Abstract
Although the rice growth response to FACE (free-air CO2 enrichment) has been widely studied and is considered important within the scientific community, few studies have attempted to examine the effects of FACE on the yield of indica rice, which is typically the parent of indica hybrids in China. The effects of FACE on the yield, yield components, biomass, N uptake and leaf photosynthesis of Yangdao 6 Hao (an indica rice) in China were examined over 2 years. The grain yield increased over 30%, the panicle number increased 12.4% on average, and the spikelet number per panicle also showed an average increase of 8.2% at elevated CO2. FACE caused a significant enhancement in both the filled spikelet percentage (+5.9%) and the individual grain weight (+3.0%). Compared with three prior FACE studies on rice, a similar enhancement of yield in hybrid indica was shown under FACE, with much a higher value than for the japonica rice cultivar (approximately + 13%) because of indica’s stronger sink generation and N uptake capacity, which help coordinate the C/N balance to avoid photosynthetic acclimation. The high enhancement of the indica rice yield under FACE holds promise for improved cultivar selection for future food security.
Since 1959, the concentrations of atmospheric carbon dioxide [CO2] have increased from approximately 318 to 400 μmol mol−1 and, depending on the anthropogenic emission rates, may reach 1000 μmol mol−1 by the end of the century1. Because the photosynthetic rate in C3 species under the current levels of ambient [CO2] is still below physiological saturation levels, it is anticipated that photosynthesis, and consequently productivity, for most crops will be stimulated by the higher atmospheric [CO2]2. In theory, an increase in [CO2] from 380 μmol mol−1 to 550 μmol mol−1 projected for the year 2050 would increase C3 photosynthesis by 38%3, as indicated by many experimental and analytical studies4,5.
Many early FACE studies have shown that rice yield increases (9–15%) were lower than expected due to photosynthetic acclimation5,6,7,8. In addition, researchers have found marked differences among rice cultivar responses to elevated [CO2]9,10,11,12. Hence, active selection and breeding for high CO2 responsiveness among rice varieties may provide a simple and direct strategy for increasing global yields and maintaining food security with climate change, but this potential has not received sufficient attention until recently13. China rice-FACE studies have already revealed hybrid rice genotypes with a greater yield enhancement (above 30%) under elevated [CO2] than conventional rice cultivars9,11. Previous FACE studies in Japan and China previously demonstrated that japonica rice exhibits a weak response to elevated [CO2]5,6,7,8. Therefore, we hope to find an indica rice genotype that has a similarly high enhancement under elevated CO2 as that exhibited by hybrid rice and that can be used under future climatic conditions.
Yangdao 6 Hao has large panicles, a high yield potential, resistance to disease and pathogens, and an anti-lodging ability, which suggest that this cultivar may be an important gene resource for rice breeding14. Hence, we chose this cultivar as the study subject. The aim of this study was to investigate whether the indica rice under consideration has a similarly strong response to elevated [CO2] as hybrid rice using FACE (free air carbon dioxide enrichment) treatment.
Results
Effects of CO2 on grain yield
FACE significantly increased the grain yield of the rice (P < 0.01) (Table 1). The enhancement of the grain yield was 36.2% and 29.6% in 2012 and 2014, respectively. There was a strong effect of year on the yield response (P < 0.05) (Table 1), but interactions between CO2 and year were not detected.
Table 1. Effect of free air CO2 enrichment (FACE) on yield and its components of Yangdao 6 Hao in the 2012 and 2014 seasons.
| Year | CO2 | Panicle number (m−2) | Spikelets per panicle | Filled ratio (%) | Single grain weight (mg) | Yield (g m2) |
|---|---|---|---|---|---|---|
| 2012 | Ambient | 206.2 ± 9.6 | 156 ± 8.6 | 79.2 ± 0.4 | 34.7 ± 0.5 | 882.7 ± 40.0 |
| FACE | 234.0 ± 13.1 | 170.9 ± 8.7 | 83.8 ± 1.2 | 35.9 ± 0.2 | 1201.8 ± 83.6 | |
| Change % | 13.5 | 9.6 | 5.8 | 3.5 | 36.2 | |
| 2014 | Ambient | 215.1 ± 17.1 | 142.1 ± 7.2 | 76.4 ± 3.1 | 34.4 ± 0.4 | 798.9 ± 66.0 |
| FACE | 239.3 ± 18.3 | 151.7 ± 3.2 | 80.9 ± 1.9 | 35.2 ± 0.3 | 1035.0 ± 118.1 | |
| Change % | 11.2 | 6.8 | 5.9 | 2.3 | 29.6 | |
| ANOVA results | ||||||
| Year | ns | ** | * | * | * | |
| CO2 | * | * | ** | ** | ** | |
| Year* CO2 | ns | ns | ns | ns | ns | |
ns, not significant; *P < 0.05; **P < 0.01.
Effects of CO2 on yield components
As shown in Table 1, the panicle number per m2 was increased to a similar extent (13.5% for 2012 and 11.2% for 2014, P < 0.05) under elevated [CO2], and there was no significant interaction between CO2 and year. The number of spikelets per panicle increased by 9.6% for 2012 and by 6.8% for 2014, and there was a strong year effect (P < 0.01). There was no interactive effect of CO2 × year on spikelets per panicle (Table 1).
For the filled spikelet percentage, FACE rice showed a similar increase in the two years (5.8% for 2012 and 5.9% for 2014). The individual grain weight increased by 3.5% and 2.3% for FACE vs. ambient plants in 2012 and 2014, respectively (Table 1). The interaction between CO2 and year was not detected for the two yield components.
Effects of CO2 on phenology, shoot and tiller biomass, and plant height
There was no change in phenology upon reaching 50% panicle emergence and grain maturity (Table 2). In contrast, FACE significantly increased the shoot and tiller biomass and plant height at maturity. When averaged across years, the shoot and tiller biomass and plant height were increased by 29.0%, 14.3% and 4.5%, respectively. There was no interactive effect of CO2 × year on phenology, shoot and tiller biomass or plant height (Fig. 1 and Table 2).
Table 2. Summary of experimental background (years, locations, test cultivars), absolute response (d) in phenology and relative responses (%) in plant height, grain yield and its components of rice crops grown under elevated [CO2] (ambient + 200 μmol mol−1) relative to ambient [CO2] in the Japanese and Chinese rice FACE experiments.
| Item | Japan FACE | Japan FACE | China FACE | China FACE | China FACE | |
|---|---|---|---|---|---|---|
| Experimental location | Iwate (39°38′N, 140°57′E) | Tsukubamirai (35°58′N, 139°60′E) | Wuxi (31°37′N, 120°28′E) | Yangzhou (32°35.5′N, 119°42′E) | Yangzhou (32°35.5′N, 119°42′E) | |
| Test variety | Akitatakomachi | Koshihikari | Wuxiangjing 14 | Shanyou 63 | Liangyoupeijiu | Yangdao 6 Hao |
| Genotype | Japonica | Japonica | Japonica | Hybrid indica | Hybrid indica | Indica |
| Experimental years | 1998–2000 | 2010–2012 | 2001–2003 | 2004–2006 | 2005–2006 | 2012 and 2014 |
| N level (g m−2) | 4, 8 or 9, 12 or 14 | 8, 12 | 12, 25, 35 | 12.5, 25 | 12.5, 25 | 22.5 |
| [CO2] target | +200 ppm | +200 ppm | +200 ppm | +200 ppm | +200 ppm | +200 ppm |
| Phenology | ||||||
| Heading | −2 d | −2 d | −3.4 d* | +1.3 d ns | −1.5 d* | 0 d ns |
| Maturity | −1–0 d | – | −5.8 d* | 0 d ns | −0.1 d ns | 0 d ns |
| Plant height | – | – | −2.2% ns | +6.7%* | +6.1%* | +4.5%* |
| Yield | +12.8%** | +16.3%* | +12.8%* | +34.1%** | +30.1%** | 32.9%** |
| Yield components | ||||||
| Panicle number per m2 | +8.6%* | +9.3%** | 18.8%** | +10.3%** | +7.8%** | +12.4%* |
| Spikelet number per panicle | +1.9%** | +2.2% ns | −7.6%** | +10.3%** | +9.6%** | +8.2%* |
| Filled spikelet percentage | +0.8% ns | +3.4%* | +4.9%** | +4.9%** | +5.3%** | +5.9%** |
| Single grain weight | +1.3%* | +0.5% ns | +1.3%** | +4.3%** | +4.4%** | +3.0%** |
| Data sources | Kobayashi et al.24, Kim et al.6 | Hasegawa et al.12 | Yang et al.8 | Liu et al.9 | Yang et al.11 | Table 2 and Fig. 1 in this paper |
Figure 1. Shoot biomass and plant height of plants grown under ambient CO2 and free-air CO2 enrichment conditions (FACE, 200 μmol mol−1 above ambient conditions) at maturity in the 2012 and 2014 seasons.
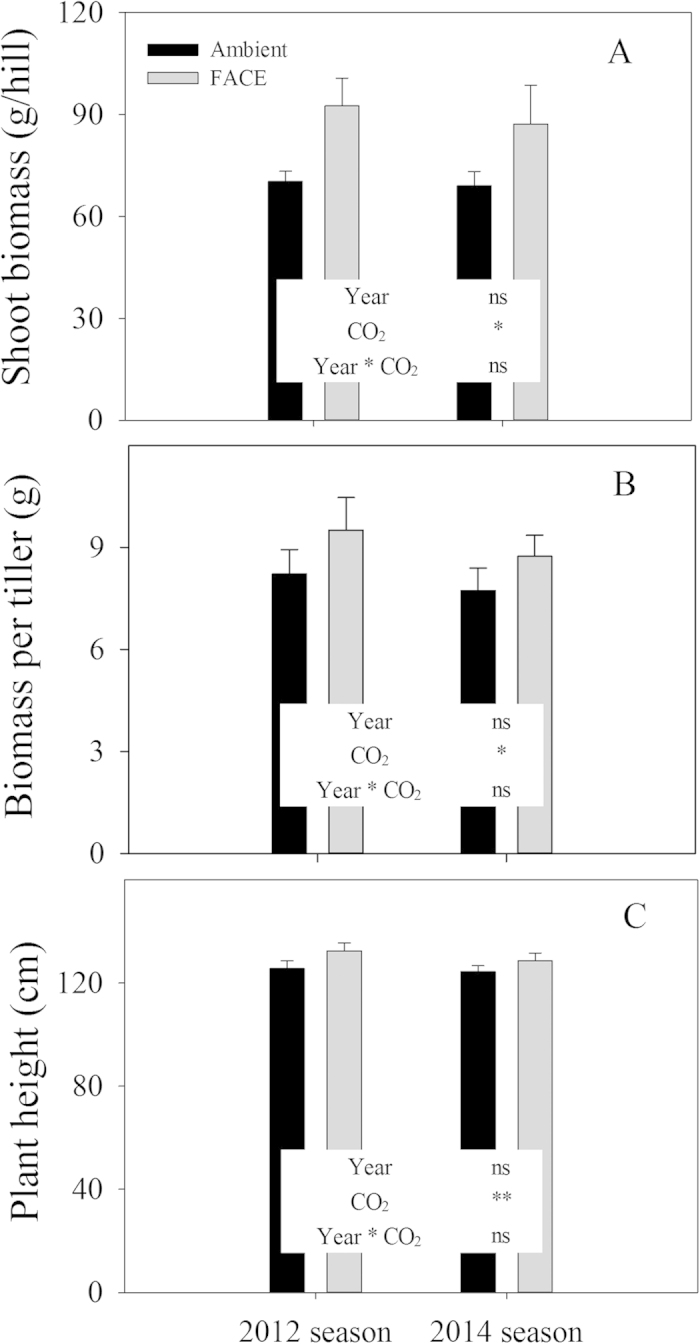
*Indicates P < 0.05 between CO2 levels. ns, not significant; *P < 0.05; **P < 0.01.
Effects of CO2 on gas exchange
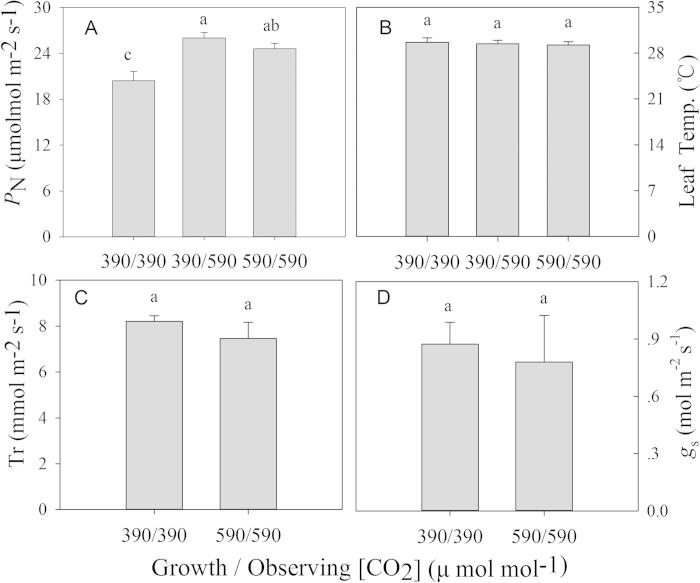
There was a significant stimulation of flag leaf photosynthesis with elevated [CO2] (relative to ambient) observed at the mid-filling stage for plants grown under either ambient or FACE conditions (Fig. 2A). There was no difference in the net photosynthetic carbon assimilation rate of plants grown under ambient and FACE conditions when measured at the same [CO2] (590 μmol mol−1), indicating that the photosynthetic efficiency was not reduced by elevated [CO2], even at the mid-filling stage (Fig. 2A). The leaf temperatures were not different during measurement. The stomatal conductance and transpiration were not significantly different under ambient CO2 and FACE conditions, although there was a declining trend due to the elevated [CO2] (Fig. 2B,C). Considering the meaningless in stomatal conductance and transpiration with elevated [CO2] for plants grown under ambient conditions, we did not show these parameters.
Figure 2. Photosynthetic gas exchange measurements taken at the respective growth [CO2] conditions (ambient and FACE: 390 and 590 μmol mol−1, respectively) on a light-saturated flag leaf at the mid-filling stage in the 2014 season.
(A) Net photosynthetic carbon assimilation rate (PN); (B) leaf temperature; (C) transpiration rate (TR); (D) stomatal conductance to water vapor (gs). 390/390 and 390/590: grown under ambient conditions and measured at 390 and 590 μmol mol−1 [CO2], respectively; 590/590: grown under FACE conditions and measured at 590 μmol mol−1 [CO2]. The mean was the average of 3 replications (n = 3) ± SD. ns, not significant; *P < 0.05; **P < 0.01.
N uptake
Averaged across two years, the N uptake in the FACE condition was increased by 15.9% during the vegetative stages (from transplanting to heading) and 15.7% during the reproductive stages (from heading to maturity) (Fig. 3). There was a clear variation between the two growth periods (Fig. 3), but the CO2 effect and stage interactions were not significant.
Figure 3. Shoot N uptake during the vegetative (from transplanting to heading) and reproductive (from heading to maturity) stages of rice grown under ambient CO2 and free-air CO2 enrichment (FACE, 200 μmol mol−1 above ambient) conditions in the 2012 and 2014 seasons.
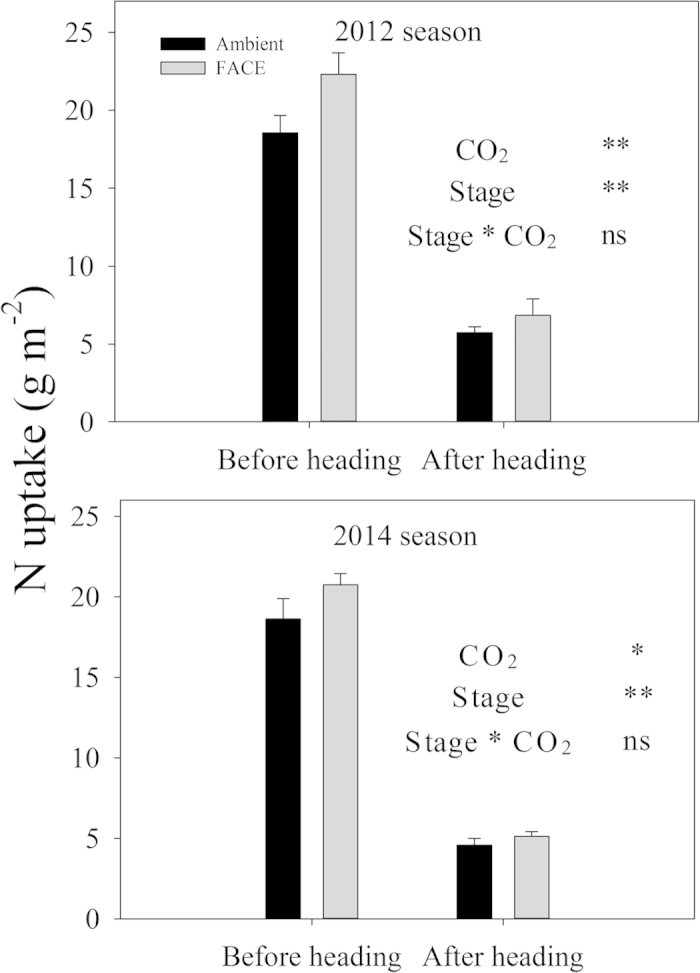
ns, not significant; *P < 0.05; **P < 0.01.
Discussion
The 2-year FACE study showed that elevated [CO2] increased the yield of Yangdao 6 Hao by over 30% (Table 1), which was a similar enhancement to that of hybrid rice in previous reports of China FACE9,11. This value is far higher than the range reported in inbred japonica rice FACE studies5,8,10,12. Undoubtedly, this study reveals the potential for taking full advantage of higher [CO2] levels for inbred rice varieties.
The FACE condition significantly enhanced the panicle density by 12.4% in Yangdao 6 Hao (Table 2), 10.3% in Shanyou 63 and 7.8% in Liangyoupeijiu9,11, all of which are smaller than the 18.8% increase in Wuxiangjing 14 in China Wuxi FACE8. The different responses to FACE among these varieties may be associated with differences in the dimensions of the leaf laminae9. Compared to rice cultivars with small and erect leaf blades (e.g., Wuxiangjing 14), Yangdao 6 Hao, Shanyou 63 and Liangyoupeijiu, with large and drooping leaves, would suffer more from mutual shading during crop development, thus presumably resulting in a weak stimulation of CO2 induction for tillering and the resulting panicle number9.
In the present study, the number of spikelets per panicle in Yangdao 6 Hao increased by 8.2%, which was slightly lower than in hybrid indica but higher than in japonica under FACE conditions (Table 2). Shimono et al. reported that the N uptake before the heading stage was closely correlated with the spikelet density rather than the [CO2] and the cultivar type10. N uptake by Yangdao 6 Hao increased by 15.9% before the heading stage under elevated [CO2]. The enhanced N uptake before heading was beneficial to increasing the spikelet number for Yangdao 6 Hao under FACE (Tables 1 and 2). In addition, the substantial enhancement in panicle size in this study was supported by the corresponding responses of plant height (Fig. 1A) and shoot biomass (Fig. 1B, Table 2) to elevated CO2, which were consistent with the findings that the height and tiller biomass were correlated positively and significantly with panicle size15. The spikelet number per panicle is the result of the difference in the number of differentiated and degenerated spikelets8. It is well accepted that cytokinins are mainly produced in the plant root and distributed in the shoot by the transpiration stream16,17, which impacts rice spikelet formation and development. The decreased spikelet number of japonica rice varieties with a low response to elevated CO2 in China FACE (Table 2) may be attributed to the decrease in root activity that reduces cytokinin synthesis18,19. This condition is unlikely to be the case for Yangdao 6 Hao, in which the spikelet number per panicle and the N uptake of the vegetative and reproductive stages were significantly increased by the elevated CO2. These findings suggest that Yangdao 6 Hao can maintain root activity for cytokinin synthesis under elevated CO2. However, the potential physiological and molecular mechanisms underlying the different responses to elevated CO2 require further study.
Elevated [CO2] increased the actual grain sink per panicle (filled spikelet ratio × spikelet number × per panicle grain weight) by 18.0% in this study (Table 2), which is slightly lower than in hybrid rice but higher than the values obtained in previous FACE studies6,8,9. Obviously, grain-filling abilities are related to photosynthate assimilation after heading. At the mid-filling stage, Yangdao 6 Hao maintained a strong increase in the net photosynthetic carbon assimilation rate and avoided photosynthetic acclimation under elevated [CO2] (Fig. 2A). This response is similar to that exhibited by the hybrid rice Shanyou 63, for which elevated [CO2] resulted in an increased spikelet number and grain weight, increased sink:source ratio, and continued stimulation of photosynthesis up to grain maturity20. Overall, these results suggest that the greater response of this rice line to elevated [CO2] may be associated with enhanced panicle sinks relative to sources and the ability to maintain photosynthetic capacity during grain development. Under FACE conditions, these rice lines can avoid the photosynthetic acclimation that is common in C3 cereals5,12,20,21. As a result of the balance between carbon and nitrogen metabolism within the leaf under elevated [CO2], the greater sink and the significant enhancement of N uptake during the filling stage (Fig. 2), these plants avoided the suppression of photosynthetic system genes and the resulting decrease in photosynthetic capacity20,21. Maintaining photosynthetic efficiency during the grain-filling stage ensures a strong yield enhancement under FACE conditions.
This is the first study to confirm that an inbred indica genotype exhibits a yield enhancement similar to that of hybrid rice under elevated [CO2]. To ensure food security in the future, additional indica genotypes with potentially strong responses to elevated [CO2] should be evaluated to take full advantage of the predicted increases in [CO2].
Materials and Methods
Research site
The experiment was conducted at the FACE facility located in Zhongcun Village (119°42’0”E, 32°35’5”N), Yangzhou City, Jiangsu Province, a typical Chinese rice-growing region22. The soil was classified as Shajiang-Aquic Cambiosol with a sandy loam texture. The soil properties at 0–15 cm relevant to this experiment are as follows: bulk density 1.16 g cm−3, soil organic carbon 18.4 g kg−1, total nitrogen 1.45 g kg−1, available phosphorous 10.1 mg kg−1, available potassium 70.5 mg kg−1, and pH 6.823.
The operation and control systems for the FACE facilities were the same as those used at the Japan FACE site24: three identical octagonal rings with the target [CO2] concentration at the center 200 μmol mol−1 higher than ambient conditions (hereinafter referred to as FACE) and three comparison rings of ambient [CO2]. During the 2012 and 2014 seasons, the average daytime [CO2] levels at the canopy height during the experiment were 378 and 577 μmol mol−1 and 394 and 590 μmol mol−1 for the ambient and elevated FACE rings, respectively. The average temperature during the growing season was 24.4 °C and 22.1 °C for 2012 and 2014, respectively.
Rice cultivation
An indica rice (Oryza sativa) line (Yangdao 6 Hao) was selected in this study. Seeds were sown at ambient [CO2] on May 20, 2012 and 2014. The seedlings were then manually transplanted to ambient and elevated FACE rings at a density of one seedling per hill on June 21. The spacing of the hills was 16.7 cm × 25 cm (equivalent to 24 hills m−2). N was applied as a basal dressing (40% of the total) 1 day prior to transplanting and as a top dressing at early tillering (30% of the total) and at the panicle initiation (PI) stage (30% of the total) at 22.5 g N m−2. Phosphorous (P) and potassium (K) were applied as a compound fertilizer at 9 g P2O5 m−2 and 9 g K2O m−2; both P and K were applied as a basal dressing 1 day before transplanting.
Photosynthetic gas exchange measurement
Before the measurements, we measured the Chl content in 5–6 flag leaves per treatment plot non-destructively using a Chl meter (SPAD-502, Konica Minolta Optics, Inc., Japan). We then used two leaves with representative Chl content for the gas exchange measurements with a portable photosynthesis system with blue and red LED light sources (LI-6400, LI-COR Bioscience, USA). This measurement was conducted between 09:30 and 14:30 h on September 21, 2014 (mid-filling stage); the block temperature in the cuvette was fixed at 28 °C, and the photosynthetic photon flux density was fixed at 1,800 μmol m−2 s−1, with a flow rate of 500 μmol s−1. A 6400-01 CO2 injector attached to the main system was used to control the [CO2] in the cuvette. PN at the respective [CO2] (390 and 590 μmol mol−1 for the control plants and 590 μmol mol−1 for the FACE plants) was recorded after the stomatal conductance had stabilized.
Rice sampling and biomass measurements
The rice plants were sampled at the heading stage and at grain maturity. Six hills per plot were randomly selected and destructively sampled. The samples were separated into green and senescent leaves, stems (including leaf sheaths), and panicles.
All the plant parts were oven-dried at 80 °C to constant weight before being weighed. The N content in plant tissue was measured using an elemental analyzer (PE 2400, Series II CHNS/O, US). The N uptake was determined by multiplying the N content by the biomass of the panicles, stems and leaves.
The grain yield and yield components were measured according to Zhu et al.20. At the maturity stage, the grain yield component characteristics (i.e., panicles per m2, spikelet number per panicle, filled spikelet percentage and individual grain weight) were tested using six hills of rice. In addition, a 1.5 m2 area of rice was harvested at ground level and separated into straw and grain components. The collected grains (seeds) were soaked in 1.00 specific gravity tap water, and the number of sunken and floated grains were counted to determine the filled spikelet percentage. The dry weight of the ripened (sunken) grains was measured after they were oven dried at 80 °C for 72 h. The weight per grain and grain yield were expressed by incorporating a 14% moisture content basis8.
Statistical analysis
The experimental design was a split plot arranged within a randomized complete block with 3 replications (three rectangular paddy fields). Using the software Statistical Package for the Social Sciences 19.0 (SPSS Inc., Chicago, USA), we first performed an analysis of variance for the main factors of [CO2] and year on the yield and its components, including biomass and plant height, as shown in Table 1 and Fig. 1. We also performed an analysis of variance for the main factors of [CO2] and stage on N uptake, illustrated in Fig. 3. In addition, post hoc comparisons were performed to detect the effects of [CO2] on the gas-exchange parameters, as shown in Fig. 2.
Additional Information
How to cite this article: Zhu, C. et al. An indica rice genotype showed a similar yield enhancement to that of hybrid rice under free air carbon dioxide enrichment. Sci. Rep. 5, 12719; doi: 10.1038/srep12719 (2015).
Acknowledgments
The work was supported by the National Basic Research Program (973 Program, 2014CB954500), the National Natural Science Foundation of China (Grant Nos 41301209, 31370457, 31261140364, and 31201126) and the Natural Science Foundation of Jiangsu Province in China (Grant Nos BK20131051 and BK20140063). The FACE system instruments were supplied by the National Institute of Agro-Environmental Sciences and the Agricultural Research Center of Tohoku Region (Japan).
Footnotes
Author Contributions C.W. and X.X. conceived the experiments; J.Z. and G.L. performed the experiments; X.X. and C.W. co-wrote the paper; D.W., J.Z. and G.L. analyzed the data and edited the manuscript. All authors reviewed the manuscript.
References
- Meinshausen M. et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Change 109, 213–241 (2011). [Google Scholar]
- Nasser R.R., Fuller M.P. & Jellings A.J. Effect of elevated CO2 and nitrogen levels on lentil growth and nodulation. Agron. Sustain. Dev. 28, 175–180 (2008). [Google Scholar]
- Long S.P. et al. Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628 (2004). [DOI] [PubMed] [Google Scholar]
- Ainsworth E.A. & Long S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372 (2005). [DOI] [PubMed] [Google Scholar]
- Long S.P. et al. Food for thought: Lower than expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921 (2006). [DOI] [PubMed] [Google Scholar]
- Kim H.Y. et al. Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Res. 83, 261–270 (2003). [Google Scholar]
- Chen G.Y. et al. Photosynthetic acclimation in rice leaves to free-air CO2 enrichment related to both ribulose-1,5-bisphosphate carboxylation limitation and ribulose-1,5-bisphosphate regeneration limitation. Plant Cell Physiol. 46, 1036–1045 (2005). [DOI] [PubMed] [Google Scholar]
- Yang L.X. et al. The impact of free-air CO2 enrichment (FACE) and N supply on yield formation of rice crops with large panicle. Field Crops Res. 98, 141–150 (2006). [Google Scholar]
- Liu H.J. et al. Yield formation of CO2-enriched hybrid rice cultivar Shanyou 63 under fully open-air field conditions. Field Crops Res. 108, 93–100 (2008). [Google Scholar]
- Shimono H. & Bunce J.A. Acclimation of nitrogen uptake capacity of rice to elevated atmospheric CO2 concentration. Ann. Bot. 103, 87–94 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.X. et al. Yield formation of CO2-enriched inter-subspecific hybrid rice cultivar Liangyoupeijiu under fully open-air field condition in a warm sub-tropical climate. Agr. Ecosyst. Environ. 129, 193–200 (2009). [Google Scholar]
- Hasegawa T. et al. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 40, 148–159 (2013). [DOI] [PubMed] [Google Scholar]
- Ziska L.H. et al. Food security and climate change: on the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. P. Roy. Soc. B. Biol. Sci. 279, 4097–4105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.X. et al. Combining predominance of elite restorer lines Yangdao 6. J. Yangzhou Univ. (Agricultural and Life Science Edition). 29, 49–53 (2008). (In Chinese with English Abstract). [Google Scholar]
- Ling Q.H. et al. New theory in rice production. Science Press, Beijing (in Chinese) (1994).
- Ashikari M. et al. Cytokinin oxidase regulates rice grain production. Science 309 (5735), 741–745 (2005). [DOI] [PubMed] [Google Scholar]
- Aloni R. et al. Root-synthesized cytokinin in Arabidopsis is distributed in the shoot by the transpiration stream. J. Exp. Bot. 56 (416), 1535–1544 (2005). [DOI] [PubMed] [Google Scholar]
- Yang L.X. et al. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on growth, morphology and physiology of rice root at three levels of nitrogen fertilization. Glob. Change Biol. 14, 1844–1853 (2008). [Google Scholar]
- Shimono H. et al. Genotypic variation in rice yield enhancement by elevated CO2 relates to growth before heading, and not to maturity group. J. Exp. Bot. 60, 523–532 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.W. et al. Biochemical and molecular characteristics of leaf photosynthesis and relative seed yield of two contrasting rice cultivars in response to elevated [CO2]. J. Exp. Bot. 65, 1535–1544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B.D. et al. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 22, 567–582 (1999). [Google Scholar]
- Zhu C.W. et al. The temporal and species dynamics of photosynthetic acclimation in flag leaves of rice (Oryza sativa) and wheat (Triticum aestivum) under elevated carbon dioxide. Physiol. Plant. 145, 395–405 (2012). [DOI] [PubMed] [Google Scholar]
- Xie B.H. et al. Influences of free-air CO2 enrichment (FACE), nitrogen fertilizer and crop residue incorporation on CH4 emissions from irrigated rice fields. Nutr. Cycl. Agroecosys. 93, 373–385 (2012). [Google Scholar]
- Kobayashi K. et al. In Ecological Studies Series. 87–104 (Springer Verlag, Berlin, 2006).