Abstract
Background and Objectives
This study aims to investigate the clinical features, angiographic findings, and outcomes of younger Korean ST-segment elevation myocardial infarction (STEMI) patients.
Subjects and Methods
We analyzed major adverse cardiac events (MACE) in the Korea Acute Myocardial Infarction Registry from November 2005 to October 2010. The registered patients were divided into two groups; young age group (<65 years) and old age group (≥65 years).
Results
The young age group included 5281 patients (age, 53±7.8 years), and the old age group included 4896 patients (age, 74.3±6.5 years). Male gender, smoking, family history, dyslipidemia, and metabolic syndrome were more frequently observed in the young age group than in the old age group (89.5% vs. 59.3%, p<0.001; 77.3% vs. 47.2%, p<0.001; 11% vs. 4.6%, p<0.001; 11.2% vs. 7.7%, p<0.001; 67.6% vs. 62.9%, p<0.001). Most of the young Korean adults with STEMI complained of typical chest pain (89.8%), and they had a shorter symptom-to-door time (12±53.2 hours vs. 17.3±132 hours, p=0.010). The young age group showed a favorable prognosis, which was represented by the MACE, compared with the old age group at one month (1.8% vs. 2.8%, p=0.028), six months (6.8% vs. 8.2%, p<0.001), and twelve months (10.1% vs. 11.9%, p=0.025). However, there was no significant difference in the adjusted MACE rate at one month {hazard ratio (HR) 0.95, 95% confidence interval (CI) 0.60-1.51, p=0.828} and twelve months (HR 0.86, 95% CI 0.68-1.10, p=0.233).
Conclusion
Younger Korean adults with STEMI have clinical outcomes similar to old aged patients, and therefore, they should be treated intensively like the elderly patients.
Keywords: Myocardial infarction, Young adult, Prognosis
Introduction
Acute Myocardial Infarction (Ami) Is One Of The Most Common Causes Of Death Worldwide, And It Is More Common In Persons Of Advancing Age.1) Because Of World Population Ageing, Many Countries Have Attempted To Reduce The Incidence And Mortality Rate Of Ami. The Representative Primary And Secondary Prevention Measures For Cardiovascular Disease Are Smoking Cessation, Weight Reduction, Lowering The Blood Pressure, And Decreasing The Glucose And Cholesterol Levels. In Korea, Like Other Developed Countries, A National Effort Has Resulted In A Decrease In The Overall Incidence Of Ami Over The Last Few Years.2) However, There Is No Significant Change In The incidence of AMI in younger patients.
Previous studies showed an approximately 2 to 10% incidence of AMI in younger patients.3),4),5) Remarkable changes in lifestyle and diet, and improvement in the socio-economic status have been noted in Korea over decades. These changes have led to obesity, increased levels of blood pressure, glucose, and cholesterol in younger Korean adults. As a result, these patients are in an increased atherothrombotic state.
Because the emerging risk factors, clinical manifestations, and outcomes of acute ST-segment elevation myocardial infarction (STEMI) in younger Korean adults are unclear, this study aims to investigate the clinical profiles of younger STEMI patients through a one-year follow-up.
Subjects and Methods
Korea Acute Myocardial Infarction Registry (KAMIR) is a Korean, prospective, open, observational, multicenter on-line registry of AMI supported by the Korean Society of Cardiology. The collected data were merged with an intention to improve the statistical power. Protocols and details of KAMIR have been published elsewhere.6) The registry protocols were verified and approved by the Institutional Review Board of each participating center. AMI was diagnosed by the characteristic presentation, serial changes on electrocardiogram (ECG) suggesting infarction, and an increase in cardiac enzymes.7) STEMI was defined as a new ST elevation in ≥2 contiguous leads, measuring >0.2 mV in leads V1-3 or 0.1 mV in all other leads, or a new left branch bundle block on a 12-lead ECG with a concomitant increase in troponin-I or -T.
Study design and patient population
From November 2005 to October 2010, 27852 patients with a final diagnosis of AMI were enrolled in the KAMIR. Among them, we selected the patients with STEMI and excluded the patients whose recorded data, including demographic features, angiographic findings, and procedure details, were invalid or incomplete. We divided the patients into two groups according to the age at admission: young age group (under the age of 65 years) and old age group (65 years of age or older).8) Finally, a total of 10177 patients were enrolled in this study.
Study variables
Demographic data and baseline clinical characteristics including age, gender, body mass index (BMI) at admission, presenting symptoms, classical cardiovascular risk factors {hypertension (HTN), diabetes mellitus (DM), dyslipidemia (DL), smoking status, and family history of coronary heart disease (CHD)}, and other co-morbidities were identified. Initial vital signs including systolic blood pressure, diastolic blood pressure, and heart rate were measured. Obesity was defined as BMI ≥25 kg/m2.9) Metabolic syndrome was defined according to the revised National Cholesterol Education Program Adult Treatment Panel III criteria.10) Attending physicians and/or cardiologists evaluated the patients using the Killip classification and also analyzed the electrocardiogram findings in all patients. Blood samples for baseline laboratory tests were collected at admission or before percutaneous coronary intervention (PCI). Also, blood was collected for lipid profile after an overnight fast. The left ventricular ejection fraction (LVEF) was measured by two-dimensional electrocardiography using the biplane Simpson's method before discharge.11)
Initial treatment strategy for STEMI included primary PCI, facilitated PCI, thrombolysis, or conservative treatment, which was at the discretion of the attending physicians and/or cardiologists. Primary PCI was defined as emergent PCI performed within 12 hours after admission,12) and/or PCI in patients with continuing ischemic symptoms, cardiogenic shock, and acute severe heart failure, even after 12 hours.13),14),15) During the index PCI, the interventional cardiologists had made some important decisions about using balloon pre-dilatation and intracoronary stents. Pre-procedure and post-procedure coronary flow in the target vessel was graded according to the classification used in the Thrombolysis In Myocardial Infarction (TIMI) trials.16) All of the patients with STEMI were treated with optimal medications which were recommended by the evidence-based guidelines during hospitalization and after discharge.
Clinical outcomes
We analyzed the major adverse cardiac events (MACE) defined as a composite of cardiac death, myocardial infarction (MI), and repeated PCI (target lesion or target vessel revascularization, or non-target vessel revascularization) or coronary bypass graft (CABG). Cardiac death was defined as death due to pump failure, arrhythmia, or mechanical complications including ventricular septal rupture and free wall rupture. Follow-up data were obtained by reviewing the medical records and/or telephone interview of patients. All data were entered into an electronic web-based case-report form.
Statistical analysis
For discrete variables, differences were expressed as counts and percentages, and differences between groups were analyzed using the Chi-square test (or Fisher's exact) as appropriate. For continuous variables, differences between groups were evaluated using the unpaired t-test or Mann-Whitney rank-sum test. Multivariate Cox regression analyses were performed using all available variables that could be of potential relevance in determining the impact of different age groups on clinical outcomes. In addition, multivariate logistic regression analyses were performed to determine the factors predicting cumulative one-year MACE in the young and old age groups. First, all of the variables such as the initial clinical characteristics, angiographic findings, and procedural findings were analyzed by the univariate logistic regression analysis. From this analysis, we constructed the multivariate logistic regression model using variables that were clinically significant (p<0.05). Hazard ratios and 95% confidence intervals were identified. All of the analyses were 2-tailed, with clinical significance defined as p<0.05. Statistical analysis was performed with SPSS 20.0 for Windows (SPSS-PC, Chicago, IL, USA).
Results
Baseline clinical characteristics
Among the 10177 patients, the young age group included 5281 patients (age, 53±7.8 years) and the old age group included 4896 patients (age, 74.3±6.5 years). Baseline characteristics are listed in Table 1. The young age group had a higher proportion of males and a higher incidence of obesity than the old age group, and patients in the young age group complained of typical chest pain more frequently. Smoking, family history, dyslipidemia, and metabolic syndrome were more frequently observed in the young age group than in the old age group (77.3% vs. 47.2%, p<0.001; 11% vs. 4.6%, p<0.001; 11.2% vs. 7.7%, p<0.001; 67.6% vs. 62.9%, p<0.001). The patients in the young age group were more frequently classified into the Killip class I (78.4% vs. 66.6%, p<0.001) and had a higher prevalence of sinus rhythm (92% vs. 87.1%, p<0.001), in comparison with the patients in the old age group.
Table 1. Baseline clinical characteristics.
| Young age group (n=5281) |
Old age group (n=4896) |
p | |
|---|---|---|---|
| Age (years) | 53±7.8 | 74.3±6.5 | <0.001 |
| Sex | |||
| Male | 4729 (89.5) | 2904 (59.3) | <0.001 |
| Female | 552 (10.5) | 1992 (40.7) | <0.001 |
| BMI (kg/m2) | 24.8±3.3 | 23.3±3.6 | <0.001 |
| Obesity | 2109 (39.9) | 1174 (24.0) | <0.001 |
| WC (cm) | 87.9±8.6 | 85.8±23.4 | <0.001 |
| HC (cm) | 93.8±15.9 | 91.2±25.9 | <0.001 |
| Symptom-to-door time (hours) | 12±53.2 | 17.3±132 | 0.010 |
| Symptom | 4747 (89.9) | 4159 (84.9) | <0.001 |
| Chest pain | 4742 (89.8) | 4112 (84.0) | <0.001 |
| Dyspnea | 1026 (19.4) | 1255 (25.6) | <0.001 |
| Previous angina | 1815 (34.4) | 1886 (38.5) | <0.001 |
| Risk factor | |||
| Hypertension | 1950 (36.9) | 2720 (55.6) | <0.001 |
| Diabetes mellitus | 1148 (21.7) | 1363 (27.8) | <0.001 |
| Smoking | 4084 (77.3) | 2311 (47.2) | <0.001 |
| Dyslipidemia | 590 (11.2) | 377 (7.7) | <0.001 |
| Metabolic syndrome | 3569 (67.6) | 3079 (62.9) | <0.001 |
| Family history | 580 (11.0) | 227 (4.6) | <0.001 |
| IHD history | 495 (9.4) | 636 (13.0) | <0.001 |
| Heart failure | 26 (0.5) | 85 (1.7) | <0.001 |
| Cerebrovascular disease | 164 (3.1) | 409 (8.4) | <0.001 |
| Peripheral vascular disease | 15 (0.3) | 43 (0.9) | <0.001 |
| Physical findings | |||
| SBP (mmHg) | 128.5±28.2 | 125±28.6 | <0.001 |
| DBP (mmHg) | 80.1±17.7 | 75.8±16.7 | <0.001 |
| Heart rate (/min) | 76.8±18.6 | 75.9±20.3 | 0.013 |
| Killip class | |||
| I | 4141 (78.4) | 3261 (66.6) | <0.001 |
| II-IV | 1140 (21.6) | 1635 (33.4) | <0.001 |
| ECG findings | |||
| Sinus rhythm | 4861 (92.0) | 4262 (87.1) | <0.001 |
| AV block (II/III) | 121 (2.3) | 181 (3.7) | <0.001 |
| AF/AFL | 108 (2.0) | 218 (4.5) | <0.001 |
| VT/VF | 13 (0.2) | 13 (0.3) | 0.847 |
Data are expressed as number (%) or mean±standard deviation. BMI: body mass index, WC: waist circumference, HC: hip circumference, IHD: ischemic heart disease, SBP: systolic blood pressure, DBP: diastolic blood pressure, ECG: electrocardiogram, AV: atrioventricular, AF: atrial fibrillation, AFL: atrial flutter, VT: ventricular tachycardia, VF: ventricular fibrillation
Baseline laboratory findings
Baseline laboratory findings and echocardiographic findings are listed in Table 2. The levels of creatinine kinase-MB (CK-MB), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and LVEF were higher in the young age group (199.3 ng/mL vs. 171.5 ng/mL, p<0.001; 191.2 mg/dL vs. 178.3 mg/dL, p<0.001; 145.2 mg/dL vs. 108.1 mg/dL, p<0.001; 122.8 mg/dL vs. 112.8 mg/dL, p<0.001; 52% vs. 49.4%, p<0.001). The levels of creatinine, glucose, high-sensitivity C-reactive protein, and N-terminal pro-B-type natriuretic peptide were lower in the young age group (1.1 mg/dL vs. 1.2 mg/dL, p=0.041; 169.7 mg/dL vs. 173.7 mg/dL, p=0.007; 8.1 mg/dL vs. 10.8 mg/dL, p=0.002; 1001 pg/mL vs. 2834.1 pg/mL, p<0.001). There were no significant differences in the troponin-I, troponin-T, and high-density lipoprotein cholesterol (HDL-C) between the two groups. The estimated glomerular filtration rate by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation,17) which considers the age and sex, was higher in the young age group (93.7 mL/min/1.73m2 vs. 78.5 mL/min/1.73m2, p<0.001).
Table 2. Baseline laboratory and echocardiographic findings.
| Young age group (n=5281) |
Old age group (n=4896) |
p | |
|---|---|---|---|
| Troponin I (ng/mL) | 64±179.1 | 60.6±103.4 | 0.290 |
| Troponin T (ng/mL) | 7.1±39.4 | 7.5±34.9 | 0.755 |
| CK-MB (ng/mL) | 199.3±323.2 | 171.5±263.7 | <0.001 |
| Creatinine (mg/dL) | 1.1±1.3 | 1.2±1.5 | 0.041 |
| eGFR (mL/min/1.73m2) | 93.7±13.0 | 78.5±11.5 | <0.001 |
| Glucose (mg/dL) | 169.7±74.9 | 173.7±75 | 0.007 |
| Total cholesterol (mg/dL) | 191.2±43.8 | 178.3±43.7 | <0.001 |
| Triglyceride (mg/dL) | 145.2±120.7 | 108.1±85.6 | <0.001 |
| HDL-cholesterol (mg/dL) | 44.7±24.0 | 45.2±14.3 | 0.246 |
| LDL-cholesterol (mg/dL) | 122.8±40.7 | 112.8±37.4 | <0.001 |
| hs-CRP (mg/dL) | 8.1±37.7 | 10.8±44 | 0.002 |
| NT-proBNP (pg/mL) | 1001±3381.4 | 2834.1±5705.5 | <0.001 |
| LVEF (%) | 52±11.3 | 49.4±12 | <0.001 |
Data are expressed as mean±standard deviation. CK-MB: creatine kinase-MB, eGFR: estimated glomerular filtration rate, HDL: high-density lipoprotein, LDL: low-density lipoprotein, hs-CRP: high-sensitivity C-reactive protein, NT-proBNP: N-terminal pro B-type natriuretic peptide, LVEF: left ventricular ejection fraction
Treatment modalities for ST-segment elevation myocardial infarction
There Was No Significant Difference In The Rate Of Undergoing Primary And Facilitated Pci (Table 3). While Patients In The Young Age Group Underwent Thrombolysis More Frequently (7.5% Vs. 4.9%, P<0.001), Patients In The Old Age Group Were More Frequently Treated Conservatively (10.6% Vs. 7.2%, P<0.001).
Table 3. Treatment strategy, baseline coronary angiographic findings, and procedural characteristics in patients who underwent CAG.
| Young age group (n=5281) |
Old age group (n=4896) |
p | |
|---|---|---|---|
| Primary PCI | 4321 (81.8) | 3977 (81.2) | 0.442 |
| Facilitated PCI | 153 (2.9) | 123 (2.5) | 0.232 |
| Thrombolysis | 397 (7.5) | 241 (4.9) | <0.001 |
| Conservative treatment | 382 (7.2) | 518 (10.6) | <0.001 |
| Door-to-balloon time (min) | 106.4±54.3 | 106.6±52.4 | 0.847 |
| Number of involved vessels (%) | |||
| 1 | 2771 (52.8) | 1969 (41.4) | <0.001 |
| 2 | 1496 (28.5) | 1495 (31.5) | 0.001 |
| 3 | 904 (17.2) | 1146 (24.1) | <0.001 |
| LM complex | 69 (1.3) | 130 (2.7) | <0.001 |
| LM isolated | 7 (0.1) | 12 (0.3) | 0.172 |
| Target lesion location (%) | |||
| LAD | 2874 (54.9) | 2387 (50.4) | <0.001 |
| LCX | 509 (9.7) | 435 (9.2) | 0.356 |
| RCA | 1803 (34.5) | 1837 (38.8) | <0.001 |
| LM | 46 (0.9) | 76 (1.6) | 0.001 |
| ACC/AHA types (%) | |||
| Type A | 206 (4.3) | 169 (3.9) | 0.341 |
| Type B1 | 986 (20.6) | 722 (16.7) | <0.001 |
| Type B2 | 1370 (28.6) | 1247 (28.8) | 0.826 |
| Type C | 2222 (46.4) | 2185 (50.5) | <0.001 |
| Pre-procedure TIMI flow 0 | 2807 (56.2) | 2510 (55.1) | 0.309 |
| Post-procedure TIMI flow 3 | 4612 (93.9) | 4047 (90.8) | <0.001 |
| Stent type (%) | |||
| Bare metal stent | 381 (7.9) | 430 (10.0) | <0.001 |
| Paclitaxel-eluting stent | 1139 (23.6) | 1041 (24.1) | 0.536 |
| Sirolimus-eluting stent | 1556 (32.2) | 1262 (29.2) | 0.002 |
| Zotarolimus-eluting stent | 818 (16.9) | 760 (17.6) | 0.388 |
| Everolimus-eluting stent | 542 (11.2) | 456 (10.6) | 0.320 |
| Other DES | 424 (8.8) | 389 (9.0) | 0.687 |
| Stent length (mm) | 24.4±6.6 | 24.7±6.6 | 0.018 |
| Stent diameter (mm) | 3.5±0.6 | 3.3±0.6 | <0.001 |
| Total stents per patient (n) | 1.4±0.7 | 1.5±0.8 | 0.002 |
| Multi-vessel PCI | 761 (15.2) | 733 (16.3) | 0.154 |
| PCI success rate (%) | 5044 (99.0) | 4541 (99.0) | 0.922 |
Data are expressed as number (%) or mean±standard deviation. CAG: coronary angiogram, PCI: percutaneous coronary intervention, LM: left main, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, ACC/AHA: American College of Cardiology/American Heart Association, TIMI: thrombolysis In myocardial infarction, DES: drug-eluting stent
Baseline coronary angiographic findings and procedure characteristics
The young and old age groups showed a similar door-to-balloon time (106.4 min vs. 106.6 min, p=0.847) (Table 3). The younger adults had a fewer number of diseased vessels, and a lower percentage of left main (LM) complex disease and American College of Cardiology/American Heart Association (ACC/AHA) lesion type C. In the young age group, the left anterior descending artery was involved more frequently, while the right coronary artery was involved more frequently in the old age group. Shorter, larger, and fewer number of stents were used in young patients (24.4 mm vs. 24.7 mm, p=0.018; 3.5 mm vs. 3.3 mm, p<0.001; 1.4 vs. 1.5, p=0.002) during the index PCI. Drug-eluting stents, especially sirolimus-eluting stents, were used more frequently in the young age group. Although there was no significant difference in pre-procedure TIMI flow 0, the younger patients had a higher incidence of post-procedure TIMI flow 3.
Comparison of the prescribed medications
We compared the prescribed medications at the time of discharge (Table 4). The use of aspirin, clopidogrel, beta-blockers, angiotensin-converting enzyme inhibitors, and statin was more frequent in the young age group (98.5% vs. 97.7%, p=0.003; 99.7% vs. 95.9%, p=0.033; 80.1% vs. 74.6%, p<0.001; 68.9% vs. 63.9%, p<0.001; 77.2% vs. 73.4%, p<0.001).
Table 4. Comparison of the prescribed medications.
| Young age group (n=5222) |
Old age group (n=4693) |
p | |
|---|---|---|---|
| Aspirin | 5166 (98.5) | 4617 (97.7) | 0.003 |
| Clopidogrel | 5073 (99.7) | 4533 (95.9) | 0.033 |
| Cilostazol | 1674 (31.9) | 1430 (30.3) | 0.074 |
| Dual anti-platelet therapy | 3595 (68.8) | 3296 (70.2) | 0.134 |
| Triple anti-platelet therapy | 1627 (31.2) | 1397 (29.8) | 0.134 |
| Beta-blockers | 4199 (80.1) | 3524 (74.6) | <0.001 |
| ACE inhibitors | 3611 (68.9) | 3021 (63.9) | <0.001 |
| Angiotensin II receptor blockers | 866 (16.5) | 926 (19.6) | <0.001 |
| Calcium channel blockers | 359 (6.8) | 311 (6.6) | 0.599 |
| Statin | 4049 (77.2) | 3468 (73.4) | <0.001 |
| Nitrate | 2468 (47.1) | 2121 (44.9) | 0.030 |
| Nicorandil | 1155 (22) | 1062 (22.5) | 0.589 |
| Loop diuretics | 751 (14.3) | 1319 (27.9) | <0.001 |
| Spironolactone | 367 (7.0) | 511 (10.8) | <0.001 |
| DM medications | |||
| Oral hypoglycemic agents | 443 (8.4) | 356 (7.5) | 0.094 |
| Insulin | 65 (1.2) | 58 (1.2) | 0.957 |
Data are expressed as number (%). ACE: angiotensin-converting enzyme, DM: diabetes mellitus
Clinical outcomes
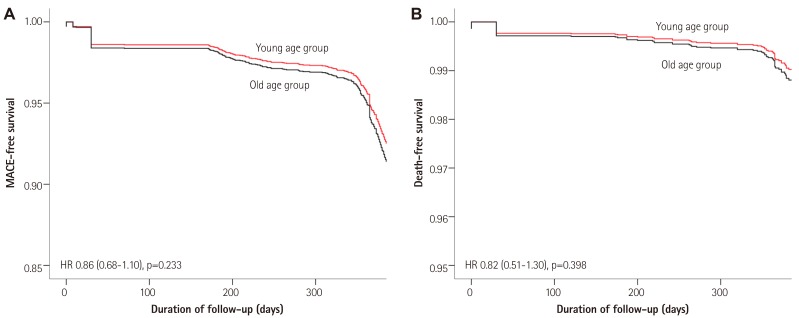
Table 5 shows in-hospital mortality, complications, and cumulative clinical outcomes during the follow-up period. According to our analyses of cumulative clinical outcomes, patients in the young age group stayed in the coronary care unit for a shorter time period (3.2 days vs. 3.9 days, p<0.001). The old age group had a higher rate of in-hospital death (3.5 % vs. 0.7 %, p<0.001) compared with the young age group. The rates of one-month, six-month, and twelve-month MACEs were lower in the young age group. The adjusted MACE rates, by Cox regression analyses, at one month and twelve months were not different between the two age groups (Table 6). These results were consistent with the adjusted all-cause death rate (Fig. 1).
Table 5. Cumulative clinical outcomes.
| Young age group (n=5281) |
Old age group (n=4896) |
p | |
|---|---|---|---|
| CCU stay (days) | 3.2±3 | 3.9±4.1 | <0.001 |
| In-hospital death (%) | 37 (0.7) | 171 (3.5) | <0.001 |
| In-hospital complications (%) | 549 (10.4) | 767 (15.7) | <0.001 |
| Acute kidney injury | 11 (0.2) | 44 (0.9) | <0.001 |
| Cardiogenic shock | 162 (3.1) | 293 (6.0) | <0.001 |
| Atrial fibrillation | 34 (0.6) | 65 (1.3) | <0.001 |
| Major bleeding | 13 (0.2) | 25 (0.5) | 0.029 |
| One-month follow-up (%) | n=4491 | n=3988 | |
| Major adverse cardiac events | 79 (1.8) | 111 (2.8) | 0.028 |
| Cardiac death | 9 (0.2) | 38 (1.0) | <0.001 |
| MI | 18 (0.4) | 28 (0.7) | 0.059 |
| Repeat PCI | 41 (0.9) | 38 (1.0) | 0.849 |
| CABG | 11 (0.2) | 7 (0.2) | 0.488 |
| Non-cardiac death | 6 (0.1) | 21 (0.5) | 0.001 |
| Six-month follow-up (%) | n=3681 | n=3410 | |
| Major adverse cardiac events | 246 (6.8) | 263 (8.2) | <0.001 |
| Cardiac death | 12 (0.3) | 65 (2.0) | <0.001 |
| MI | 24 (0.7) | 33 (1.0) | 0.098 |
| Repeat PCI | 195 (5.4) | 153 (4.7) | 0.246 |
| CABG | 11 (0.3) | 7 (0.2) | 0.490 |
| Non-cardiac death | 12 (0.3) | 49 (1.5) | <0.001 |
| Twelve-month follow-up (%) | n=3254 | n=2847 | |
| Major adverse cardiac events | 327 (10.1) | 337 (11.9) | 0.025 |
| Cardiac death | 17 (0.5) | 81 (2.9) | <0.001 |
| MI | 34 (1.1) | 36 (1.3) | 0.421 |
| Repeat PCI | 261 (8.1) | 208 (7.3) | 0.296 |
| CABG | 17 (0.5) | 15 (0.5) | 0.981 |
| Non-cardiac death | 20 (0.6) | 66 (2.3) | <0.001 |
Data are expressed as number (%) or mean±standard deviation. CCU: coronary care unit, MI: myocardial infarction, PCI: percutaneous coronary intervention, CABG: coronary artery bypass grafting
Table 6. Cox regression analyses of age difference (young age group vs. old age group) with one-month and twelve-month MACEs.
| Model | One-month MACEs | Twelve-month MACEs | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) |
p | Hazard ratio (95% CI) |
p | |
| Unadjusted | 0.94 (0.71-1.25) | 0.677 | 0.77 (0.66-0.89) | 0.001 |
| Adjusted for sex | 0.85 (0.63-1.15) | 0.295 | 0.73 (0.62-0.86) | <0.001 |
| Adjusted for selected variables* | 0.90 (0.65-1.24) | 0.517 | 0.74 (0.62-0.88) | 0.001 |
| Multivariable adjusted† | 0.95 (0.60-1.51) | 0.828 | 0.86 (0.68-1.10) | 0.233 |
*The variables of baseline clinical characteristics; sex, BMI, presence of typical symptoms, chest pain and dyspnea, history of previous angina, initial vital signs (systolic blood pressure, diastolic blood pressure, and heart rate), Killip classification on admission, symptom-to-door time, arrhythmia, cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, smoking, family history of coronary heart disease, and previous ischemic heart diseases), cerebrovascular or peripheral arterial diseases, and heart failure. †The variables considered potentially relevant on univariate analyses were included; all of the variables mentioned above as the selected variables, treatment strategy, door-to-balloon time, angiographic findings (number of diseased vessels, lesion location and type), procedural findings (stent type, length and diameter, and number of implanted stents), cardiovascular medications (aspirin, clopidogrel, beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, Angiotensin II receptor blockers, statin, nitrate, spironolactone, and diuretics), renal insufficiency (estimated glomerular filtration rate<60 mL/min/1.73m2), and left ventricular dysfunction (left ventricular ejection fraction<40%). MACE: major adverse cardiac event , CI: confidence interval
Fig. 1. Adjusted MACE-free survival (A) and death-free survival at 12 months by multivariate Cox regression analyses (B). MACE: major adverse cardiac event, HR: hazard ratio.
In the multivariate logistic regression analysis, the presence of multivessel disease, renal insufficiency, and LM complex lesion were independent predictors of MACE at one year after STEMI in younger adults (Table 7). On the contrary, complete multivessel revascularization during the index PCI in younger STEMI patients was associated with favorable outcomes.
Table 7. Multivariate logistic regression analysis for predicting the one-year major adverse cardiac events in patients of the young age and old age groups.
| Young age group | Old age group | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) |
p | Hazard ratio (95% CI) |
p | |
| Multivessel disease | 1.95 (1.37-2.78) | <0.001 | 1.76 (1.20-2.57) | 0.004 |
| Renal insufficiency | 3.20 (1.03-9.94) | 0.044 | 0.73 (2.67-1.99) | 0.537 |
| LM complex lesion | 2.72 (1.01-7.30) | 0.048 | 1.25 (0.57-2.75) | 0.584 |
| Multivessel revascularization | 0.56 (0.34-0.92) | 0.023 | 1.23 0.77-1.97) | 0.384 |
| LV dysfunction (LVEF<40%) | 1.41 (0.88-2.26) | 0.155 | 2.09 (1.39-3.14) | <0.001 |
| Diabetes mellitus | 1.24 (0.87-1.75) | 0.236 | 1.60 (1.14-2.26) | 0.007 |
| Using drug-eluting stent | 0.70 (0.42-1.18) | 0.186 | 0.60 (0.38-0.94) | 0.025 |
| ACE inhibitors | 1.23 (0.78-1.93) | 0.368 | 0.62 (0.41-0.95) | 0.027 |
CI: confidence interval, LM: left main: LV: left ventricular, LVEF: left ventricular ejection fraction, ACE: angiotensin-converting enzyme
Discussion
This is the first, large, multicenter, prospective study in younger Korean patients with STEMI. About half of the STEMI patients (52%) were less than 65 years of age. Previous studies have demonstrated that the young age group has a higher proportion of males, smokers, DL, family history, and a lower incidence of HTN and DM than the old age group.3),5),18),19) The results of our study in younger Korean adults were in agreement with those of previous studies in many aspects (Table 1). Cigarette smoking was the most common and modifiable cause of AMI in younger patients in our study. Smoking is a major independent risk factor for ischemic heart disease, cerebrovascular disease, and total atherosclerotic cardiovascular disease.20) It is well known that smoking has an adverse effect on serum lipids, and it is associated with insulin resistance,21) enhanced prothrombotic state, elevated fibrinogens, and increased platelet activity.22) The relative benefits of smoking cessation are equivalent in young and old patients.23) Since the rate of smoking in younger STEMI patients is high, it is more important to encourage smoking cessation in patients of the young age group than in those of the old age group.
The other risk factor among younger adults with STEMI was obesity. Obesity has been known to be an independent risk factor for CHD.24) It causes some changes in lipid metabolism, elevation of TG, LDL-C, and lowers the concentration of HDL-C.25) We observed that the incidence of dyslipidemia was higher in younger Korean STEMI patients than in older Korean STEMI patients. Finally, the incidence of metabolic syndrome was higher in younger adults with STEMI. For these metabolically unhealthy obese younger adults with STEMI, regular exercise and weight reduction are necessary to improve the metabolic state. Because younger adults with STEMI had higher levels of TC, TG, and LDL-C (Table 2), it is clear that the use of statin in younger STEMI patients will be beneficial in treating lipid abnormalities.
The younger STEMI patients complained of typical chest pain more frequently, but they experienced angina less frequently before hospitalization. According to the analysis of the initial coronary angiogram, the young age group had a higher frequency of single vessel disease than the old age group. These observations suggested that the proposed mechanism causing AMI in the young age group was sudden interruption of coronary blood flow before preconditioning caused by plaque rupture, followed by rapid progression of atherothrombosis rather than atherosclerosis. Shorter, larger, and fewer number of stents were used in the younger adults, and this indicated that thrombosis generally affected a single, large, and short segment of the coronary artery. On the other hand, the elderly patients experienced severe atherosclerosis leading to multi-vessel disease, LM disease, and systolic dysfunction, which were correlated with poor prognosis.3)
Since typical symptoms were experienced more frequently by younger patients, they visited the emergency room much earlier. The higher level of CK-MB in the younger adults indirectly indicated this finding. Actually, our data showed a shorter symptom-to-door time in young patients than in old patients. Meanwhile, there was no significant difference in door-to-balloon time between younger patients and elderly patients in this study. Consequently, the patients in the young age group may have more chance of reducing the ischemic time. However, unfortunately, the mean door-to-balloon time exceeded 90 minutes in both groups. To improve the prognosis of Korean STEMI patients, the physicians and/or the interventionist must make an effort and should pay attention to the methods that can reduce the door-to-balloon time. In the multivariate logistic regression analysis, we determined the independent predictive factors of one-year MACE in the young age group (Table 7). They were multivessel disease, renal insufficiency, and LM complex lesion, but these factors were not statistically different from those in the old age group except for multivessel disease. We could not determine the reason for this discrepancy.
In the present study, we analyzed the clinical outcomes of AMI patients such as the in-hospital outcomes and out-of-hospital outcomes (Table 5). As expected, the young age group showed excellent prognosis in terms of in-hospital death and complications, one-month, six-month, and twelve-month MACE. There were significant differences in the incidence of cardiac death and noncardiac death between the two groups; however, the young age group did not have a lower incidence of MI, repeat PCI, and CABG compared to the old age group. Because there were significant differences in the baseline clinical characteristics between the two groups, they exerted an influence on the prognosis. When we performed multivariate Cox regression analyses using all of the affecting variables, the age difference was not important as before (Table 6). There was no significant clinical benefit in the young STEMI patients at the one-month and twelve-month follow-up (Fig. 1). We concluded that younger patients should be treated continuously like elderly patients with close follow-up. The elderly patients, regardless of their age, should also be treated aggressively.
Our study had several limitations. First, since our study included only diseased patients, we could not accurately identify the risk factors for AMI in young healthy individuals. Second, the long-term prognosis would be more important in young AMI patients; however, a maximum one-year follow-up data were available in the current study. Third, a randomized prospective study, which shows that intensive medical treatment of younger adults improves the clinical outcomes, is needed.
In conclusion, younger Korean STEMI patients included a higher proportion of males and had higher incidences of smoking, obesity, DL, metabolic syndrome, and family history compared with elderly patients. The young age group had more favorable in-hospital outcomes. However, after adjustment for the potential confounders, the clinical outcomes of patients in the young age group were not superior to those of patients in the old age group at the one-year follow-up. Therefore, younger patients should also consistently receive intensive medical treatment like the old aged patients for preventing the MACE.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Kim RB, Kim BG, Kim YM, et al. Trends in the incidence of hospitalized acute myocardial infarction and stroke in Korea, 2006-2010. J Korean Med Sci. 2013;28:16–24. doi: 10.3346/jkms.2013.28.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman FH, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry) J Am Coll Cardiol. 1995;26:654–661. doi: 10.1016/0735-1097(95)00254-2. [DOI] [PubMed] [Google Scholar]
- 4.Choudhury L, Marsh JD. Myocardial infarction in young patients. Am J Med. 1999;107:254–261. doi: 10.1016/s0002-9343(99)00218-1. [DOI] [PubMed] [Google Scholar]
- 5.Morillas P, Bertomeu V, Pabón P, et al. Characteristics and outcome of acute myocardial infarction in young patients. Cardiology. 2007;107:217–225. doi: 10.1159/000095421. [DOI] [PubMed] [Google Scholar]
- 6.Lee SR, Jeong MH, Ahn YK, et al. Clinical safety of drug-eluting stents in the Korea acute myocardial infarction registry. Circ J. 2008;72:392–398. doi: 10.1253/circj.72.392. [DOI] [PubMed] [Google Scholar]
- 7.French JK, White HD. Clinical implications of the new definition of myocardial infarction. Heart. 2004;90:99–106. doi: 10.1136/heart.90.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia LC, Naik RH. Clinical profile of acute myocardial infarction in elderly patients. J Cardiovasc Dis Res. 2013;4:107–111. doi: 10.1016/j.jcdr.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005;94:1–12. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 12.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 13.Schömig A, Mehilli J, Antoniucci D, et al. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA. 2005;293:2865–2872. doi: 10.1001/jama.293.23.2865. [DOI] [PubMed] [Google Scholar]
- 14.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 15.Wu AH, Parsons L, Every NR, Bates ER Second National Registry of Myocardial Infarction. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2) J Am Coll Cardiol. 2002;40:1389–1394. doi: 10.1016/s0735-1097(02)02173-3. [DOI] [PubMed] [Google Scholar]
- 16.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbash GI, White HD, Modan M, et al. Acute myocardial infarction in the young--the role of smoking. The Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Trial. Eur Heart J. 1995;16:313–316. [PubMed] [Google Scholar]
- 19.Jinnouchi H, Sakakura K, Wada H, et al. Clinical features of myocardial infarction in young Japanese patients. Int Heart J. 2013;54:123–128. doi: 10.1536/ihj.54.123. [DOI] [PubMed] [Google Scholar]
- 20.Jee SH, Suh I, Kim IS, Appel LJ. Smoking and atherosclerotic cardiovascular disease in men with low levels of serum cholesterol: the Korea Medical Insurance Corporation Study. JAMA. 1999;282:2149–2155. doi: 10.1001/jama.282.22.2149. [DOI] [PubMed] [Google Scholar]
- 21.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–1130. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 22.Newby DE, Wright RA, Labinjoh C, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 23.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 24.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 25.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]