Abstract
Background: Vitamin A deficiency (VAD) is associated with increased mortality. To prevent VAD, WHO recommends high-dose vitamin A supplementation (VAS) every 4–6 months for children aged between 6 months and 5 years of age in countries at risk of VAD. The policy is based on randomized clinical trials (RCTs) conducted in the late 1980s and early 1990s. Recent RCTs indicate that the policy may have ceased to be beneficial. In addition, RCTs attempting to extend the benefits to younger children have yielded conflicting results. Stratified analyses suggest that whereas some subgroups benefit more than expected from VAS, other subgroups may experience negative effects.
Methods and Results: We reviewed the potential modifiers of the effect of VAS. The variable effect of VAS was not explained by underlying differences in VAD. Rather, the effect may depend on the sex of the child, the vaccine status and previous supplementation with vitamin A. Vitamin A is known to affect the Th1/Th2 balance and, in addition, recent evidence suggests that vitamin A may also induce epigenetic changes leading to down-regulation of the innate immune response. Thus VAS protects against VAD but has also important and long-lasting immunological effects, and the effect of providing VAS may vary depending on the state of the immune system.
Conclusions: To design optimal VAS programmes which target those who benefit and avoid those harmed, more studies are needed. Work is ongoing to define whether neonatal VAS should be considered in subgroups. In the most recent RCT in older children, VAS doubled the mortality for males but halved mortality for females. Hence, we urgently need to re-assess the effect of VAS on older children in large-scale RCTs powered to study effect modification by sex and other potential effect modifiers, and with nested immunological studies.
Keywords: Vitamin A, child mortality, vaccines, heterologous effects
Key Messages
Vitamin A deficiency is associated with increased mortality from infectious diseases, and to prevent vitamin A deficiency, high-dose vitamin A capsules are recommended twice per year to children between 6 months and 5 years of age in more than 100 countries considered at risk of vitamin A deficiency.
The effect of vitamin A supplementation is assumed to be always beneficial, reducing deaths due to vitamin A deficiency.
A number of observations contradict the interpretation that vitamin A supplementation acts merely to prevent vitamin A deficiency. The effect of vitamin A supplementation is not associated with the degree of deficiency, and the effect is modified by factors unrelated to deficiency, such as sex of the child and the vaccines he or she has received.
The existing evidence supports that besides preventing vitamin A deficiency, vitamin A supplementation also has long-lasting immunological effects; and, worryingly, the effect of vitamin A supplementation may be harmful in some subgroups.
To create optimal vitamin A supplementation policies, we urgently need to re-assess the effect of vitamin A supplementation in large-scale RCTs powered to study effect modification by sex and other potential effect modifiers, and with nested immunological studies.
Introduction: the vitamin A enigma
Vitamin A deficiency (VAD) was common in high-income countries around the time of World War II, and in low-income countries in the mid-1980s, and attentive clinicians observed that this condition was linked to increased overall morbidity and mortality.1
In the late 1980s and early 1990s, eight randomized controlled trials (RCTs) of vitamin A supplementation (VAS) were conducted in low-income countries.2 Six showed marked effects on overall mortality of providing VAS. The RCTs which showed the strongest effect were two from India and Indonesia using smaller weekly3 doses or daily dosing through fortification;4 the other RCTs used high-dose supplements. Two high-dose RCTs from India5 and Sudan6 found no effect.
A meta-analysis of these eight original RCTs was conducted and concluded that VAS—including the two studies with no effect— reduced overall mortality by 23%.2 The P-value for homogeneity of effects across the RCTs was 0.088 and reasons for the variation in effect were explored.2 It was concluded that the variation was not explained by the degree of underlying VAD, nor by sex or age at supplementation.2 WHO-recommended programmes providing high-dose VAS twice per year to children aged between 6 months and 5 years became widespread7 and are still carried out in more than 100 countries considered at risk of VAD.8
The common interpretation is that VAD is associated with increased mortality—and that VAS works by preventing and treating VAD. The lack of effect in two of the original eight trials and the lack of association between the degree of VAD and the effect of VAS should perhaps have raised concern about this compelling but rather simple interpretation. After the eight original RCTs, there have been few RCTs of VAS to children above 6 months of age; once an intervention becomes policy, it is considered unethical to test it in RCTs, since these imply depriving some children of a recommended intervention.
There have been numerous RCTs carried out to test whether the observed beneficial effects of VAS could be extended to younger children. Most RCTs providing VAS to children between 1 and 5 months of age found no beneficial effects of providing VAS, even when VAD was prevalent (reviewed in9). Several RCTs of neonatal VAS (NVAS) have yielded conflicting results.10 Very recently, three WHO-commissioned RCTs of NVAS from Ghana,11 Tanzania12 and India13 were individually powered to show a 15% reduction in mortality by 6 months of age, but found no overall benefit; the random effect meta-analysis estimate of these three trials by 6 months of age and 12 months of age being 1.03 [95% confidence interval (CI) 0.88-1.19) and 1.02 (0.92-1.13), respectively. Furthermore, two new large RCTs of VAS to children above 6 months of age in Guinea-Bissau and India found no overall beneficial effect of VAS.14, 15
All RCTs except one14 hypothesized that the overall effect of VAS would be beneficial. Hence, when so many recent RCTs produce unexpected results there is reason to pause and reflect. Moreover, many of the RCTs found interactions between VAS and various background factors. Noteworthy, VAS was even more beneficial than anticipated in some subgroups, but worryingly associated with negative effects in other subgroups. The subgroup analyses have usually been dismissed as post hoc analyses. However, since VAS is being provided to millions of children with little or no assessment of the effect, it should be a concern when our expectations of universal beneficial effects are contradicted and potential explanations should be pursued.
We therefore reviewed the factors which may modify the effect of VAS on mortality, the strength of evidence and potential underlying biological explanations, to inspire further discussion and inform future studies to define optimal policies.
Methods and Results
We reviewed the existing literature on the mortality effect of high-dose VAS to children aged above 6 months and to neonates, respectively. For studies in children aged above 6 months, we used as a starting point the meta-analysis of RCTs in children above 6 months of age conducted in 1993,2 adding subsequent large RCTs which reported mortality as the main outcome. For studies in neonates, we included all available RCTs. Data are presented separately for the two age groups unless not relevant.
Potential modifiers of the effect of VAS
Potential modifiers of the effect of VAS are summarized in Table 1.
Table 1.
Potential effect modifiers of high-dose vitamin A supplementation
| Potential modifier | Effect |
|---|---|
| Vitamin A deficiency | No strong data support that VAD is an important effect modifier of VAS |
| Sex | VAS in early infancy may have a less beneficial effect for females than for males, at least in settings with no maternal VAS and low HIV prevalence, where children receive first BCG and then DTP. The evidence in older children is less clear |
| Vaccines | VAS given close in time to DTP vaccine seems to be associated with increased female mortality. In contrast, VAS given close in time to measles vaccine may benefit females more than males. The combination of VAS, a live and an inactivated vaccine may be harmful for males |
| Repeated vitamin A | There is some evidence to suggest that a first dose of VAS primes for a beneficial response to subsequent doses of VAS in females |
| Length of follow-up | Length of follow-up is an important indicator of other exposures and may modify the effect of VAS depending on the nature of these other exposures |
| Birthweight | The interaction between VAS and birthweight has been inconsistent and seem more likely to be explained by confounding |
| Season | Season is a potential effect modifier of VAS, which may be linked to pathogen exposure and could provide clues about the biological mechanisms behind the variable effect of VAS |
Vitamin A deficiency
According to current understanding, VAD should be a strong modifier of the effect of VAS on mortality: a beneficial effect of VAS should be seen in populations suffering from VAD and the effect should be stronger with increasing prevalence of VAD, whereas no effect is expected in populations with adequate vitamin A status.
VAS above 6 months of age
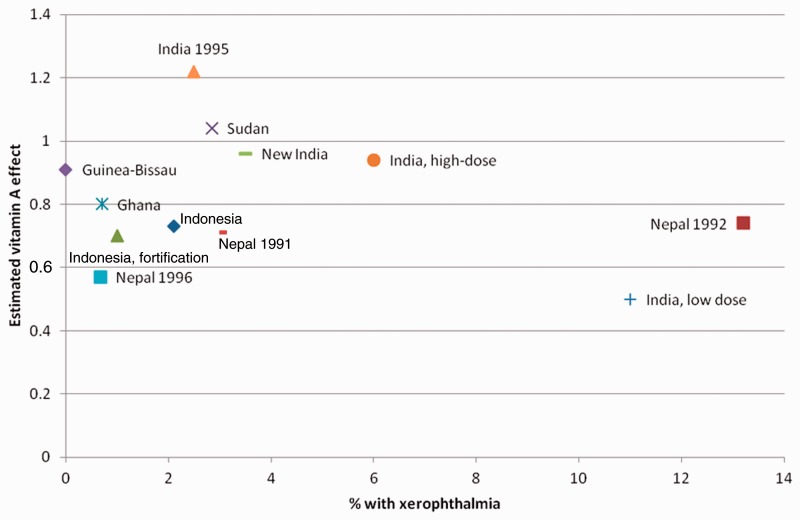
The assessment of the importance of VAD for the effect of VAS is hampered by the lack of good indicators of VAD and the fact that studies have collected different indicators. However, most of the original eight RCTs in children above 6 months of age were conducted in populations where xerophthalmia, a quite unequivocal clinical indicator of VAD, was present.2 There was no indication in these RCTs that the effect of VAS depended on the prevalence of VAD in the population.2 For instance, no effect of VAS was seen in India where around 6% of the children suffered from xerophthalmia,5 whereas a 19% (2–32%) mortality reduction was seen in Ghana, where less than 1% had signs of xerophthalmia16 (Figure 1). In two subsequent RCTs from the 1990s, there was again no strong association between the effect of VAS and degree of underlying VAD.17,18 In the two new RCTs in children above 6 months of age, the Indian RCT was conducted in a population with 3.5% of control children having Bitot’s spots, a clinical manifestation of xerophthalmia.15 The Guinea-Bissau RCT was conducted in a population with no xerophthalmia but more than 60% of the children having low levels of serum retinol-binding protein.19 None of these studies found overall beneficial effects (Figure 1).
Figure 1.
The relative risk comparing vitamin A vs no vitamin A by prevalence of xerophthalmia in the original eight, the two subsequent, and the two new trials of vitamin A supplementation to children above 6 months of age2,14,15,17,18 (modified from Beaton et al.2).
NVAS
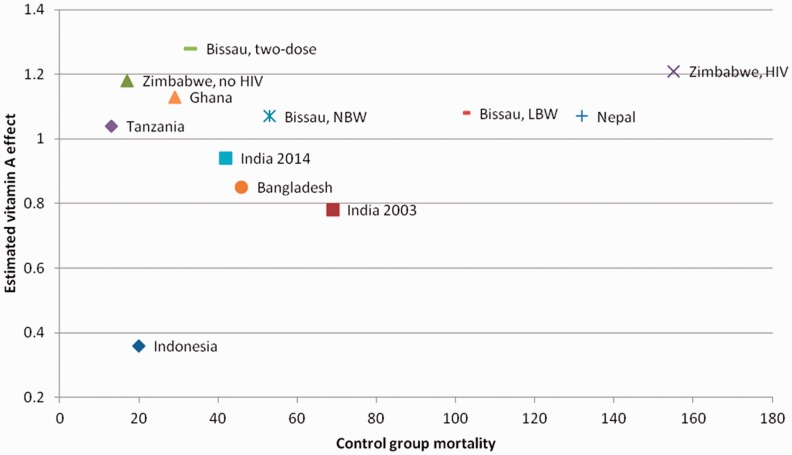
NVAS has been interpreted to be most beneficial in populations with potential VAD as assessed by maternal night blindness, but not all trials have data and the results are not consistent. For instance, the RCT showing the strongest effect was conducted in Indonesia in a setting with no maternal VAD. Baseline mortality has been used as an indicator of VAD, and was available for all trials. As seen from Figure 2, the effect of NVAS is not more beneficial with higher baseline mortality rate in the control group.
Figure 2.
The effect of neonatal vitamin A supplementation by control group mortality.11–13,20,22,23,25,26,36,37,47,70.
Conclusion
Though the interpretation should be cautious due to the lack of good indicators of VAD (apart from xerophthalmia), there are no strong data to support that VAD is an important modifier of the effect of VAS on mortality. If anything, the total body of evidence (Figures 1 and 2) contradicts the current interpretation that the overall effect of VAS on mortality is explained by prevention and treatment of VAD.
Sex of the child
VAS above age 6 months
The original eight RCTs which stratified data by sex reported no evidence of sex-differences in the response to VAS.2 Of the two new RCTs, the Indian RCT found no sex differences but the Guinea-Bissau RCT, in which VAS was provided with vaccines, found a strong interaction between VAS and sex, with a more beneficial effect in females than in males14 (Table 2). Noteworthy, in this RCT there were no sex differences in vitamin A status at enrolment.19
Table 2.
Estimates of the effect of vitamin A supplementation on mortality by age group and sex
| Author, country and year of publication | Overall effect estimate(95% CI) | Males | Females |
|---|---|---|---|
| At-birth supplementation | |||
| Humphrey, Indonesia 199622 | 0.36 (0.16–0.87) | 0.15 (0.03–0.68) | 0.84 (0.26–2.77) |
| Rahmatullah, India 20032 3 | 0.78 (0.63–0.96) | 0.70 (0.52–0.94) | 0.87 (0.65–1.17) |
| Benn, Guinea-Bissau 20083 6 | 1.07 (0.79–1.44) | 0.84 (0.55–1.27) | 1.39 (0.90–2.14) |
| Klemm, Bangladesh 20084 7 | 0.85 (0.73–1.00) | 0.89 (0.72–1.10) | 0.81 (0.65–1.00)* |
| Benn, Guinea-Bissau 20103 7 | 1.08 (0.79–1.47) | 0.74 (0.55–1.22) | 1.42 (0.94–2.15) |
| Kirkwood, Zimbabwe 20102 4 | Not provided | 1.19 (1.00–1.42) | 0.93 (0.78–1.14)*# |
| Benn, Guinea-Bissau 201420 | 1.28 (0.91–1.81) | 1.35 (0.84–2.16) | 1.21 (0.73–2.01) |
| Edmond, Ghana 201411 | 1.13 (0.98–1.31) | 1.19 (0.97–1.47) | 1.08 (0.87–1.34)** |
| Masanja, Tanzania, 20141 2 | 1.04 (0.93–1.17) | 0.96 (0.82–1.12) | 1.16 (0.97–1.38)** |
| Mazumder, India 20141 3 | 0.94 (0.86–1.02) | 0.94 (0.82–1.08) | 0.93 (0.83–1.05) |
| 1-11 months | |||
| Pathwardhan, Jordan 196671 | 0.50 (0.13–1.94) | 0 (one placebo death) | 0.59 (0.15–2.30) |
| Sommer, Indonesia 198672 | 0.83 (0.51–1.37) | 0.59 (0.33–1.06) | 1.06 (0.62–1.81) |
| West, Nepal 199570 | 1.11 (0.86–1.42) | 1.24 (0.86–1.78) | 0.98 (0.68–1.42) |
| Mahalanabis, Bangladesh 199773 | 1.06 (0.52–2.18) | 0.70 (0.21–2.36) | 1.40 (0.59–3.34) |
| Fisker, Guinea-Bissau 20141 4 *** | 0.73 (0.44–1.22) | 1.22 (0.57–2.61) | 0.46 (0.22–0.97) |
| 12 months+ | |||
| Sommer, Indonesia 198672 | 0.66 (0.44–0.97) | 0.59 (0.37–0.95) | 0.80 (0.46–1.40) |
| West, Nepal, 19914 6 | 0.70 (0.57–0.87) | 0.77 (0.55–1.09) | 0.65 (0.48–0.89) |
| Daulaire, Nepal 199274 | 0.74 (0.55–0.99) | 0.72 (0.48–1.08) | 0.76 (0.48–1.19) |
| Herrera, Sudan 19926 | 1.06 (0.82–1.37) | 1.25 (0.85–1.83) | 0.93 (0.66–1.31) |
| Ghana Vast Study Team, Ghana 19931 6 | 0.81 (0.68–0.98) | 0.73 (0.59–0.92) | 0.90 (0.71–1.15) |
| Awasthi, India 20131 5 | 0.96 (0.89–1.03) | 0.99 (0.91–1.07) | 0.93 (0.86–1.00) |
| Fisker, Guinea-Bissau 20141 4 *** | 1.68 (0.70–4.06) | 10.7 (1.37–83.6) | 0.43 (0.11–1.66) |
All major studies that have addressed the effect of vitamin A supplementation by sex. Some of the studies of children aged 12 months and older have also included younger children, but apart from Dr. Sommer’s study it is not possible to extract the sex-specific mortality ratios for separate age groups.
*These trials were factorial trials with maternal supplementation as well.
**These trials had maternal supplementation programmes ongoing.
***The only trial which provided VAS with vaccines. Estimates by age group calculated by the authors.
#combined HIV positive and HIV negative cohort.
NVAS
Two of the three RCTs in Guinea-Bissau found that males benefited more than females from NVAS. In a combined analysis of the three Guinea-Bissau RCTs, NVAS tended to reduce infant mortality in males, but was associated with 35% (95% CI 4-75%) higher infant mortality in females (P = 0.01 for interaction between NVAS and sex).20 Vitamin A status was assessed in one of these RCTs and, if anything, males had better vitamin A status than females.21
Similar tendencies with a better effect of NVAS in males were seen in the RCTs from Indonesia, India 2003 and Tanzania12,22,23 (Table 2). In contrast, in the RCTs from Zimbabwe and Ghana the effect tended to be worse for males. In a sex-stratified analysis of the total study population in the Zimbabwe RCT, NVAS was associated with 19% (0-42%) increased mortality in males.24 However, the Zimbabwean RCT was a 2-by-2 factorial trial with maternal post-partum VAS and NVAS, conducted in a population with a very high HIV prevalence. The mortality analysis was mainly driven by deaths among children of HIV-infected mothers,25,26 and significantly more of these HIV-related deaths were among males.25 Hence, the sex-stratified estimates from Zimbabwe are not comparable with the estimates from the other RCTs.27 In the Ghana RCT, half of the mothers also received post-partum VAS11—a policy which has now been abandoned.
The RCTs providing VAS between 1 and 11 months of age have generally found a less beneficial effect in females than in males (Table 2, reviewed in28).
Conclusion
The main body of evidence suggests that VAS in early infancy has a less beneficial effect for females than for males in settings with low HIV prevalence and no maternal VAS (the current policy). The evidence in older children is less clear; there were no strong sex differences in the older RCTs, but a recent RCT found that VAS to older children was better for females than for males.14 There is no indication that sex differences in VAD prevalence can explain these results.
Vaccines
Many studies have shown that the live measles vaccine (MV, recommended at 9 months of age) is associated with strong beneficial effects on survival, which cannot be explained by prevention of measles infection, so-called non-specific effects.29–31 These effects are most pronounced for females. In contrast, though protective against the target diseases, the inactivated diphtheria-tetanus-pertussis vaccine (DTP, recommended at 6, 10 and 14 weeks of age) may have negative non-specific effects, increasing the mortality of females absolutely and relative to males (reviewed in32). These trends, combined with the observation that VAS was associated with beneficial effect in children aged above 6 months but had no effect or even a tendency for a negative effect when given to children between 1 and 5 months of age, led us to propose the hypothesis that VAS amplifies the non-specific effects of vaccines, being beneficial in the context of MV, but harmful when given in the context of DTP vaccine.9 We asked for permission to test this hypothesis within RCTs in which vaccine data had been collected, or there was sufficiently high timely vaccination coverage to make reasonable assumptions about the participating children’s vaccination status.
VAS above 6 months of age
We obtained permission to test our hypothesis in a re-analysis of one of the eight original VAS RCTs, the Ghana RCT. We found that VAS had only a beneficial effect in children with no record of vaccination at enrolment. Among vaccinated children, the effect of VAS differed between males and females, and VAS had a negative effect in measles-vaccinated females who were missing one or more doses of DTP at enrolment, but usually received these doses during follow-up.33
Two observational studies from Guinea-Bissau have assessed the effect of VAS in campaigns in relation to vaccination status. In 2003, VAS was provided together with missing vaccines to children above 6 months of age, and children receiving VAS with DTP had 3.43 (95% CI 1.36-8.61) times higher mortality than those who received VAS alone, and 3.04 (1.31-7.07) times higher mortality than those who did not participate in the campaign.34 In two VAS campaigns in 2007 and 2008, the effect of VAS in children who had DTP vaccine as their last vaccine was worse than for children who had MV as their last vaccine.35
Last, a RCT was conducted in Guinea-Bissau to test the effect of VAS provided at vaccination contacts. We hypothesized that compared with placebo, VAS with MV would be beneficial, particularly for females. We found the expected trend for females—both when MV was the only vaccine and when MV and DTP were given simultaneously—but surprisingly the opposite trend was seen for males. The interaction between VAS and sex was particularly pronounced in children who received DTP and MV simultaneously. We did not find support for the hypothesis that VAS provided with DTP was bad for females, but at least one-third of the DTP-vaccinated children received MV during the 6-month-follow-up and this may have masked the effect of VAS with DTP.14
NVAS
The NVAS RCTs provided an additional possibility for testing the hypothesis of negative interaction between vitamin A and DTP vaccine, by testing the effect of NVAS vs placebo once the children start receiving DTP (around 6 weeks of age). In a combined analysis of the three NVAS trials from Guinea-Bissau,20,36,37 NVAS compared with placebo was associated with 63% (9-144%) increased mortality in females once they received DTP vaccine (38 and unpublished).
Unfortunately our request to test the hypothesis in the other NVAS RCTs with relevant data was declined, and for those RCTs we have had to rely on circumstantial evidence.
The Zimbabwean NVAS trial did not report results by vaccination status but, judged by the survival curves, NVAS was associated with increased mortality after the first months of life—just as seen in Guinea-Bissau and compatible with a negative interaction between NVAS and DTP vaccine.26 The Ghanaian trial reported that the effect of NVAS was 0.81 (95% CI 0.50-1.32) before the children received DTP-containing vaccines, but 1.36 (0.96-1.92) after DTP (P = 0.089 for interaction).12 In the Asian NVAS RCTs, vaccination coverage was lower and mortality was concentrated in the first months of life. Hence, a possible negative interaction between NVAS and subsequent DTP vaccine would be less apparent. Also, the non-specific effects of receiving first Bacillus Calmette–Guérin (BCG) and then DTP, as recommended by WHO, are quite different from receiving BCG and DTP at the same time; receiving BCG with DTP apparently ameliorates the negative effects of DTP in females.39–41 Hence, we would not expect NVAS followed by BCG + DTP, as often done in Asia,13 to be associated with negative effects in females similar to those of NVAS + BCG followed by DTP alone. Based on the Indian RCT from 2003, these authors published a separate paper on the potential non-specific effects of vaccines.42 Unfortunately, this paper eliminated the deaths in the first week of life, and the estimates for NVAS vs placebo by vaccination status cannot be deduced. The Indian 2014 RCT reported an analysis of mortality to 6 months of age by DTP vaccination status,13 but unfortunately the analysis suffered from severe survival bias and the results are not valid to draw conclusions from (Benn et al., Lancet, in press). The three new WHO-commissioned NVAS RCTs11–13 have not yet been analysed with respect to vaccines and sex but, in all three RCTs, NVAS was associated with increased female mortality in the second half of infancy, from 6-11 months [meta-analysis estimate = 1.17 (0.98-1.40)],11–13 compatible with a more negative effect in females after DTP vaccination.
In the Guinean NVAS RCTs, a subgroup of participants participated in an early MVRCT, being randomized to early MV or no such vaccine at 4.5 months of age. We would have predicted that NVAS would have a beneficial effect in the context of MV. However, we found that NVAS was associated with strong negative effects in children who received early MV, particularly so in males.29,43 All children in the RCT had received the third DTP vaccine just 4 weeks prior to MV. This finding, and the finding that VAS was negative for males who received DTP and MV at the same time,14 indicate that the combination of NVAS and live and inactivated vaccines may be harmful for males.14,43
Conclusion
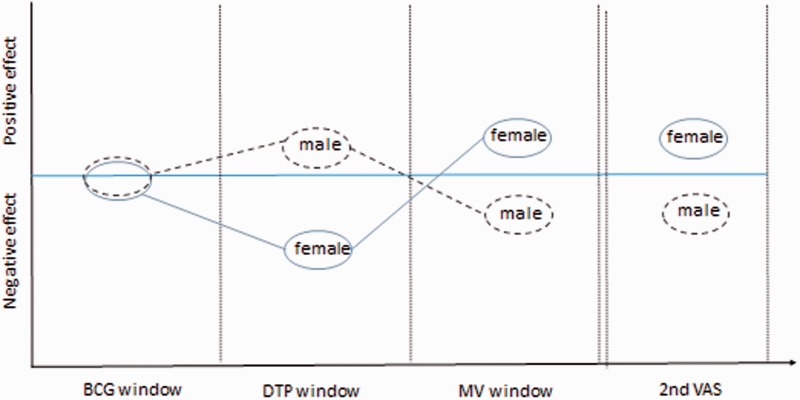
DTP has been associated with increased female mortality from non-targeted diseases, and most RCTs, backed by all available observational studies, suggest that VAS given close in time to DTP vaccine is associated with increased mortality, particularly in females.33–38 In contrast, VAS given close in time to MV may benefit females more than males14 (Figure 3). Interactions between vitamin A and vaccines hold the potential to explain why there were no strong sex differences and why VAS was more beneficial in the original eight RCTs conducted in the pre-vaccination era. It could also help explain why the African NVAS RCTs, conducted in urban settings with high vaccination coverage and sequential administration of BCG and DTP, have found more negative effects than the Asian NVAS RCTs conducted in predominantly rural areas with lower coverage and more BCG-DTP co-administration.
Figure 3.
Potential modification of vitamin A effects by vaccinations and repeated dosing.
Repeated dosing of vitamin A
All studies which have reported data on the effect of the first vs subsequent doses of VAS by sex have found that a first dose of VAS may prime for a more beneficial response to subsequent VAS in females, but not in males.14,35,44,45 This includes observational studies but also two RCTs (Table 3). In the Guinea-Bissau RCT in children above 6 months of age, VAS vs placebo was associated with a mortality rate ratio (MRR) of 0.18 (0.05-0.62) in females who had received VAS on a previous occasion, but 0.82 (0.35-1.89) in those who had not (P = 0.05 for interaction between VAS and prior VAS). In males the opposite tendency was seen, reflected in a three-way interaction between VAS, sex and prior VAS (P = 0.007) (Table 3).14
Table 3.
The effect of repeated dosing with high-dose vitamin A supplementation
| Author, country and year of publication | Group | Effect of first dose | Effect of second dose |
|---|---|---|---|
| Mortality rate ratio | Mortality rate ratio | ||
| (95% CI) | (95% CI) | ||
| Fisker, Guinea-Bissau 201144 | Overall | 1.10 (0.64–1.90) | 0.54 (0.31–0.94) |
| Males | 0.57 (0.23–1.42) | 0.73 (0.35–1.51) | |
| Females | 1.67 (0.81–3.42) | 0.37 (0.16–0.89) | |
| Fisker, Guinea-Bissau 20141 4 | Overall | 0.99 (0.57–1.77) | 0.80 (0.41–1.58) |
| Males | 1.19 (0.53–2.65) | 5.98 (1.34–26.7) | |
| Females | 0.82 (0.35–1.89) | 0.18 (0.05–0.62) |
The two original VAS RCTs which reported the effect by dosing round found better effect after subsequent doses of VAS. In Ghana, the mortality ratio after the first dose of VAS vs placebo was 0.99 (0.78-1.67) but declined to 0.68 (0.55–0.82) after subsequent doses.16 In Nepal, the mortality ratio was 0.76 (0.50-1.15) in the first 4 months of the study and 0.67 (0.63-0.73) in the subsequent rounds.46
Conclusion
There is some evidence from both RCTs and observational studies to suggest that a first dose of VAS primes for a beneficial response to subsequent doses of VAS in females; in the Guinean RCT there was also some indication that males may be primed negatively. Hence, females may benefit more than males from repeated dosing of VAS (Figure 3), and this may explain some of the stronger beneficial effect of VAS seen in females after the first year of life.14 To our knowledge there are no data which contradict the hypothesis that a first dose of VAS primes for a more beneficial response to subsequent doses in girls.
Length of follow-up
The outcome of potential effect modification by both prior VAS and subsequent vaccines would depend on the length of follow-up after VAS.
VAS above 6 months of age
The original VAS RCTs in children above 6 months of age almost all had follow-up for 12 months. The length of follow-up did not seem to play a major role.2
NVAS
In all six African NVAS RCTs, the tendency for a negative effect of NVAS on overall mortality only became apparent after the first months of life. In the Asian RCTs, nearly all mortality occurred in the first 1-2 months of life and/or these studies only followed children to 6 months of age, apart from the India 2014 RCT. The beneficial effect was strongest in the first months of life in the Asian RCTs as well, and in the India 2014 RCT there was a more negative effect in the second part of infancy, as seen in the African RCTs.13
Conclusion
The data from all RCTs are compatible with an increasingly negative effect of NVAS with increasing age/length of follow-up. This could be partly due to negative interaction with DTP vaccine. Thus, the length of follow-up is an important indicator of other exposures and could explain some of the heterogeneity in the NVAS RCTs, since the African NVAS RCTs had follow-up to 12–24 months whereas almost all the Asian NVAS RCTs only had follow-up to 4–6 months of age.
Birthweight
NVAS
Potential effect modification by birthweight has been assessed in some of the NVAS trials. NVAS had an increasingly negative effect with increasing birthweight/ponderal index in Guinea-Bissau and Zimbabwe,20,26,36,37 but not in Ghana.11 In the India 2003 RCT, the beneficial effect was limited to children with a low birthweight, but in contrast the most beneficial effect in Indonesia, India 2014 and to some extent Bangladesh was seen in the children weighing above 2500 g.13,22,47
Conclusion
Low nutritional status indices may be associated with VAD and a better effect of VAS in the smallest children could be expected. However, this is not the case. In Guinea-Bissau, higher birthweight is also a marker of early DTP vaccination.40 This could help explain the observed negative interaction between NVAS and higher birthweight seen in many of the African RCTs. The observed inconsistent interaction between NVAS and birthweight is more likely to be explained by confounding factors.
Season
Very few studies have addressed potential interaction between VAS and season, but in the Guinean RCTs it was done systematically. Guinea-Bissau has two quite distinct seasons, the dry season from December to May, and the rainy season from June to November.
VAS above 6 months of age
In the Guinea-Bissau RCT, the effect of VAS was not different in the dry [MRR = 0.79 (0.43-1.45)] and the rainy season [MRR = 1.06 (0.56-2.02)].14
NVAS
One of the Guinean NVAS RCTs showed interaction between NVAS and season, the effect of NVAS being beneficial in the dry but not the rainy season.36 This finding was not corroborated, however, in the two subsequent RCTs.20,37 In a small RCT, comparing NVAS with oral polio vaccine (OPV) in males only, the NVAS group experienced a cluster of deaths in the rainy season. However, due to the trial design it is not possible to say if this was caused by receiving NVAS or by not receiving OPV.48
Conclusion
Season is a potential effect modifier of VAS, which could be studied retrospectively in existing datasets overall and by sex, and which should be considered in future studies. Season is an evident marker of differences in pathogen exposure, and clarification of the relationship between season and the effect of VAS could provide clues to understanding more about the biological mechanisms underlying the variable effects of VAS.
Biological mechanisms
The results of the RCTs, which have been disappointing apart from the initial VAS RCTs in older children2 and a few RCTs in neonates in Asia,22,23,47 the lack of association between the degree of VAD and the effect of VAS, and the observed effect modifiers such as sex, vaccines and repeated doses of VAS (summarized in Table 1) suggest that the effect of VAS may not merely be mediated by restoration of vitamin A levels. These high-dose supplements may have long-lasting imprinting effects on the immune system.49
It is well described that vitamin A has several direct inhibitory short-term effects on the immune system. In line with this, vitamin A inhibits Th1 and Th17 responses, induces T regulatory cells, decreases pro-inflammatory cytokine production by the innate immune system and inhibits differentiation and maturation of monocytes into dendritic cells.50–52 In addition, it has recently been shown that ATRA (all-trans retinoic acid, the active metabolite of vitamin A) in vitro can induce epigenetic histone modifications in monocytes, leading to reduced gene transcription and down-regulated cytokine production.53 Epigenetic changes were long-lasting, raising the possibility that VAS could induce a long-term down-regulation of cytokine production by monocytes. Apart from histone modifications, changes in DNA methylation (another important epigenetic mark) could also play a causal role in the down-regulation of cytokine production, especially since ATRA in other cell types has been shown to be a regulator of DNA methylation. 54–56
The fact that BCG vaccination has opposite immunological effects to ATRA57 and is associated with strong reductions in all-cause mortality58,59 indicates that VAS could have negative effects on the immune system, maybe contributing to increased all-cause mortality observed for certain subgroups, e.g. females who receive DTP after NVAS. However, in other subgroups VAS had no effect, or beneficial effects on mortality were observed. It could be speculated that these contradictory effects are due to differences in epigenetic modulation in the different subgroups. Also, a down-regulated innate immune response may be beneficial in situations in which infections induce immunopathology. This could have contributed to the very strong beneficial effect of VAS observed in many of the original studies, where the pathogen exposure may have been both different and heavier. This could also contribute to explaining the conflicting results of the NVAS trials done in different regions with presumably different pathogen exposure.
Modification of epigenetic marks is a complex process that is dependent on many factors and can be induced or undone during different clinical conditions. Research assessing epigenetic changes induced in the innate immune system by vitamin A or vaccinations is only beginning and many questions are still to be answered,60 but it seems plausible that the observed variable effect of VAS could at least partially be due to epigenetic modifications. It also seems plausible that the outcome of such modifications may differ depending on the state of the immune system and the clinical conditions and challenges it faces.61 Noteworthy, observed immunological interactions between ATRA and BCG in vitro53 and between NVAS and DTP in both animals62 and humans63 and between NVAS and early MV in humans64 supports the hypothesis that these interventions interact. Thus, the effect of VAS may differ depending on the vaccine programme in a country. For instance, the exposure and the sequence of vaccination with BCG and DTP differed considerably between the different NVAS trial sites and such differences may have contributed to the divergent results.49
Discussion
VAD is associated with increased mortality, and high-dose VAS has been assumed to reduce overall mortality in children between 6 months and 5 years of age by more than 20% by preventing VAD. However, we are facing an enigma. Two new RCTs have not found any effect on mortality.14,15 In addition, RCTs providing VAS to younger children have had conflicting results; recently three WHO-commissioned NVAS RCTs hypothesized to find 15% reduction in overall mortality found no overall effect and even a tendency for negative effects in the two African RCTs.11–13
In a situation with considerable heterogeneity of results in rigorously conducted RCTs, it is clear that there must be important effect modifiers. This review has identified several potential effect modifiers, which may help explain the heterogeneity: sex, vaccines and repeated doses of VAS may all influence the effect of VAS (summarized in Table 1). In contrast, the effect did not differ in any systematic way by length of follow-up, birthweight or season.
To our knowledge we have presented all available data, with focus on data derived from RCTs, supported by observational studies where available. Unfortunately very few groups with the exception of ours have conducted subgroup analyses to explain the enigma. We have aimed to test our findings from subgroup analyses and the derived hypotheses in other groups’ datasets. However, this has usually not been possible.A clear weakness of the present review is therefore that we have had to rely on circumstantial evidence, such as for instance DTP vaccination coverage being low in the Asian NVAS trials, rather than on the ideal evidence from a statistical analysis testing whether the effect of NVAS in the Asian trials differed by DTP vaccination status within the trial.
Indeed, recent immunological research has supported the conclusion that VAS has important immunological effects and may interact with other immune-modulatory interventions. The studies identified that both VAS and vaccines may induce long-lasting epigenetic modulations of the innate immune system. For instance, BCG vaccine is associated with an up-regulation of the innate immune response,57 whereas ATRA is associated with a down-regulation, and BCG and ATRA interacted.53 Thus, it is entirely plausible that interventions with effects on the immune system, such as vitamin A and vaccines, may interact biologically; in fact it would be surprising if they did not. It also seems plausible that many other factors which affect the immune system may also modify the effect of VAS.
In a recent paper it was suggested that the lack of effect of VAS in the recent Indian RCT in older children was caused by changes in disease pattern and because the high doses are not efficient at increasing serum retinol levels and reducing VAD. A policy shift was proposed, towards increasing frequent regular intake of vitamin A, replacing the high-dose supplements.65 The Micronutrient Initiative is making plans for down-scaling vitamin supplementation in countries which—according to vitamin A status data—may no longer be in need of biannual VAS.66 The Global Alliance for Vitamin A, GAVA, recommends continuing VAS until < 5% of all children are suffering from VAD.67 These proposals are all based on the interpretation that VAS primarily works by preventing VAD.
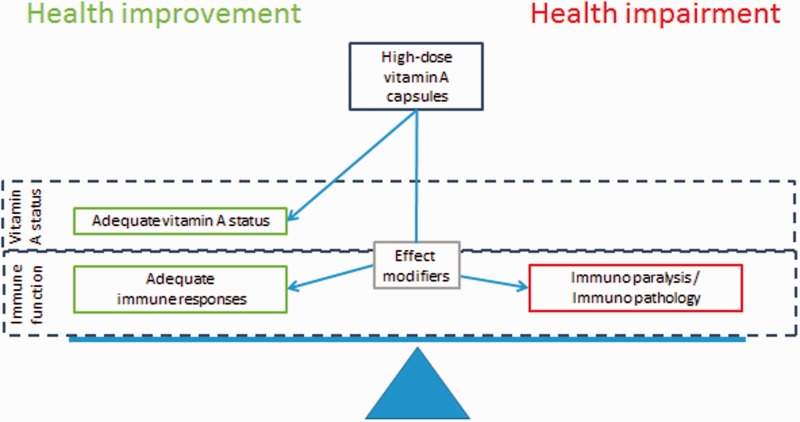
It is unquestionablya good idea to improve the diet and increase the intake of vitamin A among children in low-income countries, but we think that the underlying idea that VAS is all about preventing VAD is too simplistic.68 Whereas there is no doubt that VAD leads to increased mortality and VAS can reduce VAD and improve health, VAS also affects the immune system, often in complex interactions with other factors which influence the immune system. As illustrated in Figure 4, VAS prevents VAD and this is probably always beneficial. However, depending on the effect of VAS on the immune system, the overall net effect of providing VAS can be much more beneficial than anticipated from the VAD-preventing effect. Some subgroups may benefit from VAS even if they are not suffering from VAD (Figure 4), and stopping high-dose VAS in favour of increased dietary intake may lead to increased mortality in these subgroups. On the other hand VAS can also be harmful, if negative effects on the immune system outweigh the benefits by preventing VAD (Figure 4). This is particularly likely to happen if the prevalence of VAD is low, and therefore it is worrying that GAVA recommends continuing VAS to all children until < 5% of all children are suffering from VAD.67,68
Figure 4.
Vitamin A supplementation may affect both vitamin A status and immune function.
The negative RCTs have created an enigma—and that enigma obliges us to conduct more research and create optimal, evidence-based, vitamin A policies. In the first place, much can be gained by re-analysis of existing datasets. Second, although VAS to children above 6 months of age has been WHO policy for decades and RCTs have been judged unethical,69 the time has come for conducting new RCTs. Such RCTs should compare 4–6-monthly repeated high-dose VAS vs frequent low-dose VAS (for instance weekly doses) vs placebo. Since the vaccination programme has been the delivery channel for VAS in many countries, it would make sense to start by testing the effect of providing VAS with the first dose of MV at 9 months of age. Likewise RCTs of providing VAS with MV could be carried out in the countries that have introduced a second dose of MV in the second year of life. Ideally RCTs should be large-scale multi-centre trials, sized to assess potential effect modification by sex, vaccines and additional VAS. Only this way can we optimize the use of vitamin A, harvest the benefits and avoid harming some children.
Acknowledgements
This work was supported by the European Research Council (ERC-2009-StG, grant agreement no. 243149 to CSB). CVIVA is funded by the Danish National Research Foundation (DNRF108), which also paid the Open Access publication charges.
Conflict of interest: None declared.
References
- 1.Semba RD. Vitamin A as “anti-infective” therapy, 1920–1940. J Nutr 1999;129:783–91. [DOI] [PubMed] [Google Scholar]
- 2.Beaton GH, Martorell R, McCabe G, L’abbe KA, Edmonston B, Ross AC. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. University of Toronto, 1993. [Google Scholar]
- 3.Rahmathullah L, Underwood BA, Thulasiraj RD, et al. Reduced mortality among children in Southern India receiving a small weekly dose of vitamin A. N Engl J Med 1990;323:929–35. [DOI] [PubMed] [Google Scholar]
- 4.Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih, Karyadi D. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. Am J Clin Nutr 1988;48:1271–76. [DOI] [PubMed] [Google Scholar]
- 5.Vijayaraghavan K, Radhaiah G, Prakasam BS, Sarma KV, Reddy V. Effect of massive dose vitamin A on morbidity and mortality in Indian children. Lancet 1990;336:1342–45. [DOI] [PubMed] [Google Scholar]
- 6.Herrera MG, Nestel P, el Amin A, Fawzi WW, Mohamed KA, Weld L. Vitamin A supplementation and child survival. Lancet 1992; 340:267–71. [DOI] [PubMed] [Google Scholar]
- 7.WHO/UNICEF. Vitamin A Supplements: A Guide to Their Use in the Treatment and Prevention of Vitamin A Deficiency and Xerophthalmia. Geneva: WHO, 1997. [Google Scholar]
- 8.UNICEF. Vitamin A Supplementation. A Decade of Progress. New York, NY: UNICEF, 2007. [Google Scholar]
- 9.Benn CS, Bale C, Sommerfelt H, Friis H, Aaby P. Hypothesis: Vitamin A supplementation and childhood mortality: amplification of the non-specific effects of vaccines? Int J Epidemiol 2003;32:822–28. [DOI] [PubMed] [Google Scholar]
- 10.Gogia S, Sachdev HS. Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database Syst Rev 2011;10:CD007480. [DOI] [PubMed] [Google Scholar]
- 11.Edmond KM, Newton S, Shannon C, et al. Effect of early neonatal vitamin A supplementation on mortality during infancy in Ghana (Neovita): a randomized, double-blind, placebo-controlled trial. Lancet 2015;385:1315–23. [DOI] [PubMed] [Google Scholar]
- 12.Masanja H, Smith ER, Muhihi A, et al. Effect of neonatal vitamin A supplementation on mortality in infants in Tanzania (Neovita): a randomized, double-blind, placebo-controlled trial. Lancet 2015;385:1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazumder S, Taneja S, Bhatia K, et al. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): a randomized, double-blind, placebo-controlled trial. Lancet 2015;385:1333–42. [DOI] [PubMed] [Google Scholar]
- 14.Fisker AB, Bale C, Rodrigues A, et al. High-dose Vitamin A with vaccination after 6 months of age: a randomized trial. Pediatrics 2014;134:e739–48. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomized trial. Lancet 2013;381:1469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross DA. Vitamin A and childhood mortality. Ghana Vitamin A Supplementation Trials Study Team. Lancet 1993;342:861. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal DK, Pandey CM, Agarwal KN. Vitamin A administration and preschool child mortality. Nutr Res 1995;15:669–80. [Google Scholar]
- 18.Pant CR, Pokharel GP, Curtale F, et al. Impact of nutrition education and mega-dose vitamin A supplementation on the health of children in Nepal. Bull World Health Organ 1996;74:533–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Danneskiold-Samsoe N, Fisker AB, Jorgensen MJ, et al. Determinants of vitamin A deficiency in children between 6 months and 2 years of age in Guinea-Bissau. BMC Public Health 2013;13:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benn CS, Diness BR, Balde I, et al. Two different doses of supplemental vitamin A did not affect mortality of normal-birth-weight neonates in Guinea-Bissau in a randomized controlled trial. J Nutr 2014;144:1474–79. [DOI] [PubMed] [Google Scholar]
- 21.Fisker AB, Lisse IM, Aaby P, et al. Effect of vitamin A supplementation with BCG vaccine at birth on vitamin A status at 6 wk and 4 mo of age. Am J Clin Nutr 2007;86:1032–39. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey JH, Agoestina T, Wu L, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 1996;128:489–96. [DOI] [PubMed] [Google Scholar]
- 23.Rahmathullah L, Tielsch JM, Thulasiraj RD, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomized trial in southern India. BMJ 2003; 327:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkwood B, Humphrey J, Moulton L, Martines J. Neonatal vitamin A supplementation and infant survival. Lancet 2010;376:1643–44. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey JH, Iliff PJ, Marinda ET, et al Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis 2006;193:860–71. [DOI] [PubMed] [Google Scholar]
- 26.Malaba LC, Iliff PJ, Nathoo KJ, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr 2005;81:454–60. [DOI] [PubMed] [Google Scholar]
- 27.Benn CS, Fisker A, Aaby P. Heterogeneous effects on child survival in neonatal vitamin A supplementation trials. Lancet 2011;377:1314–15. [DOI] [PubMed] [Google Scholar]
- 28.Benn CS, Lund S, Fisker A, Jorgensen MJ, Aaby P. Should infant girls receive micronutrient supplements? Int J Epidemiol 2009;38:586–90. [DOI] [PubMed] [Google Scholar]
- 29.Aaby P, Martins CL, Garly ML, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomized controlled trial. BMJ 2010;341:c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013;34:431–39. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Meeting of the strategic advisory group of experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec 2014;89:221–36. [PubMed] [Google Scholar]
- 32.Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA. Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana Vitamin A Supplementation Trial. Am J Clin Nutr 2009;90:629–39. [DOI] [PubMed] [Google Scholar]
- 34.Benn CS, Martins C, Rodrigues A, et al. The effect of vitamin A supplementation administered with missing vaccines during national immunization days in Guinea-Bissau. Int J Epidemiol 2009;38:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisker AB, Aaby P, Bale C, et al. Does the effect of vitamin A supplements depend on vaccination status? An observational study from Guinea-Bissau. BMJ Open 2012;2:e000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn CS, Diness BR, Roth A, et al. Effect of 50 000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomized placebo controlled trial. BMJ 2008;336:1416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benn CS, Fisker AB, Napirna BM, et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomized controlled trial. BMJ 2010;340:c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benn CS, Rodrigues A, Yazdanbakhsh M, et al. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine 2009;27:2891–98. [DOI] [PubMed] [Google Scholar]
- 39.Aaby P, Nielsen J, Benn CS, Trape JF. Sex-differential and non-specific effects of routine vaccinations in a rural area with low vaccination coverage: an observational study from Senegal. Trans R Soc Trop Med Hyg 2015;109:77–84. [DOI] [PubMed] [Google Scholar]
- 40.Aaby P, Ravn H, Roth A, et al. Early diphtheria-tetanus-pertussis vaccination associated with higher female mortality and no difference in male mortality in a cohort of low birthweight children: an observational study within a randomized trial. Arch Dis Child 2012;97:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO/SAGE. http://www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf
- 42.Moulton LH, Rahmathullah L, Halsey NA, Thulasiraj RD, Katz J, Tielsch JM. Evaluation of non-specific effects of infant immunizations on early infant mortality in a southern Indian population. Trop Med Int Health 2005;10:947–55. [DOI] [PubMed] [Google Scholar]
- 43.Benn CS, Martins CL, Fisker AB, et al. Interaction between neonatal vitamin A supplementation and timing of measles vaccination: a retrospective analysis of three randomized trials from Guinea-Bissau. Vaccine 2014;32:5468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisker AB, Aaby P, Rodrigues A, Frydenberg M, Bibby BM, Benn CS. Vitamin A supplementation at birth might prime the response to subsequent vitamin A supplements in girls. Three year follow-up of a randomized trial. PLoS One 2011;6:e23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakymenko D, Benn CS, Martins C, et al. The impact of different doses of vitamin A supplementation on male and female mortality. A randomized trial from Guinea-Bissau. BMC Pediatr 2011;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West KP, Jr, Pokhrel RP, Katz J, et al. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 1991;338:67–71. [DOI] [PubMed] [Google Scholar]
- 47.Klemm RDW, Labrique AB, Christian P, et al. Newborn Vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics 2008;122:e242–e50. [DOI] [PubMed] [Google Scholar]
- 48.Lund N, Biering-Sorensen S, Andersen A, et al. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomized trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC Pediatr 2014;14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benn CS. Combining Vitamin A and vaccines: convenience or conflict? Dan Med J 2012;59:B4378. [PubMed] [Google Scholar]
- 50.Wada Y, Hisamatsu T, Kamada N, Okamoto S, Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflamm Bowel Dis 2009;15:1548–56. [DOI] [PubMed] [Google Scholar]
- 51.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J Immunol 2014;192:2953–58. [DOI] [PubMed] [Google Scholar]
- 52.Tsai YC, Chang HW, Chang TT, Lee MS, Chu YT, Hung CH. Effects of all-trans retinoic acid on Th1- and Th2-related chemokines production in monocytes. Inflammation 2008;31:428–33. [DOI] [PubMed] [Google Scholar]
- 53.Arts RJ, Blok BA, van Crevel R, et al. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J Leukoc Biol 2015, May 1. pii: jlb.6AB0914-416R. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 54.Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol 2013;24:701–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazi F, Travaglini L, Carotti D, et al. Retinoic acid targets DNA-methyltransferases and histone deacetylases during APL blast differentiation in vitro and in vivo. Oncogene 2005;24:1820–30. [DOI] [PubMed] [Google Scholar]
- 56.Cheong HS, Lee HC, Park BL, et al. Epigenetic modification of retinoic acid-treated human embryonic stem cells. BMB Rep 2010;43:83035. [DOI] [PubMed] [Google Scholar]
- 57.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biering-Sorensen S, Aaby P, Napirna BM, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr Infect Dis J 2012;31(3):306–08. [DOI] [PubMed] [Google Scholar]
- 59.Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011;204:245–52. [DOI] [PubMed] [Google Scholar]
- 60.Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol 2014;29C:1–7. [DOI] [PubMed] [Google Scholar]
- 61.Raiten DJ, Ashour FA, Ross AC, et al. Inflammation and Nutritional Science for Programs/Policies and Interpretation of Research Evidence (INSPIRE). J Nutr 2015;145:1039S–108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jørgensen MJ, Hein-Kristensen L, Hempel C, et al. The effect of vitamin A supplementation and diphtheria–tetanus–pertussis vaccination on parasitaemia in an experimental murine malaria model. Scand J Infect Dis 2011;43:296–303. [DOI] [PubMed] [Google Scholar]
- 63.Jørgensen MJ, Fisker AB, Sartono E, et al. The effect of at-birth vitamin A supplementation on differential leucocyte counts and in vitro cytokine production: an immunological study nested within a randomized trial in Guinea-Bissau. Br J Nutr 2013;109:467–77. [DOI] [PubMed] [Google Scholar]
- 64.Jensen KJ, Sondergaard M, Andersen A, et al. A randomized trial of an early measles vaccine at 4(1/2) months of age in Guinea-bissau: sex-differential immunological effects. PLoS One 2014;9:e97536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mason J, Greiner T, Shrimpton R, Sanders D, Yukich J. Vitamin A policies need rethinking. Int J Epidemiol 2015;44:283–98. [DOI] [PubMed] [Google Scholar]
- 66.http://www.sightandlife.org/fileadmin/data/Magazine/2013/27_1_2013/Congress_report_a_framework_for_shifting_from_universal_vitamin_a_supplementation.pdf.
- 67.GAVA. http://wphna.org/wp-content/uploads/2014/12/2014-11-GAVA-rebuttal-of-IJE-commentary-here.pdf.
- 68.Benn CS, Fisker AB, Aaby P. Response to: J Mason et al. Vitamin A policies need rethinking. Int J Epidemiol 2015;44:366–67. [DOI] [PubMed] [Google Scholar]
- 69.Thorne-Lyman A, Fawzi WW. Improving child survival through vitamin A supplementation. BMJ 2011;343:d5294. [DOI] [PubMed] [Google Scholar]
- 70.West KP, Jr, Katz J, Shrestha SR, et al. Mortality of infants < 6 mo of age supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am J Clin Nutr 1995;62:143–48. [DOI] [PubMed] [Google Scholar]
- 71.Patwardhan VN, Kamel WW, Pharaon H. Studies on Vitamin A Deficiency in Infants and Young Children in Jordan. WHO report. Geneva: WHO, 1966. [Google Scholar]
- 72.Sommer A, Tarwotjo I, Djunaedi E, et al. Impact of vitamin A supplementation on childhood mortality. A randomized controlled community trial. Lancet 1986;1:1169–73. [DOI] [PubMed] [Google Scholar]
- 73.Mahalanabis D, Rahman MM, Wahed MA, Islam MA, Habte D. Vitamin A megadoses during early infancy on serum retinol concentration and acute side effects and residual effects on 6 month follow-up. Nutr Res 1997;17:649–59. [Google Scholar]
- 74.Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. BMJ 1992;304:207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]