Abstract
The Clinical Practice Research Datalink (CPRD) is an ongoing primary care database of anonymised medical records from general practitioners, with coverage of over 11.3 million patients from 674 practices in the UK. With 4.4 million active (alive, currently registered) patients meeting quality criteria, approximately 6.9% of the UK population are included and patients are broadly representative of the UK general population in terms of age, sex and ethnicity. General practitioners are the gatekeepers of primary care and specialist referrals in the UK. The CPRD primary care database is therefore a rich source of health data for research, including data on demographics, symptoms, tests, diagnoses, therapies, health-related behaviours and referrals to secondary care. For over half of patients, linkage with datasets from secondary care, disease-specific cohorts and mortality records enhance the range of data available for research. The CPRD is very widely used internationally for epidemiological research and has been used to produce over 1000 research studies, published in peer-reviewed journals across a broad range of health outcomes. However, researchers must be aware of the complexity of routinely collected electronic health records, including ways to manage variable completeness, misclassification and development of disease definitions for research.
Key Messages
CPRD data have been extensively used for observational research. For example, the data were used to show that there was no association between MMR vaccine and autism, and to show an association between oral corticosteroid use and increased risk of fractures.
The CPRD has a large UK dataset bringing together longitudinal primary care medical records from participating practices. Over half of CPRD patients are eligible for linkage to additional datasets, including hospital data, national cancer registration data and national mortality records.
Quality of some data is driven by the Quality and Outcomes Framework in the UK, and data are also monitored by CPRD internal processes. Analyses described in this paper show that active (alive, currently registered) CPRD patients are representative of the UK population in terms of age and sex.
CPRD data originate from routine clinical practice, and their use for epidemiological studies typically requires extensive data processing and an understanding of the way the data are originally recorded and stored.
Data resource basics
UK primary care data for research
Over 98% of the UK population are registered with a primary care general practitioner (GP)1 and under the National Health Service (NHS), visits to the GP are free of charge. The GP is the gatekeeper of care in the UK National Health Service. GPs act as the first point of contact for any non-emergency health-related issues, which may then be managed within primary care and/or referred to secondary care as necessary. Secondary care teams also feed back information to GPs about their patients, including key diagnoses. Patient data are routinely recorded onto computers by practice staff, against a unique patient NHS number. These facets of UK primary care provide good capture of health information in a longitudinal electronic health record.
The Clinical Practice Research Datalink (CPRD)
The CPRD harnesses general practice data and produces a primary care dataset, which is one of the largest databases of longitudinal medical records from primary care in the world (Table 1). Established in London in 1987, the small Value Added Medical Products (VAMP) dataset grew to become the General Practice Research Database (GPRD) in 1993,2,3 before expanding to become the CPRD in 2012. The CPRD collates routinely collected anonymised electronic health record data from general practices who have agreed at a practice level to provide data on a monthly basis. All patients registered with the participating practices are included in the dataset, unless they have individually requested to opt out of data sharing, by asking their GP to amend their registration details on the system to disable the extraction of their data.
Table 1.
Key details about the Clinical Practice Research Datalink
| Counties participating | UK: England, Wales, Scotland and Nortdern Ireland |
| Who is included? | Patients registered at general practices that contribute data to CPRD, who have not dissented from secondary use of GP patient-identifiable data |
| What is recorded? | Demographics, diagnoses, symptoms, signs, prescriptions, referrals, immunisations, behavioural factors, tests |
| Period of data collection | 1987 to present |
| Average duration of follow-up 5.1 years | |
| Funding source | CPRD has received funding from the MHRA, Wellcome Trust, Medical Research Council, NIHR Health Technology Assessment programme, Innovative Medicines Initiative, UK Department of Health, Technology Strategy Board, Seventh Framework Programme EU, and various universities, contract research organizations and pharmaceutical companies |
Data linkage
A subset of English practices (currently 75%, representing 58% of all UK CPRD practices) have consented to participate in the CPRD linkage scheme and have provided patient-level information. Patient-level data from consenting practices are linked via a trusted third party (the Health and Social Care Information Centre4) to other existing data sources. Established linkages include Hospital Episode Statistics5 (hospitalisation data), Office for National Statistics6 (mortality data including causes of death), Index of Multiple Deprivation and Townsend scores (deprivation data)and disease registries including the National Cancer Intelligence Network,7 and the Myocardial Ischaemia National Audit Project8 (details in Supplementary Table 1, available as Supplementary data at IJE online). Other linkages are planned (see CPRD website9) and researchers can make requests for bespoke linkage for individual studies.
Uses for observational research and interventional research
Subject to the appropriate data governance and approvals, the CPRD can supply primary care and linked patient data to researchers in the UK and internationally. Through the CPRD, researchers can approach practices and patients to take part in biosample collection studies or trials. The feasibility of this work has been tested: patients from the CPRD have been recruited to a pharmacogenetic study of statin-induced myopathy,10,11 practices have been recruited to cluster randomised trials12,13 and patients have been recruited to pragmatic point-of-care randomised trials.14 The electronic health record data can be used alongside the study data to provide a full clinical picture for the recruited patients.
Ethics
The CPRD has broad National Research Ethics Service Committee (NRES) ethics approval for purely observational research using the primary care data and established data linkages. Other uses of CPRD data may require separate ethical approval. This is likely if there is any specific patient involvement in the study; for example, if the researcher wishes to ask patients to complete a questionnaire for Patient Reported Outcomes, or to conduct an interventional trial among CPRD patients.
Data governance, practice and patient confidentiality
The CPRD strives to operate within UK and European laws to protect confidentiality. Governance requirements to protect patient confidentiality where patient consent has not been obtained are respected by ensuring that patient identifiers are held separately from the clinical data and that there is separation between researchers with access to identifiable information from the primary study and those using CPRD data.
Funding sources
The CPRD is a joint venture from the Medicines and Healthcare Regulatory Agency (MHRA) and the National Institute for Health Research (NIHR).The CPRD is owned by the UK Department of Health and operates within the MHRA. The CPRD has received funding for studies from the MHRA, Wellcome Trust, Medical Research Council, NIHR Health Technology Assessment programme, Innovative Medicines Initiative, UK Department of Health, Technology Strategy Board, Seventh Framework Programme EU and various universities, contract research organizations and pharmaceutical companies.
Data resource area and population coverage
Figure 1 describes the population coverage of CPRD primary care data across England, Wales, Scotland and Northern Ireland. At the mid-year date of 2 July 2013, the dataset held information on 11.3 million patients who were deemed acceptable for research based on data quality checks (Appendix 1, available as Supplementary data at IJE online, and described below). The population of active patients (alive and currently registered) on 2 July 2013 was 4.4 million, representing 6.9% of the total UK population (based on the UK 2013 mid-year population of 64.1 million). The remaining 6.9 million records represent inactive patients who have died or are no longer registered with a participating practice. Patient numbers by age, sex, deprivation, ethnicity and region are described in Table 2.
Figure 1.
Distribution of 674 CPRD practices by region in England, and in Wales, Scotland and Northern Ireland.
Note: practices mapped are those contributing up to standard data to the dataset on 2 July 2013, based on the January 2014 dataset build
Table 2.
Demographic characteristics of acceptable CPRD patients (January 2014 dataset build), and the subset of those active on 2 July 2013
| All patients | Active | |
|---|---|---|
| No. patients | 11299221 | 4425016 |
| Men, n (%) | 5478715 (48.5) | 2183161 (49.3) |
| Women, n (%) | 5820506 (51.5) | 2241855 (50.7) |
| Age in 2013, n (%) (years) | ||
| <18 | – | 742765 (20.2) |
| 18-64 | – | 4402926 (61.8) |
| 65+ | – | 1728514 (18.1) |
| Region, n (%) | ||
| North East | 184753 (1.6) | 67639 (1.5) |
| North West | 1257846 (11.1) | 523356 (11.8) |
| Yorkshire & The Humber | 441933 (3.9) | 48480 (1.1) |
| East Midlands | 446799 (4) | 29954 (0.7) |
| West Midlands | 943011 (8.4) | 394115 (8.9) |
| East of England | 1117235 (9.9) | 306538 (6.9) |
| South West | 943295 (8.4) | 377821 (8.5) |
| South Central | 1236351 (10.9) | 544979 (12.3) |
| London | 1532066 (13.6) | 600824 (13.6) |
| South East Coast | 1130468 (10) | 474593 (10.7) |
| Northern Ireland | 275640 (2.4) | 153576 (3.5) |
| Scotland | 960121 (8.5) | 499969 (11.3) |
| Wales | 829703 (7.3) | 403172 (9.1) |
| Duration of follow-up (median years, IQR)a | 5.1 (1.8-11.1) | 9.4 (3.4-13.9) |
Active patients are alive and currently registered on 2 July 2013.
aIncludes only up to standard follow-up.
Frequency of data collection
Data collection happens as part of normal clinical care of patients in participating practices on a daily basis. The frequency of data recording is determined by patient need and varies by age, sex and underlying morbidity. Patients are included in the primary care dataset from their first until their last contact with the participating practice. Data are collected by practices and usually uploaded to the CPRD secure servers on a monthly basis. The date of last data collection corresponds to the date of the last data upload from each practice. Monthly builds of the primary care dataset are generated and made available for researchers to use.
Measures
Practice and patient data
The database structure broadly separates information into clinical, referral, immunisation, test and therapy data (see Supplementary Table 1, available as Supplementary data at IJE online). Data are recorded against practice and patient pseudo-identifiers. At the practice level, geographical region is recorded by the CPRD as one of 10 regions in England, with Wales, Scotland and Northern Ireland as separate regions (Figure 1); a practice-level deprivation score is also calculated based on practice lower super output area.
All general practice encounters are recorded electronically and practitioners are encouraged to make these records available for research. Data are collected on demographic information, prescription details, clinical events (symptoms, diagnoses), preventive care provided, tests, immunisations, specialist referrals, hospital admissions and their major outcomes, and details relating to death (details are shown in Supplementary Table 2, available as Supplementary data at IJE online).
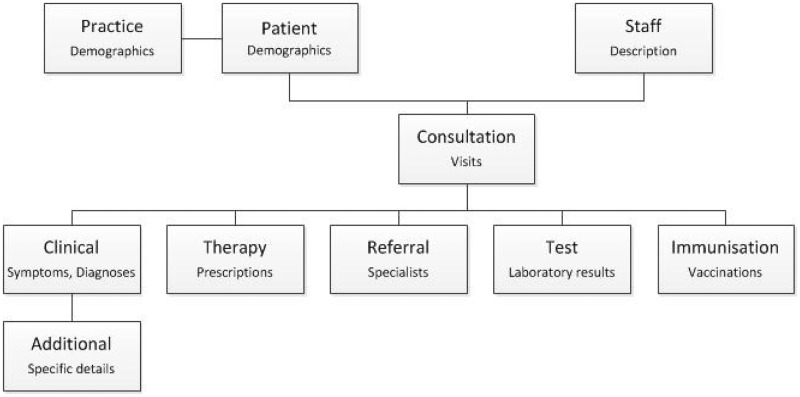
All entries to a patient record are considered as ‘consultations’, not all of which will involve a face-to-face encounter. Within a consultation multiple ‘events’ may be recorded, each with an associated date (Figure 2).
Figure 2.
Example of dataset structure.
Note: patients consult with practice staff, where clinical, therapy, referral, test and immunisation information is coded in the medical record.
Data are largely recorded by general practice staff using version 2 Read codes, a hierarchical clinical classification system containing over 96, 000 codes.15 For example, during a consultation, a GP, nurse, other healthcare professional, practice manager or administrator may enter a number of Read codes to describe a patient’s condition (e.g. lifestyle measures such as smoking status, symptoms, past medical history, diagnoses, tests performed such as blood pressure measurement, and therapies offered). Numerical data on additional clinical measures (e.g. height, weight, blood pressure, alcohol intake) can also be recorded during consultations. Prescriptions issued by the GP are automatically recorded with a product name and British National Formulary code, alongside the dosage instructions and quantity. Results of laboratory tests ordered by the GP are commonly added to the patient record via electronic links to laboratories. Data fed back to the GP from other sources may also be entered into the patient record by practice staff; this might include information from secondary care such as key diagnoses, discharge data from hospitals, or follow-up information from specialist clinics.
The GP is also able to make additional uncoded notes and observations about patients as free text. This often contains identifiable information and is not part of the standard database available to researchers.
Data resource use
Data from the CPRD (or formerly the GPRD or VAMP) have been used in the UK and internationally16 to produce close to 2000 research reports, with over 1000 published in peer-reviewed journals, across all major therapeutic areas. A bibliography is maintained by the CPRD and is available online.17 These publications cover a range of health-related research topics including pharmacoepidemiology, comparative effectiveness research, health services research, assessments of temporal trends in disease incidence, health economics, prognosis research, classical risk factor epidemiology and more recently randomised controlled trials.12,18 Publications to date include studies showing the absence of an association between measles, mumps and rubella (MMR) vaccine and autism,19 cardiovascular risk after acute infection,20 the lower risk of dementia associated with statin use,21 the risk of myocardial infarction in patients with psoriasis,22 the use of oral corticosteroids and fracture risk23 and the association between body mass index and cancer.24
Strengths and weaknesses
Strengths
The strengths of the CPRD data as a research resource lie in the breadth of coverage, size, long-term follow-up, representativeness and data quality.
Breadth of data
The CPRD primary care dataset is one of few large, ongoing databases that include data on morbidity and lifestyle variables and with a linkage to secondary care and mortality data.
Size and long- term follow-up
A key strength of this database is its size; the CPRD holds data from 674 practices and includes over 79 million person-years of follow-up (on 2 July 2013, January 2014 dataset). This allows epidemiological associations to be investigated in more detail and estimated with a higher level of statistical precision than is possible with smaller data sources, which is of particular importance for the study of rare exposures and diseases.25,26 For individual patients, there is a median prospective follow-up of 9.4 years for active patients [interquartile range (IQR) 3.4–13.9] and 5.1 years (IQR 1.8–11.1 years) (Table 2) overall, enabling research into diseases with long latency and the study of long-term outcomes.27–29
Representativeness
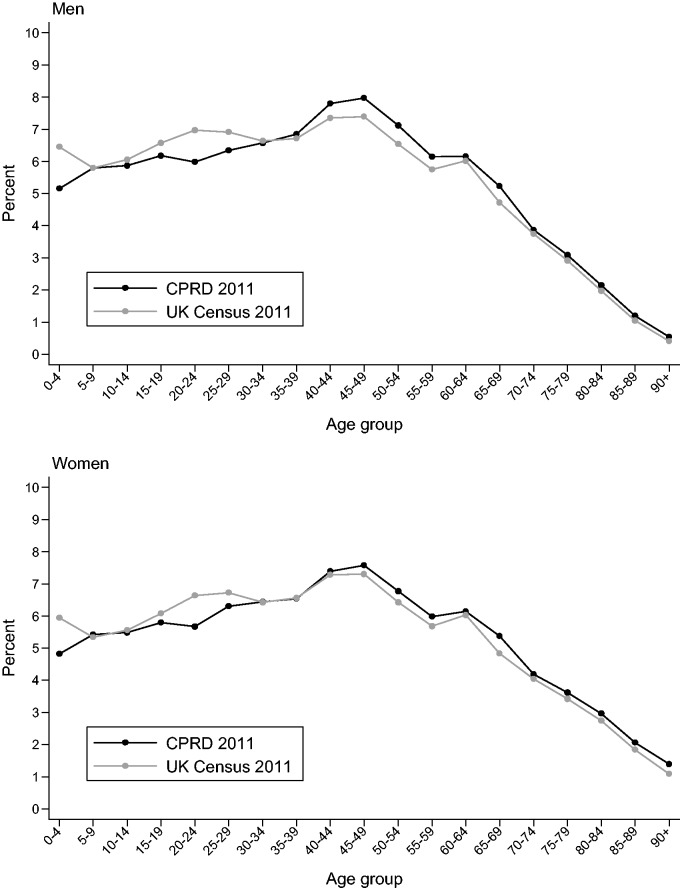
When compared with the UK census in 2011,30 CPRD patients are broadly representative of the UK population in terms of age and sex (Figure 3). Patients are also comparable to the UK census in terms of ethnicity,31 and comparable to the Health Survey for England for body mass index distribution in most patient subgroups.32 However, the CPRD may not be representative of all practices in the UK based on geography and size.33
Figure 3.
Age distribution of the CPRD primary care data on 27 March 2011 compared with UK Census data 2011, in men (top panel) and women (lower panel). These data are based on a one-million patient sample of CPRD. All patients are acceptable.
Data quality
Aspects of data quality in English general practice are enhanced by the Quality and Outcomes Framework,34 an incentive payment programme for GPs, which encourages recording of key data items (for example smoking status and the delivery of services to key patient groups). The Quality and Outcomes Framework was introduced in 2004, and completeness in recording of many variables showed subsequent improvement (Figure 4, and Supplementary Figure 1, available as Supplementary data at IJE online).
Figure 4.
Recording of key lifestyle and demographic variables by calendar year (A: ever recorded in patient follow-up; B: recorded in the past 3 years of patient follow-up). These data are based on a one-million patient sample of primary care data from the CPRD. All patients are acceptable.
Validation of the CPRD has shown high positive predictive value of some diagnoses and, where evaluated, comparisons of incidence with other UK data sources are also broadly similar.35–38 However, reporting of validation studies was often too poor to permit a clear interpretation, and the majority of studies focused on positive predictive value rather than sensitivity or specificity.39
The quality of primary care data is variable because data are entered by GPs during routine consultations, not for the purpose of research. Researchers must therefore undertake comprehensive data quality checks before undertaking a study. The CPRD provides two sets of data quality criteria: acceptability for patients and up to standard (UTS) time for practices. These criteria do not ensure data quality, but the CPRD recommends that these measures are used as a first step to selecting research-quality patients and periods of quality data recording. The acceptable patient metric is based on registration status, recording of events in the patient record, and valid age and gender. The UTS date is a practice-based quality metric based on the continuity of recording and the number of recorded deaths. The UTS date is calculated for each participating practice, corresponding to the latest date at which practices meet these minimum quality criteria (Appendix 1, available as Supplementary data at IJE online). The figures given in this paper reflect data for patients labelled as acceptable and who have at least 1 day of follow-up that is ‘up to standard’. Research into data quality has shown that, despite these criteria, there were large variations in inter-practice recording of data.40
Weaknesses
Missing data
The variability in completeness of data across patients and across time requires careful consideration; restriction to those with complete data may result in biased analyses, and imputation may not be a straightforward approach because the patterns of missingness are complex. For example, body mass index may be recorded more frequently in patients with a health issue, and blood pressure more frequently in women of reproductive age and those with existing cardiovascular disease. Complex algorithms are often required to deal with missingness, to resolve discrepancies in measures between consultations and to decide whether historical measurements, for example of body mass index, blood pressure or smoking status, are still appropriate to a patient’s disease risk much later in follow-up.32
An additional complexity of primary care data is that the absence of a Read code for disease must be interpreted as an absence of the disease itself, so whereas positive predictive value tends to be high,39 sensitivity may be lower. This potential misclassification arises partly due to patients failing to present to the GP with disease, and also from variations between GPs in coding diagnoses in the patient electronic record; if GPs enter information as free text, researchers will miss valuable information. The extent of misclassification may vary between diseases.39
Definitions
There are not generally standardised definitions for diagnoses and other details, so Read code lists and algorithms need to be developed for each study to identify exposures and outcomes of interest. This may lead to inconsistent definitions (and therefore results) between studies using the same data.
Information from secondary care
General practices receive information about patient contacts with secondary care but this information must be manually entered into the patient record. Therefore, details about hospital admissions (dates, diagnoses, tests performed, length of stay) may be incomplete.
Data not captured
Some aspects of health may be recorded very infrequently or not at all, for example level of social support, number of people in a household, over-the-counter medication use, prescriptions in secondary care, prescriptions filled, and adherence to treatments. There are also certain patient groups that are missing from primary care records, such as prisoners, private patients, some residential homes and the homeless.
Data Resource access
Access to patient level data is provided by the CPRD for health research purposes and is dependent on approval of a study protocol by the MHRA Independent Scientific Advisory Committee (ISAC).
Researchers intending to use the data should be aware that the CPRD data files contain millions of rows of data, requiring extensive data management and an in-depth understanding of the way the data are input and stored.
The CPRD provide data dictionaries and coding dictionaries to researchers, and guidance on creating code lists is available to help identify codes of interest.41 Read code repositories for electronic health record research are also now available.42,43
Details about ISAC applications and data costs are available on the CPRD website, and any other queries can be directed to the CPRD Knowledge Centre [kc@cprd.com].9
Supplementary Data
Supplementary data are available at IJE online.
Funding
LS is supported by a Wellcome Trust Senior Research Fellowship in Clinical Science grant number 098504/Z/12/Z.
Conflict of interest: AG is employed by the CPRD and TvS is an ex-employee of the CPRD. No other conflicts declared.
Supplementary Material
References
- 1.Health and Social Care Information Centre. Attribution Data Set GP-Registered Populations Scaled to ONS Population Estimates – 2011 2012. http://www.hscic.gov.uk/catalogue/PUB05054 (6 August 2014, date last accessed). [Google Scholar]
- 2.Kousoulis AA, Rafi I, de Lusignan S. The CPRD and the RCGP: building on research success by enhancing benefits for patients and practices. Br J Gen Pract 2015;65:54–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health and Social Care Information Centre (HSCIC). http://www.hscic.gov.uk/ (6 August 2014, date last accessed). [Google Scholar]
- 5.Hospital Episode Statistics. http://www.hscic.gov.uk/hes (21 January 2015, date last accessed). [Google Scholar]
- 6.Office for National Statistics. Mortality Statistics: Metadata. Cardiff, UK: ONS, 2014. [Google Scholar]
- 7.National Cancer Intelligence Network. http://www.ncin.org.uk/home (23 January 2015, date last accessed). [Google Scholar]
- 8.Herrett E, Smeeth L, Walker L, Weston C. The Myocardial Ischaemia National Audit Project (MINAP). Heart 2010;96:1264–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Clinical Practice Research Datalink (CPRD) www.cprd.com (6 August 2014, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr DF, O'Meara H, Jorgensen AL, et al. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink. Clin Pharmacol Ther 2013;94:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Meara H, Carr DF, Evely J, et al. Electronic health records for biological sample collection: feasibility study of statin-induced myopathy using the Clinical Practice Research Datalink. Br J Clin Pharmacol 2014;77:831–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horspool MJ, Julious SA, Boote J, et al. Preventing and lessening exacerbations of asthma in school-age children associated with a new term (PLEASANT): study protocol for a cluster randomised control trial. Trials 2013;14:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulliford MC, van Staa TP, McDermott L, et al. Cluster randomized trials utilizing primary care electronic health records: methodological issues in design, conduct, and analysis (eCRT Study). Trials 2014;15:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Staa TP, Dyson L, McCann G, et al. The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations of two exemplar trials. Health Technol Assess 2014;18:1–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chisholm J. The Read clinical classification. BMJ 1990;300:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YC, Wu JC, Haschler I, Majeed A, Chen TJ, Wetter T. Academic impact of a public electronic health database: bibliometric analysis of studies using the general practice research database. PLoS One 2011;6:e21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical Practice Research Datalink. CPRD Bibliography. http://www.cprd.com/bibliography/ (12 February 2015, date last accessed). [Google Scholar]
- 18.van Staa TP, Goldacre B, Gulliford M, et al. Pragmatic randomised trials using routine electronic health records: putting them to the test. BMJ 2012;344:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smeeth L, Cook C, Fombonne E, et al. MMR vaccination and pervasive developmental disorders: a case-control study. Lancet 2004;364:963–69. [DOI] [PubMed] [Google Scholar]
- 20.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med 2004;351:2611–18. [DOI] [PubMed] [Google Scholar]
- 21.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000;356:1627–31. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296:1735–41. [DOI] [PubMed] [Google Scholar]
- 23.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000;15:993–1000. [DOI] [PubMed] [Google Scholar]
- 24.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet 2014;384:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dommett RM, Redaniel T, Stevens MC, Martin RM, Hamilton W. Risk of childhood cancer with symptoms in primary care: a population-based case-control study. Br J Gen Pract 2013;63:e22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douglas I, Evans S, Rawlins MD, Smeeth L, Tabrizi SJ, Wexler NS. Juvenile Huntington's disease: a population-based study using the General Practice Research Database. BMJ Open 2013;3:pii: e002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crooks CJ, Card TR, West J. Excess long-term mortality following non-variceal upper gastrointestinal bleeding: a population-based cohort study. PLoS Med 2013;10:e1001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cotton SJ, Belcher J, Rose PK, Jaqadeesan S, Neal RD. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. Br J Cancer 2013;108:721–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalmohamed A, Bazelier MT, Van Staa TP, et al. Causes of death in patients with multiple sclerosis and matched referent subjects: a population-based cohort study. Eur J Neurol 2012;19:1007–14. [DOI] [PubMed] [Google Scholar]
- 30.Office for National Statistics. 2011 Census – Population and Household Estimates for England and Wales, March 2011. Cardiff,UK: ONS, 2012. [Google Scholar]
- 31.Mathur R, Bhaskaran K, Chaturvedi N, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf) 2013;34:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhaskaran K, Forbes HJ, Douglas I, Leon DA, Smeeth L. Representativeness and optimal use of body mass index (BMI) in the UK Clinical Practice Research Datalink (CPRD). BMJ Open 2013;3:e003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell J, Dedman DJ, Eaton SC, Gallagher AM, Williams TJ. Is the GPRD GOLD population comparable to the UK population? Pharmacoepidemiol Drug Saf 2013;22(Suppl 1):280. [Google Scholar]
- 34.Quality and Outcomes Framework. http://www.hscic.gov.uk/qof (6 August 2014, date last accessed). [Google Scholar]
- 35.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001;29:517–22. [DOI] [PubMed] [Google Scholar]
- 36.Ryan R, Majeed A. Prevalence of treated hypertension in general practice in England and Wales, 1994 and 1998. Health Stat Q 2002;16:14–18. [Google Scholar]
- 37.Ronquist G, Rodriguez LA, Ruigomez A, et al. Association between captopril, other antihypertensive drugs and risk of prostate cancer. Prostate 2004;58:50–56. [DOI] [PubMed] [Google Scholar]
- 38.Meier CR, Napalkov PN, Wegmuller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis 2000;19:834–42. [DOI] [PubMed] [Google Scholar]
- 39.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tate R, Kalra D, Boggon R, Beloff N, Puri S, Williams TJ. Data quality in European primary care research databases. Report of a workshop held in London September 2013. 2014 IEEE-EMBS International Conference on Biomedical and Health Informatics. BHI 2014;6864310:85–88. [Google Scholar]
- 41.Dave S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 2009;18:704–07. [DOI] [PubMed] [Google Scholar]
- 42.Springate DA, Kontopantelis E, Ashcroft DM, et al. ClinicalCodes: an online clinical codes repository to improve the validity and reproducibility of research using electronic medical records. PLoS One 2014;9:e99825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CALIBER Research Portal. https://www.caliberresearch.org/portal/ (23 January 2015, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.