Abstract
Nephrotic syndrome increases L-thyroxine requirements because of urinary loss of free and protein-bound thyroid hormones. We report 2 hypothyroid patients referred to us because of high serum TSH, even though the L-thyroxine daily dose was maintained at appropriate levels or was increased. The cause of nephrotic syndrome was multiple myeloma in one patient and diabetic glomerulosclerosis in the other patient. As part of the periodic controls for diabetes, urinalysis was requested only in the second patient so that proteinuria could be detected. However, as in the first patient, facial puffiness and body weight increase were initially attributed to hypothyroidism, which was poorly compensated by L-thyroxine therapy. In the first patient, the pitting nature of the pedal edema was missed at the initial examination. An endocrinologist consulted over the phone by the practitioner hypothesized some causes of intestinal malabsorption of L-thyroxine. This diagnosis would have been accepted had the patient continued taking a known sequestrant of L-thyroxine, i.e. calcium carbonate. The diagnostic workup of patients with increasing requirements of L-thyroxine replacement therapy should not be concentrated on the digestive system alone. Careful history taking and physical examination need to be thorough. Endocrinologists should not forget nephrotic syndrome that, in turn, can be secondary to serious diseases.
Key Words: Nephrotic syndrome, L-Thyroxine therapy, Hypothyroidism, Thyroid
What Is Known about This Topic?
• Nephrotic syndrome may trigger the onset of hypothyroidism or aggravate preexisting hypothyroidism, with subsequent increased requirements of the L-thyroxine dose because of urinary loss of free and protein-bound thyroid hormones.
What Does This Case Report Add?
• This case report provides detailed information on 2 cases of augmented L-thyroxine replacement because of nephrotic syndrome. In both cases, symptoms were initially supposed to be due to hypothyroidism.
Introduction
Because of the relatively high prevalence of hypothyroidism and necessity to take L-thyroxine (L-T4) replacement therapy lifelong, L-T4 is one of the medications mostly prescribed worldwide [1]. To monitor this therapy, the target range of TSH ‘should be the normal range of a third generation TSH assay’ which, if unavailable, should be 0.45-4.2 mU/l [1]; in pregnancy, however, laboratory trimester-specific ranges should be used or, if unavailable, the upper normal levels of 2.5, 3.0 and 3.5 mU/l (first, second and third trimester). Approximately 20% of patients receiving L-T4 have TSH levels above target [2], and this is one circumstance in which endocrine consultation is recommended, especially if doses of L-T4 are high [1].
A complete, detailed diagnostic workup for failure of serum TSH to be normalized is multidisciplinary, may require hospitalization and can be expensive [3,4].
Of the literature on this topic [2,3,5,6], only one article [3] mentions nephrotic syndrome as a cause of serum TSH elevation even with an increased daily dose of L-T4. It does so with just one sentence (‘patients with nephrotic syndrome who excrete large quantities of albumin may have increased thyroxine requirements due to binding of T4 to the excreted albumin’) and two references [7,8]. Another article [4] devotes 12 lines and 9 references to nephrotic syndrome, plus a mention in two tables. The very recently released American guidelines [1] fail to mention the renal etiology; however, table 10 in these guidelines contains a subsection entitled ‘Increased clearance’ [1]. These guidelines, though, mention nephrotic syndrome among the causes of decreased serum thyroxine-binding globulin (TBG) [1].
After a relatively old observation from our university hospital was published in an endocrine journal [9], other observations from the same group [10] or other groups have been published more recently in pediatric, nephrology and general medicine journals [7,8,11,12,13,14,15,16,17]. To find another patient described in an endocrine journal one has to go back 6 years [18]. This pattern of publication might have contributed to nephrotic syndrome being overlooked as one of the causes for TSH levels being above target in L-T4-replaced patients. Therefore, we think there is teaching and practical value in reporting the patients described below. Both patients were referred to our ambulatory station for possible L-T4 malabsorption.
Case Reports
Case 1
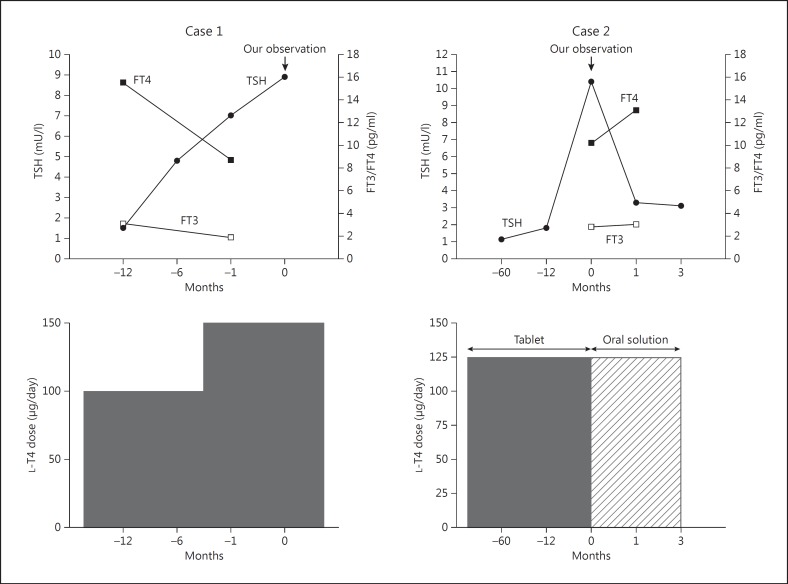
The first case concerns a 63-year-old woman who had been subjected to partial thyroidectomy for a large benign thyroid nodule 3 years before referral to S.B., R.V. and F.D.B. She was referred to us because, according to an endocrinologist consulted over the phone by her practitioner, she was likely to have some form of intestinal L-T4 malabsorption. After thyroidectomy, she was discharged with 100 μg/day of L-T4 plus 1 g/day of calcium carbonate and 7,000 IU/week of vitamin D3 because of borderline low calcemia (8.0 mg/dl, 2.0 mmol/l). She was referred because 1 month earlier her serum TSH had reached 7.0 mU/l (reference range: 0.25-4.0) under 150 μg/day (2.3 μg/kg body weight/day) of L-T4, up from 4.8 mU/l 6 months earlier and up from ≤1.5 mU/l that she used to have under 100 μg/day of L-T4 (1.5 μg/kg body weight/day). The elevation of serum TSH at 7.0 mU/l was mirrored by the decline in serum free thyroxine [FT4 = 8.7 pg/ml, 11.2 pmol/l (reference range: 8-18 pg/ml, 10.3-23.2 pmol/l) down from ≥15.5 pg/ml, 19.9 pmol/l] and free triiodothyronine [FT3 = 1.9 pg/ml, 2.9 pmol/l (reference range: 1.8-4.2 pg/ml, 2.8-6.4 pmol/l) down from ≥3.1 pg/ml, 4.8 pmol/l]. This elevation of serum TSH was also mirrored by increased levels of both triglycerides and total cholesterol (277 mg/dl, 3.1 mmol/l and 268 mg/dl, 6.9 mmol/l), while both lipids had been normal preoperatively (104 mg/dl, 1.2 mmol/l and 183 mg/dl, 4.7 mmol/l). The increment from 100 to 150 μg/day L-T4 had occurred when serum TSH increased to 4.8 mU/l (see above) (fig. 1, left panel).
Fig. 1.
Serum levels of TSH, FT3 and FT4 (upper panels), and L-T4 dose (lower panels). T0 is the time of our first observation and coincides with the switch from the tablet to the oral solution formulation of L-T4 in case 2.
The patient complained of mild asthenia, facial puffiness and body weight increase, which were attributed to subclinical hypothyroidism by her family physician. Our careful history taking [4] disclosed that she had consistently taken the L-T4 tablet with plain water early in the morning on an empty stomach, 60-90 min prior to breakfast. Calcium carbonate and vitamin D3 were taken, but only for approximately 1 year after thyroidectomy, 4-6 h after L-T4; thus, no supplements and no medications other than L-T4 were taken during the last 2 years prior to our observation. After thyroidectomy total calcemia had remained between 8.4 mg/dl, 2.1 nmol/l and 9.0 mg/dl, 2.2 nmol/l – this last value being obtained a few months prior to our observation.
At presentation, the patient had puffiness in her face, hands and feet; pedal edema was pitting (the patient admitted that her feet had not been examined until then). Signs of hypocalcemia (Chvostek's and Trousseau's signs) were absent. Biochemically, serum TSH was 8.9 mU/l, with serum FT4 (8.2 pg/ml, 10.5 pmol/l) close to the lower normal limit (fig. 1, left panel). Serum antibodies to transglutaminase, gliadin, endomysium and Helicobacter pylori were negative. The patient was hypoalbuminemic (32 g/l, reference range: 36-50) and, accordingly, she was borderline hypercalcemic (uncorrected total calcemia = 9.5 mg/dl, 2.4 mmol/l; total calcemia corrected for serum albumin = 10.1 mg/dl, 2.5 mmol/l), γ-globulins were high-normal at 14 g/l (reference range: 9-14). Proteinuria was 3.5 g/24 h (reference range: <0.15) and her urine tested positive for the Bence Jones protein, as confirmed a few days later. A diagnosis of nephrotic syndrome secondary, most likely, to multiple myeloma was made. Since the patient's relatives lived elsewhere, the patient elected to be managed somewhere else and was lost to follow-up. A posteriori, the progressive increase in calcemia of this patient, even though calcium carbonate and vitamin D had been withdrawn, was highly consistent with ongoing osteolysis.
Case 2
Case 2 concerns a 65-year-old man who was diagnosed with type 2 diabetes mellitus 22 years prior to observation by A.A. and P.F. Though his insulin dose had been increased in the previous few months (total units per day = 45), glycemic control remained poor, with the last glycosylated hemoglobin levels at 10.5%. Over the last 15 years, he had been taking 125 μg/day (1.3 μg/kg body weight/day) of L-T4 in tablet form because of hypothyroidism due to Hashimoto's thyroiditis (pretreatment TSH = 32 mU/l). The L-T4 dose was adequate since his serum TSH had remained stable in the range of 1.1-1.8 mU/l in the preceding 5 years. He used to take L-T4 fasted in the morning 1 h before breakfast. In addition, he did not take orally any drug except for an antihypertensive drug – losartan (50 mg/day).
Four months prior to our observation the patient started to complain of puffiness, ankle swelling, weight increase and mild asthenia. Nephrotic syndrome was diagnosed based on proteinuria (5.6 g/24 h, reference range: <0.15) and hypoalbuminemia (32 g/l, reference range: 36-50). Renal biopsy was performed, and histology was consistent with diabetic glomerulosclerosis.
He was referred to us because, while maintaining the said 125 μg/day L-T4, serum TSH jumped to 10.4 mU/l, with FT3 and FT4 low-normal [2.8 pg/ml, 4.3 pmol/l (reference range 2.3-4.2, 3.5-6.4) and 10.2 pg/ml, 13.1 pmol/l (reference range 8-17, 10.3-21.9), respectively; fig. 1, right panel]. In addition, mixed dyslipidemia was detected with high levels of both total cholesterol (234 mg/dl, 6.1 mmol/l) and triglycerides (267 mg/dl, 3.0 mmol/l). His family physician interpreted the association of dyslipidemia, facial puffiness and weight increase as being due to mild hypothyroidism in view of the increased level of TSH. Our biochemical screening for celiac disease (antibodies to transglutaminase, gliadin and endomysium) and H. pylori infection was negative. At physical examination, the patient presented with a puffy face and peripheral pitting edema.
Taking advantage of the availability in our nation of an oral liquid formulation of L-T4 (Tirosint® soluzione orale, IBSA Italia Srl, Lodi, Italy), which ensures better intestinal absorption of the hormone [19,20], we reasoned that the greater amount of solubilized T4 that was absorbed through the intestine might have counterbalanced the amount of circulating T4 lost with urine. Thus, we switched the patient from the tablet L-T4 to the liquid formulation, while maintaining the dose of 125 μg/day. Four weeks after the switch, serum TSH dropped to 3.3 mU/l, and FT3 and FT4 moved to the middle of the normal range (3.0 pg/ml, 4.6 pmol/l and 13.1 pg/ml, 16.9 pmol/l, respectively). Also, asthenia improved significantly. At the second check 3 months after the switch, serum TSH was 3.1 mU/l (fig. 1, right panel).
Discussion
Nephrotic syndrome is a common glomerular disease, and at least in children hypothyroidism is explicitly considered as one of its complications [11]. Onset of hypothyroidism, or aggravation of preexisting hypothyroidism with subsequent increased requirements of the L-T4 dose, stems from the loss of both protein-unbound (free) and protein-bound thyroid hormones [4]. L-T4 replacement is augmented when nephrotic syndrome (and therefore proteinuria) is uncontrolled, whereas it decreases when proteinuria decreases [14,21,22]. Bilateral nephrectomy has been reported to normalize thyroid function tests, allowing L-T4 to be discontinued, when there are no other causes of thyroid dysfunction [23,24]. Ito et al. [15] reported that the daily urinary protein excretion correlated positively with urinary T4, T3, FT4, FT3 and TBG excretion, but negatively with serum T4, T3, FT4, FT3 and TBG levels [15]. Serum albumin correlated negatively with serum TSH [15]. This negative correlation between serum albumin and serum TSH was also observed by Afroz et al. [13].
Because of albuminuria our patients were slightly hypoalbuminemic. Extremely low levels of albuminemia/analbuminemia yield spuriously low serum levels of FT4 when assayed by RIA or methods based on T4 analogues with affinity for albumin [25]. Considering that changes in FT4 were mirrored in changes in serum TSH in both patients and considering that our patients were ambulatory, as opposed to hospitalized due to serious illness, it is far beyond the scope of our report to thoroughly discuss the influence of thyroid hormone proteins (including albumin) and/or nonthyroidal illness syndrome on the assays for serum FT4, for which we refer to other example articles [26,27]. Of interest, in the context of the debated issue of treating or not treating the nonthyroidal illness syndrome with thyroid hormone, a recent meta-analysis by Liu et al. [28] assessed the efficacy of thyroid hormone replacement therapy for nephrotic syndrome patients associated with nonthyroidal illness syndrome. They found that thyroid hormone replacement increased the remission rate of the nephrotic syndrome and was not associated with any side effect.
Our first patient is similar to the one reported by Junglee et al. [8]. Their patient was a 62-year-old woman in whom serum TSH normalized only upon progressively doubling the daily dose of L-T4 (from 100 to 200 μg/day). Biochemically, she had normal FT4 (13.4 pg/ml, 17.2 pmol/l) with a TSH at the upper end of the normal range (5.17 mU/l). In addition, there was proteinuria (13.5 g/24 h), hypoalbuminemia (28 g/l; normal range: 35-50 g/l) and marked mixed dyslipidemia (total cholesterol 360 mg/dl, 9.3 mmol/l, triglycerides 664 mg/dl, 7.5 mmol/l) [8]. Renal biopsy demonstrated amyloid deposition. Serum electrophoresis and bone marrow examination were both normal, but a trace amount of Bence Jones paraprotein was detected in the urine. The final diagnosis was myeloma-associated renal amyloidosis and related nephrotic syndrome [8]. In our second patient, the nephrotic syndrome was a complication of type 2 diabetes mellitus.
The teaching value of our 2 patients is multifold. In both patients, clinical symptoms (puffiness, weight increase and asthenia) were supposed to be due to hypothyroidism, which was poorly compensated by L-T4 therapy. Indeed, the first patient's physician and an endocrinologist that he had consulted over the phone concluded her symptoms were likely the result of malabsorption of L-T4, with the edema and dyslipidemia both due to imminent progression of subclinical hypothyroidism to overt hypothyroidism. However, had physical examination been complemented by appreciation of the pedal edema and its pitting characteristic, then the said hypothesis would have been questioned. Finally, if treatment with a known sequestrant of L-T4 (i.e. calcium carbonate) had been continued, the case could have been managed by postponing calcium carbonate with respect to the ingestion of L-T4 tablets and/or by progressively increasing the daily dose of L-T4. This approach would have taken months, as serum TSH has to be checked periodically after each adjustment; in the meantime, however, a hematologic malignancy would have progressed.
Our second patient might also have been managed by increasing the daily dose of L-T4. However, we elected to switch the patient from the tablet to the oral solution L-T4 while maintaining the same daily dose. In doing so, we relied on the better intestinal absorption of liquid L-T4 so as to offset the increased urinary loss of the hormone. Indeed, the switch almost entirely compensated for the urinary loss of L-T4, as demonstrated by the decline and normalization of serum TSH 4 weeks later (10.4 vs. 3.3 mU/l).
In summary, the diagnostic workup of patients with increasing requirements of L-T4 replacement therapy should not be concentrated on the digestive system alone. Causes for such increasing requirements may not be mutually exclusive, so that two or more of them may coexist. In other terms, the endocrinologist needs to be watchful and suspicious, and exclude more serious etiologies before concluding for a single, benign cause. Careful history taking, thorough physical examinations and the inexpensive urinalysis should be part of the diagnostic workup. Reviews and major textbooks, which endocrinologists may refer to for determining causes of serum TSH failing to be normalized (or suppressed) by thyroid hormone therapy, should not forget to mention the nephrotic syndrome which, in turn, can be secondary to very serious diseases.
Disclosure Statement
S.B. and A.A. received research grants from IBSA Italia Srl. However, IBSA had no role in any phase of the writing of this paper.
References
- 1.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults Clinical practice guidelines for hypothyroidism for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200–1235. doi: 10.1089/thy.2012.0205. [DOI] [PubMed] [Google Scholar]
- 2.Jonklaas J. Treatment of hypothyroidism. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. ed 10. Philadelphia, Wolters Kluwer: Lippincott Williams and Wilkins; 2013. pp. 611–628. [Google Scholar]
- 3.Morris JC. How do you approach the problem of TSH elevation in a patient on high-dose thyroid hormone replacement? Clin Endocrinol. 2009;70:671–673. doi: 10.1111/j.1365-2265.2009.03536.x. [DOI] [PubMed] [Google Scholar]
- 4.Benvenga S. When thyroid hormone replacement is ineffective? Curr Opin Endocrinol Diabetes Obes. 2013;20:467–477. doi: 10.1097/MED.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 5.Ward LS. The difficult patient: drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arq Brasil Endocrinol Metab. 2010;54:435–442. doi: 10.1590/s0004-27302010000500002. [DOI] [PubMed] [Google Scholar]
- 6.Larsen PR, Davies TF. Hypothyroidism and thyroiditis. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. ed 10. Philadelphia: Saunders; 2005. pp. 423–455. [Google Scholar]
- 7.Fonseca V, Thomas M, Katrak A, Sweny P, Moorhead JF. Can urinary thyroid hormone loss cause hypothyroidism? Lancet. 1991;338:475–476. doi: 10.1016/0140-6736(91)90546-2. [DOI] [PubMed] [Google Scholar]
- 8.Junglee NA, Scanlon MF, Rees DA. Increasing thyroxine requirements in primary hypothyroidism: don't forget the urinalysis! J Postgrad Med. 2006;52:201–203. [PubMed] [Google Scholar]
- 9.De Luca F, Gemelli M, Pandullo E, Barberio G, Benvenga S, Trimarchi F. Changes in thyroid function tests in infantile nephrotic syndrome. Horm Metab Res. 1983;15:258–259. doi: 10.1055/s-2007-1018688. [DOI] [PubMed] [Google Scholar]
- 10.Trimarchi F, Gemelli M, Benvenga S, Genova R, De Luca F. Transient congenital hypothyroidism in an infant with congenital nephrosis of Finnish type. Acta Paediatr Scand. 1983;72:145–147. doi: 10.1111/j.1651-2227.1983.tb09684.x. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Shin JI. Complications of nephrotic syndrome. Korean J Pediatr. 2011;54:322–328. doi: 10.3345/kjp.2011.54.8.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan A, Cleper R, Krause I, Blumenthal D, Davidovits M. Hypothyroidism in children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplant. 2012;27:2171–2175. doi: 10.1093/ndt/gfr665. [DOI] [PubMed] [Google Scholar]
- 13.Afroz S, Khan AH, Roy DK. Thyroid function in children with nephrotic syndrome. Mymensingh Med J. 2011;20:407–411. [PubMed] [Google Scholar]
- 14.Trouillier S, Delèvaux I, Rancé N, André M, Voinchet H, Aumaître O. Nephrotic syndrome: don't forget to search for hypothyroidism (in French) Rev Med Interne. 2008;29:139–144. doi: 10.1016/j.revmed.2007.10.412. [DOI] [PubMed] [Google Scholar]
- 15.Ito S, Kano K, Ando T, Ichimura T. Thyroid function in children with nephrotic syndrome. Pediatr Nephrol. 1994;8:412–415. doi: 10.1007/BF00856516. [DOI] [PubMed] [Google Scholar]
- 16.Muranjan MN, Kher AS, Nadkarni UB, Kamat JR. Congenital nephrotic syndrome with clinical hypothyroidism. Indian J Pediatr. 1995;62:233–235. doi: 10.1007/BF02752333. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor K, Saha A, Dubey NK, Goyal P, Suresh CP, Batra V, Upadhayay AD. Subclinical non-autoimmune hypothyroidism in children with steroid resistant nephrotic syndrome. Clin Exp Nephrol. 2014;18:113–117. doi: 10.1007/s10157-013-0800-1. [DOI] [PubMed] [Google Scholar]
- 18.Chandurkar V, Shik J, Randell E. Exacerbation of underlying hypothyroidism caused by proteinuria and induction of urinary thyroxine loss: case report and subsequent investigation. Endocr Pract. 2008;14:97–103. doi: 10.4158/EP.14.1.97. [DOI] [PubMed] [Google Scholar]
- 19.Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012;62:631–636. doi: 10.1055/s-0032-1329951. [DOI] [PubMed] [Google Scholar]
- 20.Vita, R, Fallahi P, Antonelli A, Benvenga S. The administration of L-thyroxine as soft gel capsule or liquid solution. Expert Opin Drug Deliv. 2014;11:1103–1111. doi: 10.1517/17425247.2014.918101. [DOI] [PubMed] [Google Scholar]
- 21.Hatron PY, Wemeau JL, Guillemin R, Raviart B, Vanhille P, Devulder B. Thyroid function in the nephrotic syndrome (in French) Rev Med Interne. 1984;5:35–42. doi: 10.1016/s0248-8663(84)80076-4. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh EC, Claude JR, Rajasoorya C. Paradox of rising thyroid stimulating hormone despite increasing thyroxine dose in hypothyroidism and the association with nephrotic syndrome. Nephrology (Carlton) 2013;18:647–648. doi: 10.1111/nep.12102. [DOI] [PubMed] [Google Scholar]
- 23.Chadha V, Alon US. Bilateral nephrectomy reverses hypothyroidism in congenital nephrotic syndrome. Pediatr Nephrol. 1999;13:209–211. doi: 10.1007/s004670050594. [DOI] [PubMed] [Google Scholar]
- 24.Kacer M, Whyte DA, Boydstun I, Wilson TA. Congenital nephrotic syndrome and persistent hypothyroidism after bilateral nephrectomy. J Pediatr Endocrinol Metab. 2008;21:597–601. [PubMed] [Google Scholar]
- 25.Stockigt JR, Stevens V, White EL, Barlow JW. ‘Unbound analog’ radioimmunoassays for free thyroxin measure the albumin-bound hormone fraction. Clin Chem. 1983;29:1408–1410. [PubMed] [Google Scholar]
- 26.Csako G, Zweig MH, Glickman J, Ruddel M, Kestner J. Direct and indirect techniques for free thyroxin compared in patients with nonthyroidal illness. II. Effect of prealbumin, albumin, and thyroxin-binding globulin. Clin Chem. 1989;35:1655–1662. [PubMed] [Google Scholar]
- 27.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011;57:122–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Yan W, Xu G. Thyroid hormone replacement for nephrotic syndrome patients with euthyroid sick syndrome: a meta-analysis. Ren Fail. 2014;36:1360–1365. doi: 10.3109/0886022X.2014.949559. [DOI] [PubMed] [Google Scholar]