002) Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res. Rev. 1, 397–411 [DOI] [PubMed] [Google Scholar]
- 49. Storlien L. H., Kriketos A. D., Jenkins A. B., Baur L. A., Pan D. A., Tapsell L. C., Calvert G. D. (1997) Does dietary fat influence insulin action? Ann. N.Y. Acad. Sci. 827, 287–301 [DOI] [PubMed] [Google Scholar]
- 50. Fernández E., Martín M. A., Fajardo S., Bailbé D., Gangnerau M. N., Portha B., Escrivá F., Serradas P., Alvarez C. (2006) Undernutrition does not alter the activation of beta-cell neogenesis and replication in adult rats after partial parcreatectomy. Am. J. Physiol. Endocrinol. Metab. 291, E913–921 [DOI] [PubMed] [Google Scholar]