Abstract
Aims
To assess the laboratory policies, pathologists’ clinical practice, and perceptions about the value of second opinions for breast pathology cases among pathologists practicing in the United States.
Methods
Cross-sectional data were collected from 252 pathologists who interpret breast specimens in eight states using a web-based survey. Descriptive statistics were used to characterize findings.
Results
Most participants had >10 years of experience interpreting breast specimens (64%), were not affiliated with academic centers (73%) and were not considered experts by their peers (79%). Laboratory policies mandating second opinions varied by diagnosis: invasive cancer 65%; DCIS 56%; atypical ductal hyperplasia 36%; and other benign cases 33%. 81% obtained second opinions in the absence of policies. Participants believed they improve diagnostic accuracy (96%) and protect from malpractice suits (83%), and were easy to obtain, did not take too much time, and did not make them look less adequate. The most common (60%) approach to resolving differences between the first and second opinion is to ask for a third opinion, followed by reaching a consensus.
Conclusions
Laboratory-based second opinion policies vary for breast pathology, but are most common for invasive cancer and DCIS cases. Pathologists have favorable attitudes towards second opinions, adhere to policies, and obtain them even when policies are absent. Those without a formal policy may benefit from supportive clinical practices and systems that help obtain second opinions.
Keywords: Second opinion, clinical pathology, breast cancer, benign breast disease
INTRODUCTION
Obtaining second opinions is an established part of pathology practice and many health care institutions have implemented policies requiring them.[1-6] Second opinions may improve diagnostic accuracy and the quality of patient care,[7] as well as provide opportunities to educate physicians. For example, double reading of screening mammography by radiologists in the United States was common before the widespread use of computer aided detection [CAD)[8], and this practice is still used in Europe.[9-11] In breast pathology, many hospitals mandate a second review of pathology slides from outside laboratories before surgical interventions,[1,2,12] or for all cancer diagnoses before treatment planning.[13,14] While policies and practices are more established for malignant diagnoses, second opinions may also be important for high-risk non-malignant lesions, which have greater diagnostic disagreement than malignant lesions, to assure that cancer is not misdiagnosed and assess patient risk to guide surveillance and risk reduction strategies.[7]
Little is known about current policies and practices regarding second opinions in breast pathology. To address this gap, we undertook a survey study of pathologists who interpret breast specimens in eight U.S. states to determine details about second opinion policies, when and how second opinions are used in clinical practice, as well as pathologists’ perspectives about their value.
METHODS
Overview & Recruitment
The Breast Pathology (B-Path) study was designed to investigate the extent and impact of diagnostic variability of pathologists’ interpretations of breast biopsies in the United States. More details about this study can be found elsewhere.[15] Briefly, pathologists who interpret breast specimens were identified from multiple sources, including the Breast Cancer Surveillance Consortium (BCSC) sites in Vermont and New Hampshire, national membership directories, academic institutions and community practice groups through existing contacts of the site Principal Investigators, and Internet searches. Eligibility included having completed residency and/or fellowship training in 0pathology more than one year ago and interpreting breast specimens for at least the past year with the expectation of continuing to interpret for the next year. To compare clinical and demographic characteristics among participants and non-participants, we obtained information on the entire population of invited pathologists from Direct Medical Data, LLC.
The Institutional Review Boards at the Dartmouth College, Fred Hutchinson Cancer Research Center, Providence Health & Services of Oregon, University of Vermont, and University of Washington approved all study activities.
Key Measures
Pathologists described policies regarding the use of second opinions to interpret breast specimens, how often they actually use second opinions (regardless of policies), and what they perceive as an ideal practice. Responses were provided by diagnostic category (negative, atypical ductal hyperplasia [ADH], ductal carcinoma in situ [DCIS], invasive). Because the responses for “actual practice” and “ideal practice” were almost identical, we only report those for actual practice. We collapsed responses for “Not Applicable” (NA) and 0% to form the “no policy” category and laboratories having a second opinion policy requirement for greater than 0% of cases were considered as “having a policy”. We also report the responses for five survey questions related to the pathologists’ perceptions about the value of second opinion using a seven-point Likert scale from “Strongly agree” to “Strongly disagree” which we collapsed due to a small number of responses in some categories into the following four categories: 1) strongly disagree or disagree; 2) slightly disagree; 3) slightly agree and; 4) agree or strongly agree.
Participating pathologists were additionally provided with a hypothetical case developed by three breast pathology experts: “You are reviewing a breast needle core biopsy from a 45-year-old woman with no history of breast disease. There is an intraductal process that you consider to be borderline between atypical ductal hyperplasia (ADH) and ductal carcinoma in situ (DCIS), but you favor classifying it as ADH.” The case was followed by these questions about obtaining a second opinion: “In situations like this, what percentage of cases would you get a second opinion?”; “If you were to obtain a second opinion, would your second opinion reviewer usually be blinded to your opinion on the case?” Finally, the pathologists were asked to respond to five suggested methods they might use to resolve a difference between the first and second opinion. Responses were provided using a five-point Likert scale from “Never or almost never” to “infrequently” to “about half the time” to “frequently” to “always or almost always”.
Statistical Analyses
Descriptive statistics were calculated for pathologist and practice characteristics and for responses to questions related to second opinions. For descriptive purposes, second opinion policy requirements involving any of the four initial diagnoses were consolidated into “Any Policy” versus none at all or “No Policy”. Summary statistics are presented as frequencies and percentages. Pearson's Chi-squared test was used to analyze categorical variables. All reported p-values are two-sided and considered significant at α=0.05. Analyses were performed in R version 3.0.1 [16] using the HH [17] Likert() function for plots and in SAS 9.3 (SAS Institute Inc., Cary, NC, USA) using the FREQ procedure.
RESULTS
Participants
Of the 691 pathologists invited, 146 were ineligible and 156 could not be contacted or their eligibility verified despite multiple attempts. Among the remaining 389 pathologists, 137 (35%) declined and 252 (65%) completed the web-based B-Path survey between November 2011 and February 2013. There was no statistically significant difference between the participating pathologists and those we were unable to contact in regards to age, gender, or size of population served by their clinical laboratory (<250,000 vs. >250,000).
The majority of participants were male (63.1%), aged 40 to 59 years (70.2%), and had 10 or more years of experience interpreting breast specimens (64.3%) (Table 1). Although participants practiced in 8 states, the largest proportion worked in Washington State (36.5%). Most were not affiliated with academic medical centers (72.6%) and worked in facilities with fewer than 10 pathologists who interpret breast specimens (62.7%). Only 1.6% reported having completed fellowship training in breast pathology, with 44.0% reporting a surgical pathology fellowship. Half of the participants reported that more than 10% of their clinical case load consisted of breast specimens and 20.6% reported that their colleagues consider them experts in breast pathology. There were no statistically significant differences between demographic and practice characteristics for participants whose laboratory had a policy and those who did not.
Table 1.
Demographic and Practice Characteristics of Participating Pathologists (n = 252)
| Does Participant's Laboratory Have A Policy Requiring a Second Opinion? | ||||
|---|---|---|---|---|
| Participant Characteristics | Total n (col %) | No Policy n (row %) | Any Policy * n (row %) | p-value † |
| Total | 252 (100.0) | 85 (33.7) | 167 (66.3) | |
| Demographics | ||||
| Age at Survey (yrs) | 0.30 | |||
| 30-39 | 32 (12.7) | 9 (28.1) | 23 (71.9) | |
| 40-49 | 87 (34.5) | 25 (28.7) | 62 (71.3) | |
| 50-59 | 90 (35.7) | 32 (35.6) | 58 (64.4) | |
| 60+ | 43 (17.1) | 19 (44.2) | 24 (55.8) | |
| Gender | 0.65 | |||
| Male | 159 (63.1) | 52 (32.7) | 107 (67.3) | |
| Female | 93 (36.9) | 33 (35.5) | 60 (64.5) | |
| State of clinical practice | 0.14 | |||
| Alaska | 8 (3.2) | 4 (50.0) | 4 (50.0) | |
| Maine | 22 (8.7) | 4 (18.2) | 18 (81.8) | |
| Minnesota | 42 (16.7) | 20 (47.6) | 22 (52.4) | |
| New Hampshire | 13 (5.2) | 3 (23.1) | 10 (76.9) | |
| New Mexico | 15 (6.0) | 7 (46.7) | 8 (53.3) | |
| Oregon | 40 (15.9) | 14 (35.0) | 26 (65.0) | |
| Vermont | 20 (7.9) | 8 (40.0) | 12 (60.0) | |
| Washington | 92 (36.5) | 25 (27.2) | 67 (72.8) | |
| Training and Experience | ||||
| Laboratory size | 0.19 | |||
| < 10 Pathologists | 158 (62.7) | 58 (36.7) | 100 (63.3) | |
| ≥ 10 Pathologists | 94 (37.3) | 27 (28.7) | 67 (71.3) | |
| Fellowship training in surgical or breast pathology | 0.16 | |||
| No | 129 (51.2) | 41 (31.8) | 88 (68.2) | |
| Yes, Breast Pathology | 4 (1.6) | 0 (0.0) | 4 (100.0) | |
| Yes, Surgical | 111 (44.0) | 43 (38.7) | 68 (61.3) | |
| Both | 8 (3.2) | 1 (12.5) | 7 (87.5) | |
| Affiliation with academic medical center | 0.098 | |||
| No | 183 (72.6) | 59 (32.2) | 124 (67.8) | |
| Yes, adjunct/affiliated | 42 (16.7) | 12 (28.6) | 30 (71.4) | |
| Yes, primary appointment | 27 (10.7) | 14 (51.9) | 13 (48.1) | |
| Do your colleagues consider you an expert in breast pathology? | 0.42 | |||
| No | 200 (79.4) | 65 (32.5) | 135 (67.5) | |
| Yes | 52 (20.6) | 20 (38.5) | 32 (61.5) | |
| Clinical Practice and experience | ||||
| Breast pathology experience (yrs) | 0.12 | |||
| < 5 | 46 (18.3) | 10 (21.7) | 36 (78.3) | |
| 5-9 | 44 (17.5) | 16 (36.4) | 28 (63.6) | |
| 10-19 | 89 (35.3) | 28 (31.5) | 61 (68.5) | |
| ≥ 20 | 73 (29.0) | 31 (42.5) | 42 (57.5) | |
| Breast specimen case load (%) | 0.94 | |||
| < 10 | 126 (50.0) | 43 (34.1) | 83 (65.9) | |
| 10-24 | 104 (41.3) | 34 (32.7) | 70 (67.3) | |
| ≥ 25 | 22 (8.7) | 8 (36.4) | 14 (63.6) | |
| No. Breast cases (per week) | 0.85 | |||
| < 5 | 57 (22.6) | 20 (35.1) | 37 (64.9) | |
| 5-9 | 109 (43.3) | 34 (31.2) | 75 (68.8) | |
| 10-19 | 64 (25.4) | 24 (37.5) | 40 (62.5) | |
| ≥ 20 | 22 (8.7) | 7 (31.8) | 15 (68.2) | |
Policy was dichotomized to No Policy (includes not known and laboratories that did not require second opinion) versus
Having a Policy Requiring a Second Opinion for at Least One of Four Initial Diagnoses (in which percentage of cases requiring a second opinion was >0%)
Including invasive cancer, DCIS, atypical ductal hyperplasia (ADH), and/or benign cases.
p-value for No Policy vs Policy from the Chi-square test
Policies and Practice
Of the 252 participants, 167 (66.3%) stated that they had a policy for at least one diagnostic category. Participants reported that policies on obtaining second opinions at laboratories where they interpret specimens varied according to diagnostic category. For cases with invasive breast cancer, 65.1% of participants indicated their laboratories had policies requiring second opinions on 100% of cases (Figure 1), while 33.3% reported having no policy for invasive cancers and 1.6% responded that they did not know their policy (data not shown). Policies were less common for less severe diagnoses; 56.3% reported requiring second opinions on all cases of DCIS, 36.1% for all ADH cases, and 32.5% for all benign cases (Figure 1). Differences in policies between the four diagnostic groups were statistically significantly different (p<.001).
Figure 1.
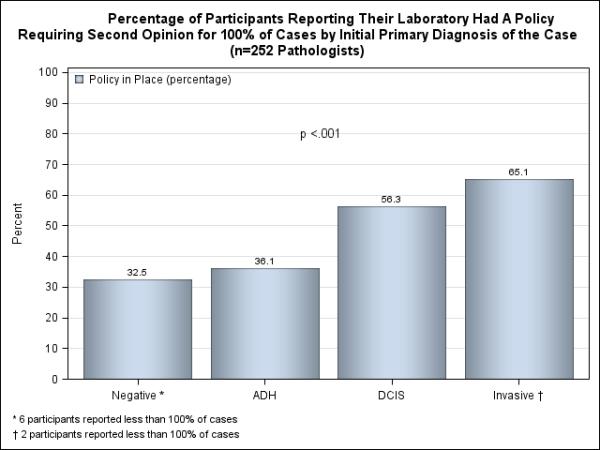
Percentage of participants reporting their laboratory had a policy requiring second opinion for 100% of the cases by initial primary diagnosis of the case.
Most participants obtained second opinions when laboratory policies were in place for some cases, even for negative (benign without atypia) diagnoses, and only a few did not practice such policies (Table 2). The far left side of Table 2 shows that even though a policy is in place, not all cases necessarily receive a second opinion for 100% of the cases. For example, among the benign without atypia (negative) breast cases, 73 of the 82 pathologists reported their actual practice was to obtain a second opinion for 100% of these cases, but for the 9 other pathologists, their practice to obtain a second opinion was less.
Table 2.
Percent of pathologist who report they would seek a second opinion by diagnosis category and second opinion policy. (n = 252 Pathologists)
| Percent of actual cases with second opinion by diagnostic category | Policy in Place | |||||
|---|---|---|---|---|---|---|
| Yes | No† | |||||
| n | col % | row % | n | col % | row % | |
| Negative (benign) | ||||||
| 0% | 1 | 1.2 | 1.6 | 60 | 35.3 | 98.4 |
| >0-50% | 6 | 7.3 | 5.5 | 104 | 61.2 | 94.5 |
| >50-<100% | 2 | 2.4 | 50.0 | 2 | 1.2 | 50.0 |
| 100% | 73 | 89.0 | 94.8 | 4 | 2.4 | 5.2 |
| Total (n) | 82 | 100.0 | 32.5 | 170 | 100.0 | 67.5 |
| ADH | ||||||
| 0% | 1 | 1.1 | 3.7 | 26 | 16.1 | 96.3 |
| >0-50% | 0 | 0.0 | 0.0 | 53 | 32.9 | 100.0 |
| >50-<100% | 2 | 2.2 | 5.1 | 37 | 23.0 | 94.9 |
| 100% | 88 | 96.7 | 66.2 | 45 | 28.0 | 33.8 |
| Total (n) | 91 | 100.0 | 36.1 | 161 | 100.0 | 63.9 |
| DCIS | ||||||
| 0% | 1 | 0.7 | 4.3 | 22 | 20.0 | 95.7 |
| >0-50% | 2 | 1.4 | 3.3 | 58 | 52.7 | 96.7 |
| >50-<100% | 5 | 3.5 | 21.7 | 18 | 16.4 | 78.3 |
| 100% | 134 | 94.4 | 91.8 | 12 | 10.9 | 8.2 |
| Total (n) | 142 | 100.0 | 56.3 | 110 | 100.0 | 43.7 |
| Invasive† | ||||||
| 0% | 1 | 0.6 | 5.0 | 19 | 21.6 | 95.0 |
| >0-50% | 4 | 2.4 | 7.1 | 52 | 59.1 | 92.9 |
| >50-<100% | 9 | 5.5 | 47.4 | 10 | 11.4 | 52.6 |
| 100% | 150 | 91.5 | 95.5 | 7 | 8.0 | 4.5 |
| Total (n) | 164 | 100.0 | 65.1 | 88 | 100.0 | 34.9 |
| At least one above§ | ||||||
| 0% | 1 | 0.6 | 5.9 | 16 | 18.8 | 94.1 |
| >0-50% | 0 | 0.0 | 0.0 | 34 | 40.0 | 100.0 |
| >50-<100% | 9 | 5.4 | 32.1 | 19 | 22.4 | 67.9 |
| 100% | 157 | 94.0 | 90.8 | 16 | 18.8 | 9.2 |
| Total (n) | 167 | 100.0 | 66.3 | 85 | 100.0 | 33.7 |
Note: Row percentages might not add up to 100 due to rounding.
* Pathologists reported the % of their breast caseload by primary diagnosis for which they usually obtain a second opinion.
Policy was dichotomized to NO (which include the Unknowns and 0%) and YES (% reported >0)
One or more second option policies; actual practice is maximum % of cases in any 4 diagnostic groups
Many pathologists also obtained second opinions for certain diagnoses in the absence of policies. Of participants reporting no second opinion policy for ADH, 83.9% obtained second opinions in at least some of the initial ADH cases, and 28.0% obtained second opinions in all their ADH cases. Of participants who reported no policy requiring second opinion for DCIS cases, 10.9% asked for second opinions for 100% of their DCIS cases, and 80.0% obtained second opinions for some of their DCIS cases. Also, among the 170 pathologists who reported that their laboratory did not have a policy for benign without atypia (negative) cases, 110 (64.8%) stated that in actual practice they obtained a second opinion for some of the cases.
Perceptions about Second Opinion
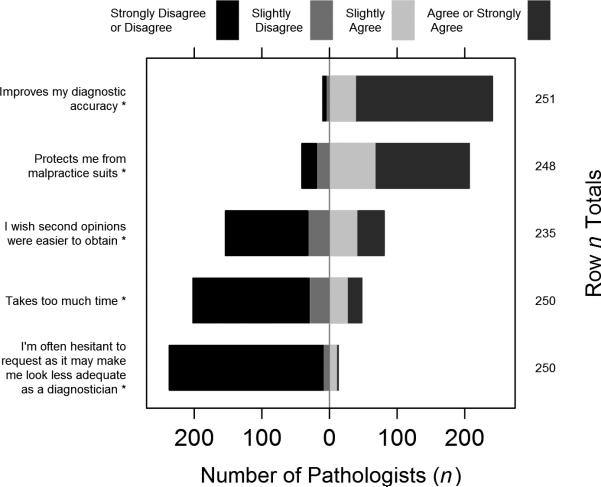
Participants overwhelmingly agreed (96.0% slightly agree or strongly agree) that asking another pathologist for a second opinion on breast cases “Improves my diagnostic accuracy” (Figure 2). 83.5% agreed that it protected them from malpractice suits. The majority felt that second opinions were easy to obtain (65.5%), did not take too much time (80.8%), and did not make them look less adequate (94.8%).
Figure 2.
Responses to the question, “What are your thoughts on asking another pathologist for a second opinion on breast cases?”
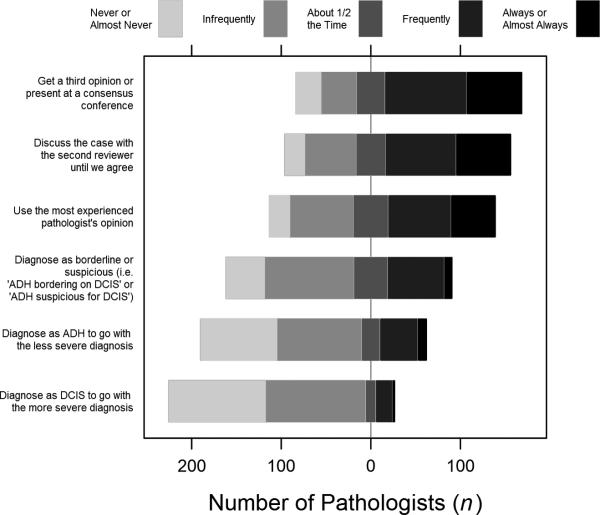
Responses to the hypothetical case with a diagnosis borderline between ADH and DCIS indicated that 2.3% of participants would not ask for a second opinion, 66.3% would ask for a second opinion in all situations like the case, while 17.5% would seek a second opinion in half or less of such situations. 52.3% reported that the second reviewer would be blinded to the first reviewer's opinion. The most common approach to resolving differences between the first and second opinion was to ask for a third opinion, based on 60.3% of the participants responding with always, almost always, or frequently following this approach (Figure 3). The majority of participants (55.2%) also reported that they would discuss the case with the two reviewers until they came to consensus. Although the least common method of resolving clinical differences was to assign the most severe diagnosis (e.g., DCIS), 7.9% reported that they always, almost always, or frequently do this.
Figure 3.
Responses to questions about resolving differences between first and second opinions for a hypothetical case.
DISCUSSION
Pathologists in our study reported that they commonly obtain second opinions in breast pathology practice, even when laboratory policies are not in place, demonstrating that they value this process. The majority of participants had greater than 10 years of experience interpreting breast specimens, but were not affiliated with academic centers and were not considered experts by their peers. Policies mandating second opinions for all cases varied by diagnosis and were less frequent for less severe diagnoses. Most participants reported that second opinions improve diagnostic accuracy and protect from malpractice suits. The most common approach to resolving differences between the first and second opinion is to ask for a third opinion, followed by reaching a consensus.
In 2000, the American Society of Clinical Pathologists published proceedings of a consensus conference on second opinions in diagnostic anatomic pathology with the purpose of developing guidelines for equitable and effective use of second opinions to prevent medical errors.[13] The guidelines are intentionally broad because the field is large and complex; thus rules made specific to one type of specimen may not be suitable for another type. However, the guidelines focus on “highly critical/ significant cases” and “problem-prone cases” and they recommend that a formal policy on second opinions be developed by respective pathology departments.
More recently 45 laboratories participating in a College of American Pathology Q-Probes Study that examined surgical pathology case reviews before sign-out found that 28.9% of the laboratories had no policy regarding second opinions compared with 33.7% of pathologists in our study.[6] Following gastrointestinal tract specimen, the second most common type of re-reviewed case in the Q-Probe Study were breast cases at 16% of the breast cases examined. Of those laboratories with a policy in the Q-Probe Study 63.6% required only 10% of cases re-reviewed. Ten percent review of surgical pathology cases is commonly suggested for Quality Assurance programs,[18] but there is little evidence that this requirement improves accuracy.
Variability was reported in the clinical practice of obtaining second opinions with differences in what types of breast cases are reviewed, how the second opinions are conducted, and how discrepancies between the two reviews are resolved. Our data indicate that the most common method to conduct a second review is to have the second reviewer blinded to the first pathologist's assessment. Published studies on second opinions also describe various methods, including both blinded [19, 20] and un-blinded reviews.[3] As part of an error reduction strategy, the second opinion should be as independent from the initial review as possible.[13]
In anatomical pathology it is difficult to set a gold standard. Some use expert opinions, while others follow cases until a more definitive diagnosis becomes apparent.[19] However, most women have breast biopsies with benign results that do not warrant further work-up, and the assessment of a biopsy specimen from a more definitive diagnosis is often biased by the original assessment.
Although researchers have shown that second opinions improve patient care and outcomes,[3] with one study specific to breast pathology,[21] studies rarely describe the details of how discrepancies are resolved. In another study, achieving consensus was the first approach to resolving discrepancies, followed by sending the case to an outside expert.[19] In another study that reports the results of a second review of all inter-laboratory-based pathology cases for one year, 9.1% (N=77) were discrepant; however, after one year of follow-up in five of the 77 cases, the original diagnoses were noted to be correct and the consultative diagnoses incorrect.[22] Because discordant second opinions may not be the correct diagnoses, it would be helpful to establish practical and/or more formal methods for resolving differences in diagnostic opinion.
In our survey, we specifically asked pathologists about their methods for addressing discrepancies between their assessment and that of the second pathologist. The most common way they resolve these differences is to ask for a third review. Their second most common resolution method was to have the two pathologists attempt to come to a consensus opinion. We speculate that the interaction that takes place between the first and second pathologists, and sometimes a third pathologist, can be a learning experience for all involved by identifying what may have been visually missed and/or understanding how other pathologists interpret specific findings on the slide.
Pathologists in our study reported finding a second review helpful, in particular, they agreed it improves their diagnostic accuracy, it protects them from malpractice suits, and they do not find requesting a second opinion difficult. Obtaining a second opinion is highly regarded by pathologists based on its frequent use even in laboratories without a policy in place.
Digital whole slide imaging (WSI) would allow pathologists to seek second opinions via the Internet, making it more accessible to rural and small pathology laboratories. However, WSI currently is not approved by the United States Food and Drug Administration. WSI will require proof of accuracy and improvements in technology before it can be used in clinical practice. [23]
In addition to its clinical value, second opinions have also been shown to be cost saving. Second opinions were used to identify misdiagnoses in a large study that reviewed prostate needle biopsies.[24] As a result, 18.7% of men avoided cancer surgery with a $1.91 cost savings for every $1.00 spent.
Given these results, we were surprised to find that few pathology laboratories require second opinion for high-risk lesions such as DCIS and ADH. We found the most common second opinion policy in breast pathology was for invasive cancers and 64% of participants reported that their laboratories require that all invasive breast cancer cases be re-reviewed. Interestingly, DCIS and high-risk lesions, such as ADH, are among the more difficult diagnoses to come to agreement on. Yet most pathology laboratories do not have policies requiring a second opinion for these cases.[12] Just as double reading in mammography with consensus or arbitration to resolve differences has been shown to increase cancer detection rate,[25,26] treatment of breast disease could easily change depending on the pathology differential diagnosis. From detection through diagnosis, accuracy is important. It is heartening to see that pathologists request second opinions often even without a policy in place. Perhaps if laboratory administrators realized that second opinions were occurring regardless of the existence of a policy they would be more likely to endorse and adopt policies and practices that would encourage uniform use of second opinions.
There are both strengths and limitations to this study. We invited a large sample to participate from a variety of geographic locations in the United States. Because most of the participating pathologists were not affiliated with an academic medical center, were not considered experts, nor were most fellowship trained, our responses may be representative of the wide range of pathologists who practice in the United States. The topic of obtaining second opinions in clinical practice is complex and the survey was kept short to obtain a high response rate from busy pathologists. Second opinion was one of many topics we asked about on the brief survey, so we did not ask in depth questions about second opinion.
The findings from our study leave many interesting questions for future research, including: What is the role of informal second opinion (curbside consult)? Are second opinions only helpful for certain pathologists or for certain cases? Should the second reviewer be more experienced than the first reviewer? What is the best method for resolving a difference between two reviews? Can digital whole slide imaging be used for second reviews to speed and improve efficiency? Are digital whole slide imaging adequately accurate compared with glass slides?
In conclusion, breast pathologists perceive that second opinions improve accuracy of diagnoses. Many pathology laboratories do not have policies in place that require second opinions, particularly for difficult or borderline diagnoses. However, pathologists in clinical practice often request second opinions regardless of whether a policy exists at their facilities. Those without a formal policy may benefit from supportive clinical practices and systems that help obtain second opinions.
TAKE HOME MESSAGES.
Many pathology laboratories do not have policies in place that require second opinions, particularly for difficult or borderline diagnoses.
Even though there are no policies in place, many pathologists value and request second opinions for difficult lesions.
Those without a formal policy may benefit from supportive clinical practices and systems that help obtain second opinions.
Acknowledgements
Acknowledgments: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01 CA140560 and KO5 CA104699. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
Footnotes
- BG, HN, PF, JE conceived and designed the study
- DW, KA, HN, BG, JE, PC, TO, AT contributed to the development of the survey and the acquisition of data
- BG, HN, JE, PF contributed to the analysis
- DW, KA, HN, BG, JE, PC, TO, AT contributed to the interpretation of data
- BG, HN, JE, TO, PF contributed to drafting the manuscript
- DW, KA, HN, BG, JE, PC, TO, AT, PF contributed to revising it critically for important intellectual content as well as approving the final version.
Contributor Information
Berta M Geller, University of Vermont Department of Family Medicine, OHPR 1 South Prospect Street Burlington, VT 05401-3444.
Heidi D Nelson, Department of Medical Informatics and Clinical Epidemiology, Oregon Health and Science University, Portland, OR, USA.
Patricia A Carney, Department of Family Medicine, Oregon Health and Science University, Portland, OR, USA.
Donald L Weaver, Department of Pathology, University of Vermont and Vermont Cancer Center, Burlington, VT, USA.
Tracy Onega, Norris Cotton Cancer Center and The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Kimberly H Allison, Department of Pathology, Stanford University School of Medicine, Palo Alto, CA, USA.
Paul Frederick, Department of Medicine, University of Washington, Seattle, WA, USA.
Anna N A Tosteson, Norris Cotton Cancer Center and The Dartmouth Institute for Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, Hanover, NH, USA.
Joann G. Elmore, Department of Medicine, University of Washington, Seattle, WA, USA.
REFERENCES
- 1.Manion E, Cohen M, Weydert J. Mandatory second opinion in surgical pathology referral material: Clinical consequences of major disagreements. Am J Surg Path. 2008;32:732–37. doi: 10.1097/PAS.0b013e31815a04f5. [DOI] [PubMed] [Google Scholar]
- 2.Kronz J, Westra W, Epstein J. Mandatory second opinion surgical pathology at a large referral hospital. Cancer. 1999;86:2426–35. [PubMed] [Google Scholar]
- 3.Gaudi S, Zarandona M, Raab S, et al. Discrepancies in dermapahtology diagnoses: The role of second review policies and dermapathology fellowship training. J Am Acad Dermatol. 2013;68:119–28. doi: 10.1016/j.jaad.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Frable W. Surgical pathology - Second reviews, institutional reviews, audits, and correlations: What's out there? Error or diagnostic variation? Arch Pathol Lab Med. 2006;130:620–24. doi: 10.5858/2006-130-620-SPRIRA. [DOI] [PubMed] [Google Scholar]
- 5.Price J, Grunfeld E, Barnes P, et al. Inter-institutional pathology consultations for breast cancer: impacty on clinical oncology therapy recommendations. Current Oncolo. 2010;17:25–32. doi: 10.3747/co.v17i1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhleb R, Bekeris L, Souers R, et al. Surgical pathology case reviews befire sign-out: A College of American Pathologists Q-Probes Study of 45 laboratories. Arch Patholo Lab Med. 2010;134:740–43. doi: 10.5858/134.5.740. [DOI] [PubMed] [Google Scholar]
- 7.Swapp R, Aubry M, Salomão D, et al. Outside case review of surgical pathology for referred patients: the impact on patient care. Arch Pathol Lab Med. 2013;137:233–4. doi: 10.5858/arpa.2012-0088-OA. [DOI] [PubMed] [Google Scholar]
- 8.Harvey S, Geller B, Oppenheimer R, et al. Increase in Cancer Detection and Recall Rates with Independent Double Interpretation of Screening Mammography. Am J Roentgenol. 2003 May 1;180(5):1461–7. doi: 10.2214/ajr.180.5.1801461. 2003. [DOI] [PubMed] [Google Scholar]
- 9.Ciatto S, Rosselli Del Turco M, Burke P, et al. Comparison of standard and double reading and computer-aided detection (CAD) of interval cancers at prior negative screening mammograms: blind review. Br J Cancer. 2003;89(9):1645–9. doi: 10.1038/sj.bjc.6601356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duijm L, Louwman M, Groenewoud J, et al. Inter-observer variability in mammography screening and effect of type and number of readers on screening outcome. Br J Cancer. 2009;100(6):901–7. doi: 10.1038/sj.bjc.6604954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duijm L, Groenewoud J, Hendriks J, et al. Independent double reading of screening mammograms in The Netherlands: effect of arbitration following reader disagreements. Radiology. 2004;231:564–70. doi: 10.1148/radiol.2312030665. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Layfield L. Prevalence of inter-institutional anatomic pathology slide review. Am J Surg Pathol. 2000;24:280–84. doi: 10.1097/00000478-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Tomaszewski J, Bear H, Connally J, et al. Consensus conference on second opinion in diagnostic anatomic pathology: Who, what, and when. Am J Clin Pathol. 2000;114:329–35. doi: 10.1093/ajcp/114.3.329. [DOI] [PubMed] [Google Scholar]
- 14.Renshaw A, Gould EW. Measuring the value of review of pathology material by a second pathologist. Am J Cin Pathol. 2006;125:737–39. doi: 10.1309/6A0R-AX9K-CR8V-WCG4. [DOI] [PubMed] [Google Scholar]
- 15.Oster N, Carney P, Allison K, et al. Development of a diagnostic test set to assess agreement in breast pathology: Practical application of the Guidelines for Reporting Reliability and Agreement Studies (GRRAS). BMC Women's Health. 2013;13:3. doi: 10.1186/1472-6874-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- 17.Heiberger R. HH: Statistical Analysis and DataDisplay: Heiberger and Holland. R package version 2.3-42. 2013 http://CRAN.R-project.org/package=HH.
- 18.Association of Directors of Anatomical and Surgicval Pathology Recommendations on quality control and quality assurance in anatomical pathology. Am J Surg Pathol. 1991;15:1007–09. doi: 10.1097/00000478-199110000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Renshaw A, Gould E. Measuring erros in surgical pathology in real-life practice. Am J Clin Pathol. 2007;127:144–52. doi: 10.1309/5KF89P63F4F6EUHB. [DOI] [PubMed] [Google Scholar]
- 20.Renshaw A, Pinnar N, Jiroteck M, et al. Blinded review as a method for quality improvement in surgical pathology. Arch Pathol Lab Med. 2002;126:961–3. doi: 10.5858/2002-126-0961-BRAAMF. [DOI] [PubMed] [Google Scholar]
- 21.Staradub V, Messenger K, Hao N, et al. Changes in breast cancer therapy because of pathology second opinions. Ann Surg Oncol. 2002;9:982–7. doi: 10.1007/BF02574516. [DOI] [PubMed] [Google Scholar]
- 22.Abt A, Abt L, Olt G. The effect of interinstitution anatomic pathology consultation on patient care. Arch Pathol Lab Med. 1995;119:514–7. [PubMed] [Google Scholar]
- 23.Onega T, Weaver D, Geller B, et al. Digitized whole slides for breast pathology Interpretation: Current practices and perceptions. Journal of Digital Imaging. 2014 Mar; doi: 10.1007/s10278-014-9683-2. DOI 10.1007/s10278-014-9683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein S, Bingham E, Rall D, et al. Losing the “war against cancer”: A need for public policy reforms. Intl J Health Serv. 1992;22(3):455–69. doi: 10.2190/14MF-42U5-T0XV-VCNU. [DOI] [PubMed] [Google Scholar]
- 25.Hofvind S, Geller BM, Rosenberg RD, et al. Screening-detected breast cancers: discordant independent double reading in a population-based screening program. Radiology. Dec. 2009;253(3):652–60. doi: 10.1148/radiol.2533090210. [DOI] [PubMed] [Google Scholar]
- 26.Dinnes J, Moss S, Melia J, et al. Effectivenss and cost-effectiveness of double reading of mammograms in breast cancer screening: findings of a systematic review. The Breast. 2001;10:455–63. doi: 10.1054/brst.2001.0350. [DOI] [PubMed] [Google Scholar]