Abstract
The Applied Research Group for Kids (TARGet Kids!) is an ongoing open longitudinal cohort study enrolling healthy children (from birth to 5 years of age) and following them into adolescence. The aim of the TARGet Kids! cohort is to link early life exposures to health problems including obesity, micronutrient deficiencies and developmental problems. The overarching goal is to improve the health of Canadians by optimizing growth and developmental trajectories through preventive interventions in early childhood. TARGet Kids!, the only child health research network embedded in primary care practices in Canada, leverages the unique relationship between children and families and their trusted primary care practitioners, with whom they have at least seven health supervision visits in the first 5 years of life. Children are enrolled during regularly scheduled well-child visits. To date, we have enrolled 5062 children. In addition to demographic information, we collect physical measurements (e.g. height, weight), lifestyle factors (nutrition, screen time and physical activity), child behaviour and developmental screening and a blood sample (providing measures of cardiometabolic, iron and vitamin D status, and trace metals). All data are collected at each well-child visit: twice a year until age 2 and every year until age 10. Information can be found at: http://www.targetkids.ca/contact-us/.
Key Messages.
TARGet Kids! is an innovative practice-based model for doing health research in early childhood, that works.
A 5-min talk with parents at the 9-month well-child visit reduced prolonged bottle use by 60% but did not reduce iron depletion.
A short behavioural counselling intervention with parents of 3-year-old children reduced the number of meals eaten in front of the television but was not effective in reducing screen time.
Eating behaviours were positively associated with serum non-HDL cholesterol levels in preschool-aged children.
Two cups of cow's milk per day is sufficient to maintain healthy vitamin D and iron stores for most young children.
Two modifiable dietary intake variables (vitamin D supplementation and cow's milk intake) are the most important determinants of serum 25-hydroxyvitamin D level in early childhood.
Why was the cohort set up?
Early childhood is a critical period in human development. The science underpinning the Developmental Origins of Health and Disease (DOHaD) hypothesis suggests that healthy growth and developmental trajectories established in the first 5 years of a child’s life are strongly associated with health outcomes throughout the life course.1,2 Many adult chronic diseases have origins in early human development, mediated by nutrition-related risk factors such as obesity, micronutrient deficiencies and related health outcomes including poor cognitive and social well-being. Treatment of established chronic disease in adults places an enormous burden on our health care system. Preventive care targeting young children in community-based primary care settings is therefore a critical strategy for chronic disease prevention and optimizing the well-being of Canadians. Despite the recent emphasis on the importance of early human development, there are significant gaps in data regarding the health of young children, and gaps in knowledge to inform clinical practice and health policy in the Canadian setting. For example, the Canadian Health Measures Survey, designed to measure the health of Canadians, excludes children under 3 years3 and has minimal data for children 3–5 years of age.4 Furthermore, a recent study of preventive interventions intended for community-based primary health care settings identified 21 screening recommendations for which there are significant gaps in evidence.5
Given that parents and their young children have trusting relationships with their child’s community-based primary health care team and have frequent contact in the first 5 years of a child’s life, the team are in a unique position to optimize children’s health.6–9 According to our provincial publicly funded immunization schedule, children visit their primary care physician at least seven times: at ages 2 months, 4 months, 6 months, 12 months, 15 months, 18 months and 4–6 years.10 Many practitioners also schedule health supervision visits at an additional three visits: at ages 9 months, 24 months and 36 months. Furthermore, our province recently implemented a billing code for developmental screening at 18 months of age.11 Our existing community-based primary health care system is well positioned to serve as a platform to engage health practitioners in a shared vision for child health, and to fill existing knowledge gaps through health surveillance and evaluation of health delivery.
With these guiding principles, our group based in Toronto, Ontario, Canada established a large child-focused primary care practice-based research network (PBRN) called the Applied Research Group for Kids (TARGet Kids!). Our aim is to improve the evidence for population health and primary prevention using a research platform embedded in primary care practice. TARGet Kids! is the only network of primary care practices in Canada collecting longitudinal data to examine growth and developmental trajectories of infants and preschool children. It is a partnership between leading child health scientists from the Paediatric Outcomes Research Team at the Hospital for Sick Children, and the Applied Health Research Centre (AHRC) at the Li Ka Shing Knowledge Research Institute, St. Michael's Hospital, and community-based primary care paediatricians and family physicians from the Faculty of Medicine at the University of Toronto. P.P, C.B. and J.M. are the lead investigators. To date, TARGet Kids! practice sites are located in Toronto, Ontario, the TARGet Kids! Methods Centre at The Hospital for Sick Children and the TARGet Kids! Data Management and Analysis Centre at the Applied Health Centre (AHRC) at the Li Ka Shing Knowledge Research Institute, St. Michael’s Hospital.
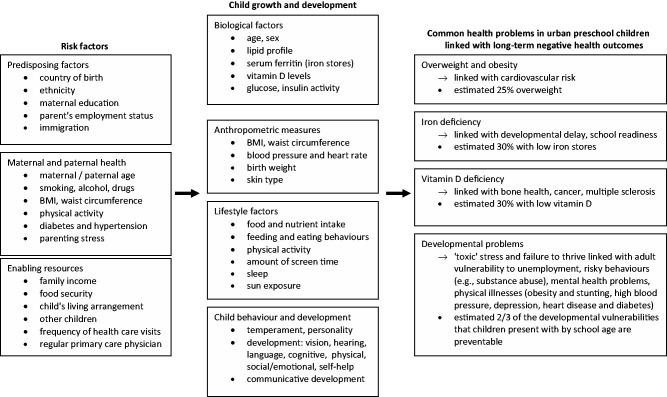
Research ethics board approval has been received for the cross-sectional and longitudinal TARGet Kids! study and for the individual randomized controlled trials. The areas of research focus in the TARGet Kids! study are shown in Figure 1.
Figure 1.
Research Focus of the TARGet Kids! study.
What are the main objectives?
The overall goals of TARGet Kids! are:
to establish a ‘proof-of concept’ community-based primary care research network
to learn from the ‘proof-of-concept’ experience and to scale up to a provincially-based network with sentinel sites to ensure representativeness;
to build partnerships between child health researchers, community-based practitioners and public health researchers and practitioners;
to build a platform to advance evidence for community-based prevention and health promotion;
to build a platform to advance population-level child health surveillance.
The specific objectives of TARGet Kids! are:
to conduct cross-sectional, longitudinal and pragmatic randomized controlled trials;
to focus on the broad domains of healthy growth and developmental trajectories in early childhood including body mass index, physical activity, sedentary behaviours, nutrition and cognitive-social-emotional-behavioural development;
to consider factors related to health equity in all analyses.
Who is in the cohort?
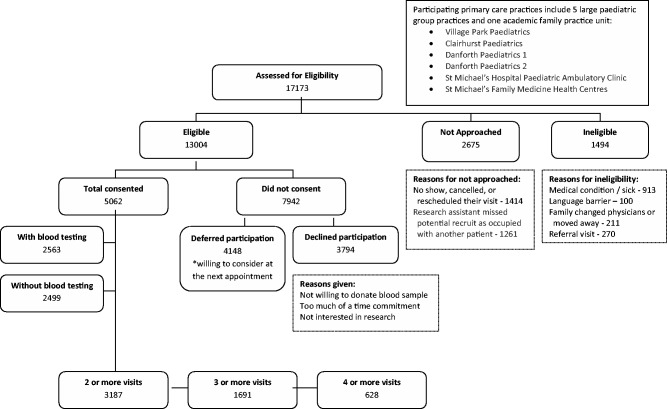
TARGet Kids! is the largest cohort under 6 years of age in Canada recruited from primary health care settings. Between June 2008 and September 2013, 17 173 children attending scheduled health maintenance visits with their primary care physician were assessed for study eligibility. The first child was enrolled into the TARGet Kids! cohort in June 2008. Between June 2008 and July 2011, 3583 children aged 1 to 5 years were enrolled in the study. In August 2011, we changed our inclusion criteria to include children in their first year of life. As of 3 September 2013, we have enrolled 5062 children under 6 years of age. There are currently five paediatric group practices and one large family practice unit involved in patient recruitment. Each practice has between three and 10 practising physicians. Participating practices were chosen based on the following criteria: (i) at least three interested and academically motivated physicians; (ii) all physicians see at least five children aged 0 to 5 years per day; and (iii) the practice cares for a population of children not yet represented either geographically or demographically.
Exclusion criteria for this TARGet Kids! cohort include: children with associated health conditions affecting growth (e.g. failure to thrive, cystic fibrosis), children with any acute or chronic conditions (other than asthma and high functioning autism), children with severe developmental delay and families who are unable to communicate in English.
Of the 13 004 children who were eligible, a total of 5062 parents consented to participate, completed all questionnaires and provided physical measures. In the first year of TARGet Kids! when having blood work was optional, our recruitment rate was 90%. We were impressed with the willingness of parents to have their children undergo blood tests, since it is outside the standard of care in Canada. Starting in December 2008, blood sample collection was no longer optional for the initial inclusion in the TARGet Kids! cohort. Blood work at subsequent follow-up visits remains optional. Although performing blood tests on this young age group is feasible, of the 5062 participants enrolled over the entire recruitment period, a blood sample has been collected and analysed for 2563 children (50.6%). The participation rate for our study and the proportion providing a blood sample reflect the realities of conducting research requiring invasive measures in young healthy children.
Figure 2 shows the patient recruitment and enrollment flow chart for the TARGet Kids! cohort. Of the 13 004 children who were eligible and whose parents were approached by research personnel, 7942 (61%) did not consent. Of those who did not consent, 3794 declined to participate and 4148 deferred participation until their next appointment. Those who defer are asked to participate at the subsequent well-child visit, whereas those who declined participation are not asked again. Of those 17 173 children scheduled for their routine visits, 2675 were not approached. Very few (1494/17 173 = 8.7%) were ineligible.
Figure 2.
Patient recruitment and enrollment flow chart for TARGet Kids! cohort from June 3, 2008 to September 3, 2013.
Baseline characteristics of the TARGet Kids! open cohort are presented in Table 1. The median age of participants at baseline is 25 months (range, 0.2–72 months) and 2646 (52%) of participants are male. Children with blood sampling are slightly older but otherwise are similar to children who did not have blood sampling. Prevalence rates among TARGet Kids! participants for common early childhood nutritional disorders, including obesity (4.4%), iron deficiency (10.2%) and iron deficiency anaemia (2.2%), are consistent with rates previously reported in Canada12,13 and other developed countries.14,15
Table 1.
Baseline characteristics of TARGet Kids! cohort recruited from June 3 2008 to September 3 2013
| Characteristics | Total (N=5062) |
With blood testing (N=2563) |
Without blood testing (N=2499) |
|||
|---|---|---|---|---|---|---|
| Frequency (N, %) | Median (Range) | Frequency (N, %) | Median (Range) | Frequency (N, %) | Median (Range) | |
| Demographics | ||||||
| Child’s age, months | 25 (0.2–72) | 26 (0.4–72) | 25 (0.2–72) | |||
| 0 – <1 years | 511 (10.1) | 199 (7.8) | 312 (12.5) | |||
| 1 – <3 years | 2441 (48.3) | 1162 (45.4) | 1279 (51.2) | |||
| 3 – <6 years | 2110 (41.7) | 1202 (46.9) | 908 (36.4) | |||
| Missing | 0 | 0 | 0 | |||
| Child's sex, male | 2646 (52.3) | 1352 (52.8) | 1294 (51.8) | |||
| Missing | 0 | 0 | 0 | |||
| Child’s place of birth | ||||||
| In Canada | 4728 (93.4) | 2369 (92.4) | 2359 (94.4) | |||
| Outside Canada | 117 (2.3) | 66 (2.6) | 51 (2.0) | |||
| Missing | 217 (4.3) | 128 (5.0) | 89 (3.6) | |||
| Maternal age, years | 36 (17–59) | 36 (17–59) | 36 (17–49) | |||
| <20 | 10 (0.2) | 6 (0.2) | 4 (0.2) | |||
| 20–24 | 88 (1.7) | 44 (1.7) | 44 (1.8) | |||
| 25–29 | 304 (6.0) | 135 (5.3) | 169 (6.8) | |||
| 30–34 | 1355 (26.8) | 638 (24.9) | 717 (28.7) | |||
| 35–39 | 1993 (39.4) | 999 (39.0) | 994 (39.8) | |||
| 40+ | 958 (18.9) | 535 (20.9) | 423 (16.9) | |||
| Missing | 354 (7.0) | 206 (8.0) | 148 (5.9) | |||
| Mother’s place of birth | ||||||
| In Canada | 3144 (62.1) | 1552 (60.6) | 1592 (63.7) | |||
| Outside Canada | 1707 (33.7) | 887 (34.6) | 820 (32.8) | |||
| Missing | 211 (4.2) | 124 (4.8) | 87 (3.5) | |||
| Maternal ethnicity | ||||||
| European (White) | 3380 (66.8) | 1665 (65.0) | 1715 (68.6) | |||
| East, South, Southeast Asian | 799 (15.8) | 412 (16.1) | 387 (15.5) | |||
| African and Caribbean | 218 (4.3) | 122 (4.8) | 96 (3.8) | |||
| Latin American | 166 (3.3) | 74 (2.9) | 92 (3.7) | |||
| West Asian/Arab/ North African | 89 (1.8) | 45 (1.8) | 44 (1.8) | |||
| Mixed ethnicity | 263 (5.2) | 160 (6.3) | 103 (4.1) | |||
| Missing | 147 (2.9) | 85 (3.3) | 62 (2.5) | |||
| Maternal education | ||||||
| Primary school | 56 (1.1) | 32 (1.3) | 24 (1.0) | |||
| High school | 453 (9.0) | 234 (9.1) | 219 (8.8) | |||
| College or university | 4399 (86.9) | 2214 (86.4) | 2185 (87.4) | |||
| Missing | 154 (3.0) | 83 (3.2) | 71 (2.8) | |||
| Maternal parity status | ||||||
| Nulliparous (1 child) | 1947 (38.5) | 913 (35.6) | 1034 (41.4) | |||
| Parous (≥2 children) | 3111 (61.5) | 1648 (64.3) | 1463 (58.5) | |||
| Missing | 4 (0.1) | 2 (0.1) | 2 (0.1) | |||
| After tax family income, Can$ | ||||||
| <$30,000 | 233 (4.6) | 122 (4.7) | 111 (4.5) | |||
| $30,000 to $79,999 | 567 (11.2) | 318 (12.4) | 249 (10.0) | |||
| $80,000 to $149,999 | 1061 (21.0) | 566 (22.0) | 495 (19.8) | |||
| $150,000 + | 1467 (29.0) | 752 (29.3) | 715 (28.6) | |||
| Missing | 1734 (34.3) | 805 (31.4) | 929 (37.2) | |||
|
Child health | ||||||
| Birthweight, kg | 3.4 (0.5–6.4) | 3.3 (0.6–6.4) | 3.4 (0.5–6.0) | |||
| < 2.5 kg | 476 (9.4) | 276 (10.8) | 200 (8.0) | |||
| 2.5–4.0 kg | 3287 (64.9) | 1777 (69.3) | 1510 (60.4) | |||
| >4.0 kg | 406 (8.0) | 207 (8.1) | 199 (8.0) | |||
| Missing | 893 (17.6) | 303 (11.8) | 590 (23.6) | |||
| Ever breastfed | 4623 (91.3) | 2333 (91.0) | 2290 (91.7) | |||
| Missing | 86 (1.7) | 52 (2.0) | 34 (1.4) | |||
| Breastfeeding duration | 10 (0–48) | 10 (0–48) | 11 (0.1–48) | |||
| 0–6 months | 901 (17.8) | 427 (16.7) | 474 (19.0) | |||
| 6–12 months | 2274 (44.9) | 1194 (46.6) | 1080 (43.2) | |||
| 12–24 months | 1103 (21.8) | 560 (21.8) | 543 (21.7) | |||
| 24 months + | 149 (2.9) | 82 (3.2) | 67 (2.7) | |||
| Never | 353 (7.0) | 178 (6.9) | 175 (7.0) | |||
| Missing | 282 (5.6) | 122 (4.8) | 160 (6.4) | |||
| Child’s BMI (N, %) | ||||||
| Weight, zBMI | 0.1 (–4.7–6.2) | 0.1 (–4.0–6.2) | 0.1 (–4.7–6.0) | |||
| Underweight (z <–1) | 681 (13.5) | 324 (12.6) | 357 (14.3) | |||
| Normal weight (–1≥ z ≤1) | 3143 (62.1) | 1621 (63.2) | 1522 (60.9) | |||
| Overweight (1> z ≤2) | 708 (14.0) | 352 (13.7) | 356 (14.2) | |||
| Obese (z >2) | 225 (4.4) | 115 (4.5) | 110 (4.4) | |||
| Missing | 305 (6.0) | 151 (5.9) | 154 (6.2) | |||
| Blood tests | ||||||
| Total cholesterol | 4.0 (1.8–7.4) | |||||
| Total cholesterol ≥5.18 SI | 144 (5.6) | |||||
| LDL cholesterol | 2.2 (0.2–5.5) | |||||
| LDL cholesterol ≥3.37 SI | 109 (4.3) | |||||
| Non-HDL | 2.8 (0.8–6.4) | |||||
| Non-HDL ≥3.76 SI | 37 (1.4) | |||||
| HDL cholesterol | 1.2 (0.3–2.7) | |||||
| Serum ferritin, µg/l | 30 (2–191) | |||||
| Haemoglobin, g/L | 122 (69–163) | |||||
| Iron deficiency (ferritin <14) | 254 (10.2) | |||||
| Iron deficiency anaemia (ferritin <14 andHgb le110) | 56 (2.2) | |||||
| Vitamin D, nmol/l | 80 (11–352) | |||||
| Vitamin D <50 nmol/l | 162 (6.7) | |||||
| Vitamin D <75 nmol/l | 992 (40.9) | |||||
| Maternal health | ||||||
| Mother's BMI | 3715 (73.4) | 23.7 (16.2–57.2) | 1893 (73.9) | 23.8 (16.2–57.2) | 1822 (72.9) | 23.5 (16.5–56.6) |
| Underweight (<18.5 kg/m2) | 107 (2.9) | 64 (3.4) | 43 (2.4) | |||
| Normal (18.5–24.9 kg/m2) | 2230 (60.0) | 1120 (59.2) | 1110 (60.9) | |||
| Overweight (25–29.9 kg/m2) | 922 (24.8) | 444 (23.5) | 478 (26.2) | |||
| Obese (≥30 kg/m2) | 456 (12.3) | 265 (14.0) | 191 (10.5) | |||
| Father’s BMI collected | 721 (14.2) | 403 (15.7) | 318 (12.7) | |||
| Missing | 626 (12.4) | 267 (10.4) | 359 (14.4) | |||
How often have they been followed up and what is attrition like?
Out of the 5062 enrolled, and of those due for follow-up (i.e. at least 18 months has passed since their last visit), longitudinal data have been collected as follows: 3187 (3187/4149 = 76.8%) have had two or more visits; 1691 (1691/2299 = 73.6%) have had three or more visits; and 628 (628/889 = 70.6%) have had four or more visits. Thus, our retention rate is more than 70%. Attrition in this follow-up rate is due to study withdrawal (reasons given include lack of time and lack of interest) and loss to follow-up as a result of an unknown address or the family having changed physicians or moved away.
We aim to ensure complete data collection and participant follow-up. As our study involves collecting observational data, a moderate amount of missing data are expected. Study variables with >20% missing data include family income, and variables with >10% missing data include mother's body mass index (BMI), prevalence of cigarette smoking and alcohol use during pregnancy. Since social desirability may affect the accuracy of self-reported smoking behaviour and alcohol use during pregnancy, other methods may be needed to determine children's exposure to these important health risks. When 5 to 20% of the data are missing, we use analytical methods including multiple imputation to determine the effect of missing data.16
What has been measured?
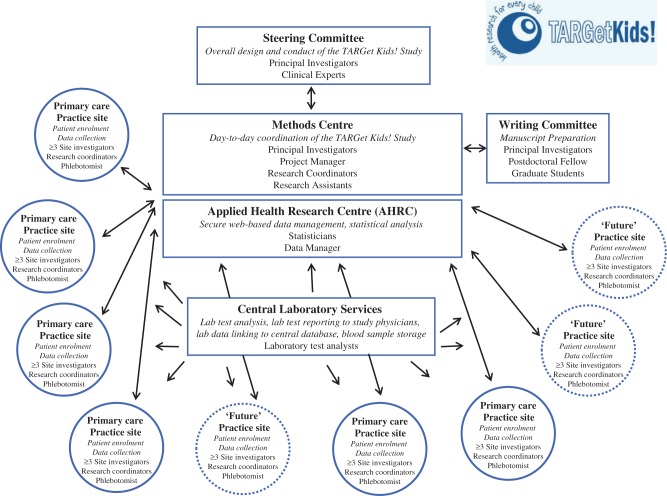
Table 2 provides an overview of the data collected. Figure 3 shows the TARGet Kids! framework. The central database is housed at AHRC. Upon enrolment, research assistants at each site enter the subject ID and identifying personal information into a web-based remote data entry system using the MediData Rave™ platform (MediData Solutions, New York, NY, USA). All study documents are transported to the Methods Centre, where trained research assistants enter the remaining data and ensure secure document storage.
Table 2.
General overview of data collected in TARGet Kids! Study
| Data |
Age at time of enrolment or follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0-6 months | 12 months | 15 months | 18 months | 2 years | 3 years | 4 years | 5 years | 6+ years | |
| PHYSICAL MEASUREMENTS | |||||||||
| Weight | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Length | ✓ | ✓ | ✓ | ✓ | |||||
| Height | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Waist circumference | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood pressure | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| BLOOD SAMPLE | |||||||||
| CBC,a lipid profile,b ferritin, vitamin D, PTH,d chemistry panel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| HEALTH & LIFESTYLE FACTORS | |||||||||
| Nutrition and Health Questionnaire (NHQ) | |||||||||
| NHQ Initial Form | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Sociodemographic variables: child's age, maternal/paternal age, sex, child's country of birth mother's/father's country of birth, employment, immigration, income, ethnicity, maternal education | |||||||||
| Child health: birth weight, gestational age | |||||||||
| Mother Health: obstetric history, weight prior to pregnancy, weight at the end of pregnancy, medication use | |||||||||
| Mother Lifestyle and Nutrition: smoking, alcohol, vitamin use during pregnancy and breastfeeding, nutrition during pregnancy | |||||||||
| NHQ v. 0 to 3 years | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Socio-demographic variables: child’s living arrangement, other children | |||||||||
| Child lifestyle and nutrition: vitamins and supplement use, breastfeeding, bottle use, nutrition, screen time, physical activity, child care, sun exposure, dental care | |||||||||
| Child health: overall health, previous diagnoses, wheezing/asthma, fractures, medication use, influenza history | |||||||||
| Mother health: overall health, medical history (previous diagnoses) | |||||||||
| Mother lifestyle and nutrition: smoking, physical activity, medication use | |||||||||
| NHQ v. 4 to 5 years | ✓ | ✓ | |||||||
| Note: similar to v. 0 to 3 years with additional response options | |||||||||
| NHQ v. 6+ years (follow-up only) | ✓ | ||||||||
| Note: similar to v. 4 to 5 years with additional response options | |||||||||
| Child lifestyle and nutrition plus school attendance | |||||||||
| NutriSTEP™ | |||||||||
| NutriSTEP™ v. 18 to 35 months | ✓ | ✓ | |||||||
| Nutrition risk score based on child’s food and nutrient intake, physical growth, developmental and physical capabilities, physical activity, food security and the feeding environment | |||||||||
| NutriSTEP™ v. 3 to 5 years | ✓ | ✓ | ✓ | ||||||
| Nutrition risk score based on physical growth, child's food and fluid intake, feeding behaviours and lifestyle factors | |||||||||
| CHILD BEHAVIOUR | |||||||||
| Infant Behaviour Questionnaire (IBQ) | ✓ | ✓ | |||||||
| To assess temperament, activity and parenting across 3 domains: surgency, negative affect and effortful control | |||||||||
| Early Child Behaviour Questionnaire (ECBQ) | ✓ | ||||||||
| To assess temperament, activity and parenting across 3 domains: surgency, negative affect and effortful control | |||||||||
| Children's Behaviour Questionnaire (CBQ) | ✓ | ✓ | ✓ | ✓c | |||||
| To assess temperament, activity and parenting across 3 domains: surgency, negative affect and effortful control | |||||||||
| To assess temperament, activity and parenting across 3 domains: surgency, negative affect and effortful control | |||||||||
| DEVELOPMENTAL SCREENING | |||||||||
| Nipissing Developmental Screening Tool (NDDS) | ✓ | ✓ | |||||||
| Developmental areas: vision, hearing, communication, gross and fine motor function, self-help and cognitive, social and emotional function | |||||||||
| Infant Toddler Checklist (ITC) | ✓ | ||||||||
| 7 areas of communicative development: emotion and use of eye gaze, communication,; use of gestures’ use of sounds, use of words, understanding of words, and use of objects | |||||||||
| PARENTING | |||||||||
| Parenting Stress Index (PSI) | ✓ | ✓ | ✓ | ||||||
aCBC = complete blood count.
bLipid profile = HDL, LDL, total cholesterol, triglycerides.
cOnly at 6 years of age.
dPTH = parathyroid hormone.
Figure 3.
The TARGet Kids! Framework.
Questionnaires
There are a total of six questionnaires administered to parents throughout the planned 10-year period of longitudinal data collection.
Nutrition and Health Questionnaire (NHQ)
The NHQ is an age-specific, parent-completed TARGet Kids! core instrument designed to capture important predictors and health outcomes for young children, not captured by the other instruments. The NHQ was developed by the lead investigators and is based on questions used in the Canadian Community Health Survey.17 It is used to obtain sociodemographic information, child's dietary intake and their eating habits, and includes questions on physical activity, screen time and sun exposure. The number of items ranges from 20 to 107, including questions that have multiple components.
Nutrition Screening Tool for Every Preschooler (NutriSTEP™)
The 17-item parent-completed NutriSTEP™27 questionnaire for children aged 3–5 years assesses nutritional risk based on child's food and fluid intake, physical growth, physical activity, sedentary behaviour and factors affecting food intake for this age group. NutriSTEP™ for toddlers aged 18–35 months includes similar constructs found in the preschooler version, with modifications to feeding environment to account for differences in age. The NutriSTEP™ has been validated in multicultural Canadian children by a registered dietitian using a detailed nutritional history and 3-day dietary recall.18
Child Behaviour Questionnaire (CBQ) – Short Form
The CBQ-SF provides a comprehensive assessment of reactive and self-regulative temperamental behaviour patterns in young children.19 The CBQ-SF assesses temperament, activity and parenting across three domains: surgency/extraversion, negative affectivity and effortful control. The CBQ-SF is a validated measure of child temperament for 3–7-year-olds.19 We are also using the versions introduced for younger children, called the Infant Behaviour Questionnaire (IBQ) and the Early Childhood Behaviour Questionnaire (ECBQ) for children aged 3–12 months and 18–36 months, respectively. All versions have 36 items.
Nipissing District Developmental Screen (NDDS)
The NDDS is an age-specific, parent-completed developmental screening tool for children between 1 and 72 months of age.20,21 The 17-item version for infants 18 months of age is currently in use by primary care physicians in Ontario, Canada (http://www.ndds.ca/ontario).
Infant Toddler Checklist (ITC)
The 24-item parent-completed ITC was developed as a screen for communication delays in children between 6 and 24 months of age. It is designed to identify seven developmental milestones of social communication including emotion and use of eye gaze, use of communication, use of gestures, use of sounds, use of words, understanding of words and use of objects.22,23
Parenting Stress Index (PSI)
The 36-item, parent-completed PSI is designed to identify potentially dysfunctional parent-child systems and includes three scales: parental distress, difficult child characteristics and dysfunctional parent-child interaction.24
Physical measures
At each visit, children and their accompanying parent have height (or length for children under 2 years old), weight and waist circumference measured using standardized protocols.25 Pre-pregnancy weight is recorded in the event that the child's mother is pregnant. Blood pressure is measured for those 2 years and older, according to the National High Blood Pressure Education Program guidelines.
Blood samples
Non-fasting blood samples (4–7 ml) are drawn by trained paediatric phlebotomists at each practice site and include the following measures: lipid profile, insulin, glucose, haemoglobin, serum ferritin, 25-hydroxyvitamin D, ApoA1, ApoB, CRP, ALT, adiponectin and leptin and other markers of nutritional status.26 Any abnormal findings are reported to the child's primary care physician and followed up by a paediatric physician member of our team (J.L.M., C.S.B., P.C.P.).
What has it found? Key findings and publications
One of the most important findings of the TARGet Kids! study is that our model for doing health research in early childhood works. Our contributions so far can be summarized according to our research foci. Data collection is ongoing and the first longitudinal analyses are in progress. A full list of publications is available on our website (http://www.targetkids.ca).
Overweight, obesity, physical activity and sedentary behaviour
In a cohort of 3-year-old children, we found that mean screen time per day was 104 min, 10% had a TV in their bedroom, 59% consumed at least one meal while watching TV and 81% had household rules about screen time.27 Eating meals in front of a screen and the mother being employed were associated with an increase in weekday screen time, and household rules about screen time were associated with a decrease in weekend screen time.27 In a pragmatic randomized controlled trial, a behavioural counselling intervention reduced the number of meals eaten in front of the screen but was not effective in reducing screen time or BMI.28 We also found that eating behaviours were positively associated with serum non-high-density lipoprotein (HDL) cholesterol levels, suggesting that interventions targeting eating behaviours in the early years may be important in promoting cardiovascular health.29 We are now assessing the influence of stroller use on adiposity and measuring physical activity levels in young children 4 months to 5 years using accelerometers. We are determining the longitudinal effects of child and parent nutrition, physical activity and sedentary behaviours on growth and cardiometabolic risk (www.clinicaltrials.gov, ID NCT01869530).
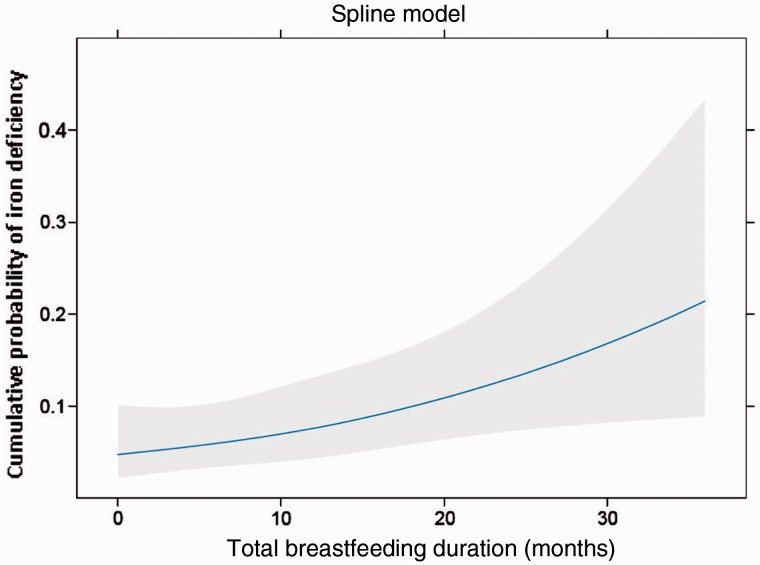
Iron deficiency
In a cohort of children aged 12 to 36 months, we found an almost 2-fold association between iron deficiency and daytime bottle-feeding compared with cup feeding.30 We also identified that increased total breastfeeding duration is associated with an increased probability of iron deficiency (Figure 4). Children who are breastfed beyond 12 months of age have a 1.7-fold increase in the odds of having iron deficiency,31 suggesting that they may benefit from enhanced screening for iron deficiency and targeted nutritional interventions. In a pragmatic randomized controlled trial, an educational intervention with parents at the 9-month well-child visit reduced prolonged bottle use by 60% but did not result in a decrease in iron deficiency.32
Figure 4.
Adjusted restricted cubic spline model of the association between total breastfeeding duration and iron deficiency. The solid line represents the predicted probability of iron deficiency as a function of total breastfeeding duration, and the grey area represents the 95% CIs for the predicted probabilities. (Reproduced with the permission from J.L. Maguire, L. Salehi, C.S. Birken, S. Carsley, M. Mamdani, K.E. Thorpe, G. Lebovic, M. Khovratovich, P.C. Parkin, Pediatrics, 2013, Vol. 131, Pages 1530-7, Copyright ã 2013 by the AAP).
We are now conducting a randomized controlled trial, called OptEC: Optimizing Early Child Development in the Primary Care Practice Setting, to compare the effect of iron treatment plus dietary counselling vs placebo plus dietary counselling in improving developmental and laboratory outcomes in young children with non-anaemic iron deficiency (www.clinicaltrials.gov, ID NCT01481766). Children enrolled in the TARGet Kids! cohort who meet eligibility criteria are approached to participate.
Vitamin D deficiency
We found that two modifiable dietary intake variables (vitamin D supplementation and cow's milk intake) are the most important determinants of serum 25-hydroxyvitamin D level in early childhood.33 We also found that two cups of cow's milk per day appears sufficient to maintain healthy vitamin D and iron stores for most young children and that wintertime vitamin D supplementation is particularly important among children with darker skin pigmentation.34 We have found that non-Western immigrant children have lower 25-hydroxyvitamin D than children from Western families.35
We have another ongoing pragmatic randomized controlled trial, called DO IT!: vitamin D Outcomes and Interventions in Toddlers, to determine whether wintertime ‘high-dose’ vitamin D supplementation (2000 IU/day) vs the ‘standard dose’ of vitamin D (400 IU/day) can prevent upper respiratory tract infections and asthma attacks in young children (www.clinicaltrials.gov, ID NCT01419262).
Child development and behaviour
We have found that the child temperament dimension of Negative Affectivity such as discomfort, fear, anger/frustration, sadness and poor soothability (as measured by the CBQ-SF) is associated with high nutrition risk (as measured by the NutriSTEP™) in young children aged 3–5 years (Abdullah K, Birken CS, Maguire JL, Lebovic G, Jenkins J, Parkin P. Temperament as a predictor of nutrition risk in preschool children. Manuscript in preparation). A future study will determine whether BMI and other health and developmental trajectories in early childhood (0–3 years) are associated with school readiness as measured by the Early Developmental Instrument (EDI) at school entry (Junior Kindergarten, age 4).
What are the main strengths and limitations?
In Canada, there are critical gaps in population surveillance of infants and preschool children. The Canadian Health Measures Survey (CHMS), the most extensive national survey, excludes children aged 0 to < 3years and has minimal data on children 3 to 5 years of age; TARGet Kids! is filling this gap. We are aware of only one paediatric PBRN conducting child health research in this age group; the American Academy of Pediatrics (AAP) Pediatric Research in Office Settings (PROS) network initiated in 1986 to study paediatric primary care problems in practice settings is well established.
Our study's greatest strength is the richness of our clinical data, possible because our study is embedded in primary care practices. Aligning our data collection with scheduled primary care visits has assured a >70% follow-up rate and our ability to collect both longitudinal and cross-sectional data. The gain in data richness by obtaining blood samples from young children comes at a cost of a lower participation rate and a possible selection bias. Parents willing to have their child provide a blood sample may have more health-seeking behaviour and therefore their children may be in better health compared with the rest of the population. Reviewing laboratory results adds to the workload for our physicians so, to ease their burden, our lead investigators are available for consultation when abnormal results are reported. An unanticipated result of requiring a blood sample at the initial visit is that previously undiagnosed conditions such as mild iron deficiency, low vitamin D levels and high cholesterol levels are being treated, thus changing the possible life course of such conditions in our cohort as compared with the general population.
Our other main strength is in our collaboration with our research partners. The AHRC provides rigorous data management, enabling us to collect large amounts of data and already to perform cross-sectional analyses. The Mount Sinai Services laboratory provides real-time laboratory test results to our study physicians, laboratory data linking to our database, and long-term blood sample storage. Early collaboration with our participating opinion-leading primary care physicians, and regular meetings with them to develop their own clinical queries, were essential to establish our PBRN.
Our study has potential limitations. Children are currently being recruited from primary care practices located in one large Canadian city (Toronto), and may not be representative of children in other settings. Many children were born to mothers with a high level of education; however, a high education is not uncommon for women of childbearing age in Toronto.36 We have balanced the possible bias from lack of representativeness against the likelihood of bias from poor response to follow-up in a more representative sample. We opted to first focus on good follow-up rates. We are now in the process of expanding the TARGet Kids! framework to other Canadian regions and recruiting additional practice sites with diverse patient populations. Until then, our data offer the best information on infants and preschool children seen in primary care practice settings in Canada. Another potential limitation is that participation in a pragmatic trial may alter a child’s exposure experimentally. Therefore, depending on the research question, trial participants will be excluded from longitudinal analyses.
We have been very successful in receiving funding from various sources, including Canadian Institutes of Health Research. Our strategic goals for the next 5 years include: scale up the network, adding sentinel sites in the region to increase representativeness; maintain the longitudinal cohort into middle childhood; and secure funding to maintain the infrastructure.
Can I get hold of the data? Where can I find out more?
TARGet Kids! welcomes collaboration with interested colleagues. For more information, please visit our website at (http://www.targetkids.ca/contact-us/) or contact Cory Borkhoff (cory.borkhoff@sickkids.ca). Once initial contact has been made, we request a short research proposal that should include information about the background, objectives, methods, timetable and budget. Approval of a proposal will be subject to review by the TARGet Kids! Scientific Committee. Collaboration will be established through a formal contract which would include mutual obligations (data sharing, rules for publication and authorship, and contribution to the TARGet Kids! infrastructure).
Funding
This work was supported by the St. Michael’s Foundation; the Sickkids Foundation; the Canadian Institutes of Health Research under the New Emerging Team (NET) Program (2008–2013 Childhood Obesity Team Grant), which allowed us to establish the TARGet Kids! infrastructure; the Canadian Institutes for Health Research, Institute of Human Development, Child and Youth Health (IHDCYH) [No. MOP 114945 to J L M , No. MOP 115059 to P C P, No. MOP 106532 to J L M ]; the Canadian Institutes for Health Research, Institute of Nutrition, Metabolism and Diabetes (INMD) [No. MOP 119375 to C S B ]; the Physician Services Incorporated Foundation; the Thrasher Fund [No. 602429 to J.L.M.]; the Danone Institute; the Dairy Farmers of Ontario; Sun Life Financial; and the University of Toronto Dean’s Fund [No. N/A to J.L.M.]. Funding agencies had no role in the design or conduct of the study, collection, management, analyses or interpretation of the results of the study, or the preparation, review or approval of the manuscript.
Acknowledgements
The authors thank all participating families for their time and involvement in TARGet Kids!, and are grateful to all practitioners who are currently involved in the TARGet Kids! research network. Steering Committee: Tony Barozzino, Brian Chisamore, Mark Feldman, Moshe Ipp; Research Coordinators: Charmaine Camacho, Diviya Elango, Julie DeGroot, Shanique Edwards, Nadia Kabir, Tarandeep Malhi, Juela Sejdo, Laurie Thompson, Mandy Tran, Weeda Zabih; Applied Health Research Centre: Magda Melo, Patricia Nguyen; and Mount Sinai Services Laboratory: Azar Azad.
Conflict of interest: None declared.
TARGet Kids! Collaboration: Co-Leads: Patricia C. Parkin, Catherine S. Birken, Jonathon L. Maguire; Scientific Advisory: Colin Macarthur, Muhammad Mamdani; Scientific Committee: Kawsari Abdullah, Laura Anderson, Cornelia M. Borkhoff, Sarah Carsley, Matthew D’Ascanio, Mikael Katz-Lavigne, Kanthi Kavikondala, Christine Koroshegyi, Grace Jieun Lee, Jessica Omand, Navindra Persaud, Meta van den Heuvel, Peter Wong; Applied Health Research Centre: Yang Chen, Gerald Lebovic, Kevin E. Thorpe; Site Investigators: Jillian Baker, Tony Barozzino, Joey Bonifacio, Douglas Campbell, Sohail Cheema, Brian Chisamore, Karoon Danayan, Paul Das, Mary Beth Derocher, Anh Do, Michael Dorey, Sloane Freeman, Keewai Fung, Charlie Guiang, Curtis Handford, Hailey Hatch, Sheila Jacobson, Tara Kiran, Holly Knowles, Bruce Kwok, Sheila Lakhoo, Margarita Lam-Antoniades, Eddy Lau, Fok-Han Leung, Jennifer Loo, Sarah Mahmoud, Rosemary Moodie, Julia Morinis, Sharon Naymark, Patricia Neelands, James Owen, Michael Peer, Marty Perlmutar, Navindra Persaud, Andrew Pinto, Michelle Porepa, Nasreen Ramji, Noor Ramji, Alana Rosenthal, Janet Saunderson, Rahul Saxena, Michael Sgro, Susan Shepherd, Barbara Smiltnieks, Carolyn Taylor, Thea Weisdors, Sheila Wijayasinghe, Peter Wong, Ethel Ying and Elizabeth Young.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1986;1:1077–81. [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 3. StatsCan. Canadian Health Measures Survey. Detailed information for January 2012 to December 2013 (Cycle 3). http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071&Item_Id=145921&lang=en (6 December 2013, date last accessed).
- 4.Roberts KC, Shields M, de Groh M, Aziz A, Gilbert J. Overweight and obesity in children and adolescents: Results from the 2009 to 2011 Canadian Health Measures Survey. Statistics Canada. Health Rep 2012;23:1–7. [PubMed] [Google Scholar]
- 5.Melnyk BM, Grossman DC, Chou R, et al. and the US Preventive Services Task Force. USPSTF perspective on evidence-based preventive recommendations for Children. Pediatrics 2012;130:e399–e407. [DOI] [PubMed] [Google Scholar]
- 6.Olson LM, Inkelas M, Halfon N, Schuster MA, O'Connor KG, Mistry R. Overview of the content of health supervision for young children: reports from parents and pediatricians. Pediatrics 2004;113:1907–16. [PubMed] [Google Scholar]
- 7. ICES Practice Atlas – Primary Care in Ontario. Toronto, ON: Institute for Clinical Evaluative Sciences, 2002.
- 8.Guttmann A, Lam K, Schultz SE, Jaakkimainen L. (eds). Primary care for children. In: Primary Care in Ontario. An ICES Practice Atlas. Toronto, ON: Institute for Clinical Evaluative Sciences, 2006. [Google Scholar]
- 9.Chien A, Coker T, Choi L, et al. What do pediatric primary care providers think are important research questions? A perspective from PROS providers. Ambul Pediatr 2006; 6:352–55. [DOI] [PubMed] [Google Scholar]
- 10. Ministry of Health and Long-term Care. Publicly Funded Immunization Schedules for Ontario. August 2011. http://www.health.gov.on.ca/en/public/programs/immunization/docs/schedule.pdf (6 December 2013, date last accessed).
- 11. Ontario Ministry of Children and Youth Services. Your Child's Enhanced 18-Month Well-Baby Visit. http://www.children.gov.on.ca/htdocs/English/topics/earlychildhood/health/your_enhanced_18-month.aspx (6 December 2013, date last accessed).
- 12.Tremblay MS, Willms JD. Secular trends in the body mass index of Canadian children. CMAJ 2000;163:1429–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Hartfield D. Iron deficiency is a public health problem in Canadian infants and children. Paediatr Child Health 2010;15:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thane CW, Walmsley CM, Bates CJ, Prentice A, Cole TJ. Risk factors for poor iron status in British toddlers: further analysis of data from the National Diet and Nutrition Survey of children aged 1.5–4.5 years. Public Health Nutr 2000;3:433–40. [DOI] [PubMed] [Google Scholar]
- 15.Brotanek JM, Gosz J, Weitzman M, Flores G. Secular trends in the prevalence of iron deficiency among US toddlers, 1976–2002. Arch Pediatr Adolesc Med 2008;162:374–81. [DOI] [PubMed] [Google Scholar]
- 16.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd edn Hoboken, NJ: Wiley, 2002. [Google Scholar]
- 17. StatsCan. Canadian Community Health Survey. 2004. http://www.statcan.gc.ca/concepts/health-sante/content-contenu-eng.htm (30 July 2013, date last accessed).
- 18.Randall Simpson JA, Keller HH, Rysdale LA, Beyers JE. Nutrition Screening Tool for Every Preschooler (NutriSTEP): validation and test-retest reliability of a parent-administered questionnaire assessing nutrition risk of preschoolers. Eur J Clin Nutr 2008;62:770–80. [DOI] [PubMed] [Google Scholar]
- 19.Putnam SP, Rothbart MK. Development of short and very short forms of the Children's Behavior Questionnaire. J Pers Assess 2006;87:102–12. [DOI] [PubMed] [Google Scholar]
- 20.Dahinten SV, Ford L. Validation of the Nipissing District Developmental Screen for Use With Children and Toddlers . Working paper.:Vancouver, BC: Consortium for Health, Intervention, Learning and Development, 2004. [Google Scholar]
- 21. Early Childhood Developmental Screening Work Group. Field Test of the Nipissing District Developmental Screen in the NWT. Yellowknife, NWT: Early Childhood Developmental Screening Work Group, Department of Health and Social Services, Yellowknife Association for Community Living, 2001.
- 22.Wetherby A, Prizant B. Communication and Symbolic Behavior Scales Developmental Profile. Baltimore, MD: Paul H. Brookes, 2002. [Google Scholar]
- 23.Wetherby A, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the 2nd year of life. J Autism Dev Disord 2004;34:473–93. [DOI] [PubMed] [Google Scholar]
- 24.Abidin RR. Parenting Stress Index . 3rd edn Odessa, FL: Psychological Assessment Resources, 1995. [Google Scholar]
- 25. Centers for Disease Control and Prevention and National Center for Health Statistics. Third National Health and Nutrition Examination (NHANES III). In: Anthropometric Procedures. Video. Pittsburgh, PA: Centers for Disease Control and Prevention and National Center for Health Statistics, 2003.
- 26. Hospital for Sick Children (SickKids) Research Ethics Board Blood. Sampling Guidelines. 2010. http://www.sickkids.ca/Research/REB/guidelines-procedures-and-policies/index.html (30 July 2013, date last accessed).
- 27.Birken CS, Maguire JL, Mekky M, et al. Parental factors associated with screen time in pre-school children in primary-care practice: A TARGet Kids! study. Public Health Nutr 2011;5:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Birken CS, Maguire JL, Mekky M, et al. Office-based randomized controlled trial to reduce screen time in preschool children. Pediatrics 2012;130:1110–15. [DOI] [PubMed] [Google Scholar]
- 29.Persaud N, Maguire JL, Lebovic G, et al. Association between serum cholesterol and eating behaviours during early childhood: a cross-sectional study. CMAJ 2013;185:E531–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutcliffe TL, Khambalia A, Westergard S, Jacobson S, Peer M, Parkin PC. Iron depletion is associated with daytime bottlefeeding in the second and third years of life. Arch Pediatr Adolesc Med 2006;160:1114–20. [DOI] [PubMed] [Google Scholar]
- 31.Maguire JL, Salehi L, Birken CS, et al. Association between total duration of breastfeeding and iron deficiency. Pediatrics 2013;131:1530–37. [DOI] [PubMed] [Google Scholar]
- 32.Maguire JL, Birken CS, Jacobson S, et al. Office-based intervention to reduce bottle use among toddlers: TARGet Kids! Pragmatic randomized trial. Pediatrics 2010;126:e343–50. [DOI] [PubMed] [Google Scholar]
- 33.Maguire JL, Birken CS, Khovratovich M, et al. Modifiable determinants of serum 25-hydroxyvitamin D status in early childhood: opportunities for prevention. JAMA Pediatr 2013;167:230–35. [DOI] [PubMed] [Google Scholar]
- 34.Maguire JL, Lebovic G, Kandasamy S, et al. The relationship between cow's milk and stores of vitamin D and iron in early childhood. Pediatrics 2013;131:e144–51. [DOI] [PubMed] [Google Scholar]
- 35.Omand JA, Darling PB, Parkin PC, et al. Non-western immigrant children have lower 25-hydroxyvitamin D than children from western families. Public Health Nutr 2013;24:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. StatsCan. Highest Level of Educational Attainment for the Population Aged 25 to 64, Percentage Distribution for Both Sexes, for Canada and Census Metropolitan Areas. http://www12.statcan.ca/census-recensement/2006/dp-pd/hlt/97-560/pages/page.cfm?Lang=E&Geo=CMA&Code=01&Table=1&Data=Dist&Sex=1&StartRec=126&Sort=15&Display=Page&CSDFilter=5000 (6 December 2013, date last accessed).