Abstract
Background
In Graves' thyrotoxicosis tachycardia, weight loss and mental symptoms are common. Recovery takes time and varies between patients. Treatment with methimazole reduces thyroid hormone levels. According to previous research, this reduction has been faster if selenium (Se) is added.
Objective
The objective was to investigate whether supplementing the pharmacologic treatment with Se could change the immune mechanisms, hormone levels and/or depression and anxiety.
Methods
We prospectively investigated 38 patients with initially untreated thyrotoxicosis by measuring the thyroid-stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), thyroid receptor antibodies and thyroid peroxidase auto-antibodies before medication and at 6, 18 and 36 weeks after commencing treatment with methimazole and levo-thyroxine, with a randomized blinded oral administration of 200 µg Se/day or placebo. The selenoprotein P concentration was determined in plasma at inclusion and after 36 weeks. The patients were also assessed with questionnaires about depression, anxiety and self-rated symptoms before medication was started and after 36 weeks.
Results
FT4 decreased more in the Se group at 18 weeks (14 vs. 17 pmol/l compared to the placebo group, p = 0.01) and also at 36 weeks (15 vs. 18 pmol/l, p = 0.01). The TSH increased more in the Se group at 18 weeks (0.05 vs. 0.02 mIU/l, p = 0.04). The depression and anxiety scores were similar in both groups. In the Se group, the depression rates correlated negatively with FT3 and positively with TSH. This was not seen in the placebo group.
Conclusions
Se supplementation can enhance biochemical restoration of hyperthyroidism, but whether this could shorten clinical symptoms of thyrotoxicosis and reduce mental symptoms must be investigated further.
Key Words: Selenium, Thyroid hormones, Auto-antibodies, Self-rated symptoms, Hospital Anxiety and Depression Scale
Introduction
Graves' disease (GD) is a common autoimmune disease. The incidence in Sweden is 21/100,000, peaking in the age group between 40-59 years [1]. Little is known about what causes the activation of the disease, but hereditary factors, smoking and female gender increase the risk [2]. Thyroid receptor antibodies (TRAb) activate the thyroid hormone receptors and thereby enhance thyroxine synthesis. This antibody is also a marker for the disease, together with elevated thyroid hormones and low thyroid-stimulating hormone (TSH). Patients typically develop physical and mental symptoms such as tachycardia, weight loss, sweating, muscle weakness, tremor and anxiety [2]. Medication blocking the thyroid hormone synthesis is one common treatment, making the patient euthyroid and offering a 50% chance of cure [3,4]. Although euthyroidism is restored during treatment, some of these patients of working age take sick leave due to lack of energy, muscle weakness and mental symptoms either for shorter periods or sometimes for months [5].
In Western Europe, selenium (Se) blood levels are low [6]. This trace element is an essential component of selenoproteins with primarily anti-oxidative functions. Humans acquire Se in foods such as fish, meat, eggs, cereals and seafood. Se concentration varies round the globe: it is low in China, while in other areas, such as in central parts of the US and in South America, the Se content in soils is higher, and residents in those areas acquire sufficient Se from vegetarian sources. The most common selenoprotein found in plasma is selenoprotein P (SePP) which constitutes about 50-60% of all Se in plasma in humans with a modest level of Se in the blood stream [7].
Low dietary Se intake and blood concentrations may have multiple effects on thyroid hormone synthesis and regulation. Firstly, Se is a necessary component within both the thioredoxin reductases and the glutathione peroxidase (GPx) family, which are powerful anti-oxidant enzymes [8]. As the thyroid hormone metabolism causes an oxidative milieu within the thyroid gland, which is enhanced during thyrotoxicosis [9], GPxs and thioredoxin reductases are required to balance this oxidative stress. Secondly, thyroid hormone synthesis, mainly thyroxine, is converted within target cells by another group of selenoproteins, the deiodinases, to active triiodothyronine and inactive thyroxine metabolites [10]. Thirdly, Se, as sodium selenite or selenomethionine, appears to influence the immune system by unknown mechanisms, as supplementation with Se decreases the levels of thyroid peroxidase auto-antibodies (TPO Ab) in autoimmune hypothyroidism [11,12]. However, other investigators have not repeated this finding [13,14]. Reports also describe how Se supplementation restores euthyroidism earlier in GD patients given methimazole plus a fixed combination of antioxidants including 60 µg Se compared to methimazole alone [15].
In this study, we examined the effect of Se on depression and anxiety scores, self-rated symptoms, thyroid hormones and antibody levels in a cohort of patients with newly diagnosed GD, following 9 months of pharmacological treatment with a randomized supplementation with 200 µg/day Se as selenized yeast or placebo.
Material and Methods
Study Design
This was a randomized prospective investigation, blinded to the patient and investigators. Half of the patients were randomized to placebo treatment (PT) and half to Se treatment (ST).
GD was confirmed by clinical symptoms and blood tests, decreased TSH, elevated free thyroxine (FT4) and free triiodothyronine (FT3) and the presence of TRAb. In 2 patients in whom TRAb were absent, an increased even distribution on a radionuclide scan was acknowledged as compatible with GD. The physician provided information about the study at the patient's first visit, and informed consent was obtained. An extra blood sample to measure the Se concentration was also acquired.
Treatment with antithyroid drugs was given with methimazole (Thacapzol®, Recip) 15 mg twice/day to block the synthesis of thyroid hormones and the replacement of thyroid hormones by levo-thyroxine (Levaxin®, Takeda) 100 µg/day starting 3 weeks after methimazole. The patients were randomized to treatment with 200 µg Se/day as yeast tablets (ST group) or to placebo (PT group) for 9 months. The tablets (SelenoPrecise, Pharma Nord ApS, Vejle, Denmark) contain organically bound Se predominantly as selenemethionine [16].
Participants
Fifty-four consecutive patients, aged 18-55, with newly diagnosed and untreated GD, were invited to participate. Eight patients declined participation, 1 patient was indicated for surgery, and 1 further patient was missed. Of the remaining 44 patients, 4 withdrew from participation; 1 more was treated with thyroidectomy and 1 with radioactive treatment. In all, 38 patients remained as our study cohort. Of these, 31 (82%) were women, and 7 (18%) were men. The mean age was 39.1 (±10.4). Twenty-seven (71%) patients did not smoke, whereas 11 were smokers (table 1). All patients were instructed not to ingest any extra compound containing Se during the study period.
Table 1.
Demographic and hormonal data at inclusion
| ST group | Range | PT group | Range | p value | |
|---|---|---|---|---|---|
| Patients, n | 19 | 19 | |||
| Age, years | 35 | 19–49 | 44 | 23–55 | 0.003 |
| Sex, F/M | 15/4 | 16/3 | n.s. | ||
| Smokers | 7 (37%) | 5 (26%) | n.s. | ||
| TSH, mIU/l | 0.01 | 0.1 – 0.2 | 0.01 | 0.1 – 0.2 | n.s. |
| FT4, pmol/l | 42.0 | 24–84 | 37.0 | 15–105 | n.s. |
| FT3, pmol/l | 18.0 | 8.6–30 | 14.5 | 6.1–30 | n.s. |
| TRAb, mIU/l | 7.4 | 1–29.8 | 4.8 | 1.3–40 | n.s. |
| TRAb, negative | 1 | 1 | n.s. | ||
| TPO Ab, kIU/l | 79.5 | 10–1,385 | 198.5 | 10–3,740 | n.s. |
| TPO, negative | 4 | 5 | n.s. | ||
| SePP, ng/ml | 46.5 | 42–86 | 49.5 | 34–13 | n.s. |
Medians and ranges are presented for age, hormones, auto-antibodies and SePP. All other values represent numbers (%), unless otherwise stated. n.s. = Not significant.
Exclusion criteria were: medication such as sedatives, planned or ongoing pregnancy, reduced intellectual capacity or dementia as observed at inclusion, severe ophthalmopathy requiring corticosteroids at inclusion, other systemic or severe disease or difficulties with the Swedish language. Also, patients with previous treatment with anti-thyroid drugs were excluded.
The participants were followed clinically and with laboratory testing of FT4, FT3, TSH, TRAb and TPO Ab at diagnosis and after 6, 18 and 36 weeks. The plasma for SePP analysis was also collected from the untreated patients at inclusion and after 36 weeks. This was centrifuged and stored as plasma at −70°C until analysed. All other laboratory samples were analysed during the clinical follow-up. At this time, Se substitution/placebo was withdrawn. Subjects had then completed the study. None of the participants withdrew from the study due to adverse events.
Procedures and Measures
Assessment
Questionnaires
An expanded version of the Symptom Questionnaire, derived from the Rivermead Post Concussions Questionnaire [17], was used to record self-rated symptoms (online suppl. table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000381768). The patient states how bothersome the symptom is on a scale from 0-4 (1 indicating that they have experienced the symptom but it is not present any more, and 4 indicating a severe, on-going problem). The maximum score is 156.
The Hospital Anxiety and Depression Scale (HADS) was used to indicate the presence of depression and anxiety. A score of <7 points on the HADS depression subscale indicates no signs of depression; a score of 8-10 indicates possible depression, and >10 points defines a more definitive case of depression. Corresponding numbers assess anxiety. The maximum summed score of 42 indicates severe depression/anxiety [18].
Laboratory Assays
Venous blood samples were collected at baseline before the neuropsychological assessment, and repeated during follow-up. The concentrations of TSH, FT4 and FT3 were measured by chemiluminescent methods on an ADVIA Centaur (Siemens Healthcare Diagnostics, Tarrytown, N.Y., USA). The total coefficient of variation (CV) for TSH was 5.4% at 0.39 mIU/l, 5.6% at 5.4 and 6.1% at 30 mIU/l, respectively. The CV for FT4 was 1.4% at 4.0 pmol/l, 5.2% at 15 pmol/l and 6.0% at 54 pmol/l. For FT3, the CV was 3.1% at 3.4 pmol/l and 3.0% at 14.9 pmol/l. TPO Ab were also measured by a chemiluminescent method on the Immulite system from the same manufacturer, with a detection limit of 5.0 kIU/l, and the CV was 6.4% at 37 kIU/l and 6.0% at 635 kIU/l. TRAb were measured by radioimmunoassay (TRAK, BRAHMS Diagnostica GmBH, Heningsdorf, Germany). The limit of detection was 0.3 mIU/l, and the CV was 14% at 3 mIU/l and 9% at 20 mIU/l. All blood samples for Se analysis were taken at inclusion and repeated when patients had been treated for 9 months. The samples were centrifuged and stored as plasma at −70°C with EDTA and were analysed together. The SePP was determined by affinity HPLC with ICP-MS detection and isotope dilution quantification of Se using a method previously described in detail [19].
Ethics
The participants gave informed consent to take part in the investigation. The research was carried out in accordance with the Declaration of Helsinki (2000). It was approved by the Committee for Medical Ethics at the Karolinska Institutet and was registered at ClinicalTrials.gov DLL-159361.
Statistics
SPSS 21 was used for the statistical analyses. Data were checked for skewness and kurtosis. Parametric methods (χ2 test and Student's t test) were used for normally distributed variables on an interval level, and non-parametric methods were used for skewed or ordinal data (the Spearman rank correlation, Wilcoxon signed-rank test and Mann-Whitney U test). Generally, the hormonal data were positively skewed. Two-tailed p values were used with a critical significance level of p < 0.05.
Results
Measures of Hormones, Auto-Antibodies and Se Concentrations
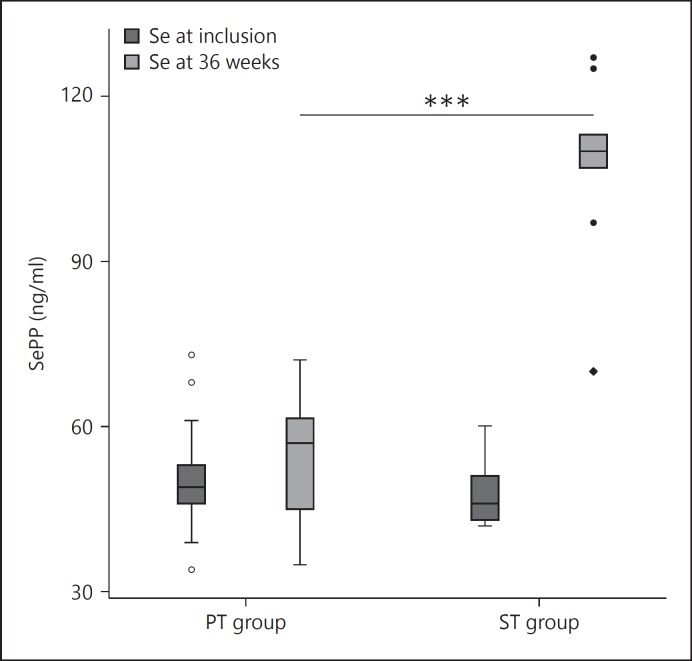
The median concentration of Se as SePP rose in the treatment group from 47 ng/ml (range 42-86) to 113 ng/ml (range 70-139), p < 0.001, whereas the levels remained unchanged in the PT group from the initial 49.5 ng/ml (range 34-73) to 57 ng/ml (range 35-72) (fig. 1). Assuming that SePP contains 50-60% of all blood Se, these mean values accord well with the mean Se level in the plasma of 221 µg/ml sampled from individuals receiving Se yeast at 200 µg Se/day from the same supplement producer [20]. The median TSH levels increased significantly more in the ST group. At 18 weeks, this group had a TSH of 0.05 mIU/l (range 0.01-20) and the PT group one of 0.02 mIU/l (range 0.01-11), Z = 2.03, p = 0.042 (table 2). Also, after 18 weeks, the FT4 levels were lower in the ST group (14 pmol/l, range 7-25) compared to the PT group with 17 pmol/l (range 12-21), Z = −2.51, p = 0.012. After 36 weeks, the FT4 in the ST group was 15 pmol/l (range 12-20), and in the PT group it was 18 pmol/l (range 13-25), Z = −2.49, p = 0.013 (table 2). No statistical differences were found in any other area of hormones or in titres of TPO Ab and TRAb (table 2).
Fig. 1.
Boxplot of SePP at inclusion and after 9 months' treatment. Comparison of groups treated with Se versus placebo. *** p < 0.001.
Table 2.
Serum concentrations of TSH, FT3, FT4, TRAb and TPO Ab for the ST and PT groups at inclusion and at weeks 6, 18 and 36
| Laboratory | ST group | PT group | p value | Ref. value |
|---|---|---|---|---|
| TSH, mIU/l | ||||
| At inclusion | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | n.s. | 0.4–5.0 |
| At 6 weeks | 0.02 (0.01–4.30) | 0.01 (0.01–5.80 | n.s. | |
| At 18 weeks | 0.05 (0.01–20.0) | 0.2 (0.01–11.0) | 0.042 | |
| At 36 weeks | 1.4 (0.0–39.0) | 0.2 (0.0–12.0 | n.s. | |
| FT3, pmol/l | ||||
| At inclusion | 18.0 (8.6–30) | 14.5 (6.1–30) | n.s. | 3.3–6.0 |
| At 6 weeks | 5.5 (3.1–11.0) | 5.7 (3.8–12.0) | n.s. | |
| At 18 weeks | 4.2 (3.2–6.1) | 4.4 (3.4–6.2) | n.s. | |
| At 36 weeks | 4.3 (3.3–6.0) | 4.1 (3.6–6.5) | n.s. | |
| FT4, pmol/l | ||||
| At inclusion | 42 (24–84) | 37 (15–105) | n.s. | 10–22 |
| At 6 weeks | 19 (11–65) | 19 (9–29) | n.s. | |
| At 18 weeks | 14 (7–25) | 17 (12–21) | 0.012 | |
| At 36 weeks | 15 (12–20) | 18 (13–25) | 0.013 | |
| TRAb, mIU/l | ||||
| At inclusion | 7.4 (1.0–29.8) | 4.6 (1.3–40.0) | n.s. | <1.0 |
| At 6 weeks | 4.8 (1.0–40.0) | 4.4 (1.0–40.0) | n.s. | |
| At 18 weeks | 2.1 (1.0–31.1) | 1.2 (1.0–40.0) | n.s. | |
| At 36 weeks | 1.0 (1.0–8.5) | 1.0 (1.0–40.0) | n.s. | |
| TPO Ab, kIU/l | ||||
| At inclusion | 80 (10–1,385) | 198 (10–3,740) | n.s. | <35 |
| At 6 weeks | 41 (10–1,115) | 218 (10–4,840) | n.s. | |
| At 18 weeks | 22 (10–873) | 81 (10–3,720) | n.s. | |
| At 36 weeks | 23 (10–610) | 59 (10–2,190) | n.s. | |
Values represent medians with ranges in parentheses, unless otherwise stated. The Mann-Whitney U test was used for comparison between the PT and the ST groups. n.s. = Not significant.
Self-Rated Symptom Measurements
The whole study population reduced their symptoms from a median score of 35.5 (range 2-85) to 22.5 (range 0-89; Z = −3.90, p < 0.001). The HADS depression subscale scores were reduced from a median score of 4.5 (range 0-15) to 2.0 (range 0-12), Z = −3.79, p < 0.001. The HADS anxiety subscale scores were reduced from a median score of 8.5 (range 1-18) to 6.0 (range 0-17), Z = −3.59, p < 0.001. There were, however, no significant differences between the ST and PT groups regarding symptom ratings.
For the ST group, TSH correlated negatively with the HADS depression subscale rs (19) −0.613, p = 0.005, as low TSH was associated with higher self-rated depression scores at the follow-up. For FT3, we found a positive correlation with symptom ratings rs (12) 0.586, p = 0.045 (the higher the FT3, the more self-rated symptoms) and HADS depression subscale rs (12) 0.797, p = 0.002 (the higher the FT3, the more self-rated depression) in the ST group, while the FT4 did not correlate with any of the self-ratings. Auto-antibodies in the ST group did not correlate to self-rated symptoms. For the PT group, neither TRAb, TPO Ab, FT4 nor FT3 correlated with any of the self-rating measures at the 9 months' follow-up.
Discussion
This was a randomized prospective investigation examining the effect of Se versus placebo on thyroid hormone levels, auto-antibodies and mental symptom ratings in patients with GD on block and replacement therapy. The main biochemical findings were a reduction of FT4 at 18 and 36 weeks, an increase of TSH at 18 weeks and an increased SePP concentration in the group of patients treated with Se.
Standard Se concentrations in blood vary. In Western Europe, these levels are generally low, and plasma concentrations of Se range between 55-117 ng/ml [21]. SePP reflects circulating Se available for biosynthesis of bioactive selenoproteins [22.] The concentration achieved in this study in the ST group was in the upper normal European range.
In a previous investigation, the FT4 and FT3 levels decreased more rapidly in GD patients given methimazole, anti-oxidants and 60 µg Se compared to methimazole alone [15]. This decrease was observed 30 and 60 days after treatment initiation, together with a significant increase of TSH after 60 days in the group receiving anti-oxidants. This may be due either to the other anti-oxidative components, vitamin C, E and β-carotene or to the non-specified form of Se given in that study. Our findings are to some extent in line with those of Vrca et al. [15], but the hormonal changes found in that study were noted at an earlier phase. In that investigation, Se increased in the group receiving supplementation from 60 to 90 µg/l. Thus, the addition of Se may have an effect on the time it takes for endocrine normalization in GD.
Our findings about FT4 and TSH might imply a reduction in disease activity in patients with GD treated with the addition of Se. As there was no change in TRAb levels between the two groups, other indirect mechanisms could be plausible. Such factors could be mediated by the immune system, by effects on oxidative stress within the thyroid gland or by deiodinase enzymes.
Se is important for initiating and enhancing immunity, but is also involved in regulating excessive immune responses, which is crucial for preventing responses that may lead to autoimmunity or chronic inflammation [23]. Se supplementation in humans also has stimulating effects on the immune system, as such supplementation has been shown to promote the differentiation of CD4+ cells to T helper-1 cells [24]. The addition of Se has also demonstrated a correlation between an enhanced expression of interleukin-2 receptors and lymphocyte proliferation. However, whether the immune system could be modulated via Se in GD remains speculative.
Another of Se's actions could be its effect on oxidation, partly via immune cells. As T cells are vulnerable to oxidative stress, and selenoproteins are necessary for these cells to proliferate in response to reactive-oxygen species, this may affect humans with subnormal Se reservoirs [25], which has been found to be the case in GD [26]. In humans, thyroid GPx contributes to the high Se content in the gland and also appears to be a regulator of thyroid hormone synthesis [27]. Moreover, a decrease in reactive-oxygen species levels 2-12 months during methimazole treatment has been demonstrated [26] as well as a correlation between Se concentration and GPx activity [15]. Thus, evidence exists that Se supplementation could increase anti-oxidative potential in GD. It is still not known whether quality-of-life, recovery or hormonal levels could be strongly modified by increased anti-oxidative potential.
We found no significant changes in TRAb or TPO Ab during Se supplementation. This is in contrast to Hashimoto's disease, where a reduction of TPO Ab has been found after Se substitution in a substantial number of studies [28,29], but not by all investigators [13]. As auto-antibodies did not change in these patients with autoimmune hyperthyroidism, Se does not appear to affect immunoglobulins in GD.
Increased SePP concentration, in its capacity as a Se transporter protein, could enhance the concentration of another group of selenoproteins such as the deiodinases. These enzymes convert FT4 to active FT3 or to inactive reverse triiodothyronine. We found a significant reduction of FT4 after 18 and 36 weeks of treatment in the ST group, but no such pattern in FT3. However, this association's effect on the deiodinases is not known. To speculate, Se addition may to some extent catalyse the conversion of FT4 to reverse triiodothyronine and by means of this defend the body from thyrotoxicosis. However, as no difference in FT3 levels between the two groups was found, we could not find proof of this mechanism.
On a self-rated functional level, we found a significant reduction but no differences between the treatment groups regarding symptom ratings, depression or anxiety. However, in the ST treatment group, a more elevated TSH indicated a lower degree of depression after 9 months. Also, FT3 correlated positively with symptom ratings and depression, namely, the more enhanced the levels of FT3, the higher the symptom ratings. No such correlations were found in the PT group. Thus, it appears that in the ST group there was a correlation between the recovery from the disease and the symptoms, while this was not the case in the PT group. The lack of significant differences regarding symptom ratings on the group level may be due to low power and a substantial range of reported symptoms on the symptom questionnaire, resulting in high variability and a lack of significant results. Another limiting factor is the age spectrum, as the PT group was older than the ST group. It may well be that this could affect recovery, which would then influence our results.
In summary, this randomized, prospective investigation showed a reduction of FT4 after 18 and 36 weeks and an increase of TSH after 18 weeks in the Se-supplemented group. In the ST group, the rates of depression were lower.
Conclusions
Se supplementation can enhance biochemical restoration of hyperthyroidism, but whether this could curtail the clinical symptoms of thyrotoxicosis, and also reduce mental symptoms, must be investigated further.
Disclosure Statement
None of the authors has any conflict of interest with the funding sources.
Supplementary Material
Supplementary data
Acknowledgements
We would like to thank all our colleagues who saw these patients during the clinical follow-up: Vibeke Bergmark, Hugo Tellez, Sam Westdal, Carina Ottoson, Anastasia Trouva, Alexandre Saric and Anett Forsberg. The statistical calculation was improved with the help of Ulf Larsson at the Research Council in Sörmland. We thank Mats Bergström for his assistance with the laboratory work-up. Marianne Hansen is acknowledged for carrying out the analyses of SePP and Stefan Arousell for helping us with figure 1. The County of Sörmland's Research Council supported the study financially. Pharma Nord kindly supplied the Se tablets as well as the placebo.
References
- 1.Abraham-Nordling M, Bystrom K, Torring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165:899–905. doi: 10.1530/EJE-11-0548. [DOI] [PubMed] [Google Scholar]
- 2.Seigel SC, Hodak SP. Thyrotoxicosis. Med Clin North Am. 2012;96:175–201. doi: 10.1016/j.mcna.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Benker G, Reinwein D, Kahaly G, et al. Is there a methimazole dose effect on remission rate in Graves' disease? Results from a long-term prospective study. The European Multicenter Trial Group of the Treatment of Hyperthyroidism with Antithyroid Drugs. Clin Endocrinol (Oxf) 1998;49:451–457. doi: 10.1046/j.1365-2265.1998.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS. Antithyroid drugs. N England J Med. 2005;352:905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- 5.Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative effectiveness of therapies for Graves' hyperthyroidism: a systematic review and network meta-analysis. J Clin Endocrinol Metab. 2013;98:3671–3677. doi: 10.1210/jc.2013-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayman MP. The argument for increasing selenium intake. Proc Nutr Soc. 2002;61:203–215. doi: 10.1079/PNS2002153. [DOI] [PubMed] [Google Scholar]
- 7.Mostert V, Selenoprotein P. Properties, functions and regulation. Arch Biochem Biophys. 2000;376:433–438. doi: 10.1006/abbi.2000.1735. [DOI] [PubMed] [Google Scholar]
- 8.Diez D, Grijota-Martinez C, Agretti P, De Marco G, Tonacchera M, Pinchera A, et al. Thyroid hormone action in the adult brain: gene expression profiling of the effects of single and multiple doses of triiodo-l-thyronine in the rat striatum. Endocrinology. 2008;149:3989–4000. doi: 10.1210/en.2008-0350. [DOI] [PubMed] [Google Scholar]
- 9.Hagenbuch B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract Res Clin Endocrinol Metab. 2007;21:209–221. doi: 10.1016/j.beem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.St Germain DL . Selenium deiodinases and endocrine function. In: Hatfield DL, editor. Selenium. Its Molecular Biology and Role in Human Health. Boston: Kluwer Academic Publishers; 2001. pp. 189–205. [Google Scholar]
- 11.Levi Y, Rassovsky Y, Agranov E, Sela-Kaufman M, Vakil E. Cognitive reserve components as expressed in traumatic brain injury. J Int Neuropsychol Soc. 2013;19:1–8. doi: 10.1017/S1355617713000192. [DOI] [PubMed] [Google Scholar]
- 12.Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- 13.Eskes SA, Endert E, Fliers E, Birnie E, Hollenbach B, Schomburg L, et al. Selenite supplementation in euthyroid subjects with thyroid peroxidase antibodies. Clin Endocrinol (Oxf) 2014;80:444–451. doi: 10.1111/cen.12284. [DOI] [PubMed] [Google Scholar]
- 14.Bonfig W, Gärtner R, Schmidt H. Selenium supplementation does not decrease thyroid peroxidase antibody concentration in children and adolescents with autoimmune thyroiditis. ScientificWorldJournal. 2010;10:990–996. doi: 10.1100/tsw.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrca VB, Skreb F, Cepelak I, Romic Z, Mayer L. Supplementation with antioxidants in the treatment of Graves´disease: the effect on glutathione peroxidase activity and concentration of selenium. Clin Chim Acta. 2004;341:55–63. doi: 10.1016/j.cccn.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Larsen EH, Hansen M, Paulin H, Moesgaard S, Reid M, Rayman M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J AOAC Int. 2004;87:225–232. [PubMed] [Google Scholar]
- 17.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242:587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Loeschner K, Hadrup N, Hansen M, Pereira SA, Gammelgaard B, Møller LH, et al. Absorption, distribution, metabolism and excretion of selenium following oral administration of elemental selenium or selenite in rats. Metallomics. 2014;6:330–337. doi: 10.1039/c3mt00309d. [DOI] [PubMed] [Google Scholar]
- 20.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 21.Ravn-Haren G, Krath BN, Overvad K, Cold S, Moesgaard S, Larsen EH, et al. Effect of long-term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) pilot study. Br J Nutr. 2008;99:1190–1198. doi: 10.1017/S0007114507882948. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 23.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffman PR. Dietary selenium modulates activation and differentiation of CD+4 T-cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010;140:1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 26.Abalovich M, Llesuy S, Gutierrez S, Repetto M. Peripheral parameters of oxidative stress in Graves' disease: the effects of methimazole and 131 iodine treatments. Clin Endocrinol (Oxf) 2003;59:321–327. doi: 10.1046/j.1365-2265.2003.01850.x. [DOI] [PubMed] [Google Scholar]
- 27.Köhrle J, Gärtner R. Selenium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:815–827. doi: 10.1016/j.beem.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008;52:1235–1246. doi: 10.1002/mnfr.200700465. [DOI] [PubMed] [Google Scholar]
- 29.Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kuvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010;20:1163–1173. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data