Abstract
IL-4/IL-13-induced alternatively activated macrophages (M(IL-4/IL-13), AAMs or M2) are known to express E-cadherin, enabling them to engage in heterotypic cellular interactions and IL-4-driven macrophage fusion in vitro. Here we show that E-cadherin overexpression in Raw 264.7 macrophages inhibits their inflammatory response to LPS stimulation, as demonstrated by a reduced secretion of inflammatory mediators like interleukin (IL)-6, tumor necrosis factor (TNF) and nitric oxide (NO). To study the function of E-cadherin in M(IL-4/IL-13) macrophages in vivo, we generated macrophage-specific E-cadherin-deficient C57BL/6 mice. Using this new tool, we analyzed immunological parameters during two typical AAM-associated Th2-driven diseases and assessed Th2-associated granuloma formation. Although E-cadherin is strongly induced in AAMs during Taenia crassiceps helminth infections and allergic airway inflammation, its deletion in macrophages does not affect the course of both Th2 cytokine-driven diseases. Moreover, macrophage E-cadherin expression is largely redundant for granuloma formation around Schistosoma mansoni ova. Overall, we conclude that E-cadherin is a valuable AAM marker which suppresses the inflammatory response when overexpressed. Yet E-cadherin deletion in macrophages does not affect M(LPS+IFNγ) and M(IL-4) polarization in vitro, nor in vivo macrophage function, at least in the conditions tested.
Depending on the microenvironment, macrophages are polarized to different subsets which have been broadly classified as M1 and M2. Classically activated macrophages (CAMs or M1) are induced by Th1 inflammatory cytokines, such as IFN-γ, and by microbial or endogenous danger-associated molecules. According to the latest nomenclature guidelines, CAMs or M1 are now classified as M(LPS), M(IFNγ) or M(LPS+IFNγ), depending on the activators used to generate them1. These inflammatory macrophages produce cytokines like IL-1β, IL-6, IL-12 and TNF and express high levels of inducible nitric oxide synthase (iNOS)2, which makes them potent effector cells to combat microorganisms and potentially also tumor cells. Macrophages are also activated by anti-inflammatory mediators, including the Th2 cytokines interleukin-4 (IL-4) and IL-13, IL-10, transforming growth factor-β (TGF-β), glucocorticoids, and immune complexes. While all these types of ‘non-M1’ macrophages are often grouped under the generic term M23, this nomenclature is often indistinct and confusing1,4. Therefore, the formerly so-called IL-4 (and/or IL-13)-induced alternatively activated macrophages (AAMs) are now termed M(IL-4) or M(IL-4/IL-13)1. M(IL-4/IL-13) inhibit Th1/M1-driven inflammatory responses, promote Th2 responses, induce angiogenesis and wound repair, and can be immunosuppressive5. However, it is not fully-understood how these diverse functions are regulated at the molecular level.
We previously identified E-cadherin as a novel IL-4/IL-13-induced, STAT6/polyamine-dependent marker for M(IL-4/IL-13)6,7. E-cadherin co-localizes with β- and p120-catenin at the cell surface, enabling M(IL-4/IL-13) macrophages to undergo homotypic adhesive interactions, leading to cell fusion upon IL-4 treatment in vitro. Macrophages still fuse in the absence of E-cadherin, but the number of nuclei in each giant cell and their size is reduced6. In fact, different IL-4-induced molecules, including E-cadherin, DC-STAMP and TREM-2, need to cooperate to induce fusion-competent macrophages8,9. Furthermore, E-cadherin+ macrophages engage in heterotypic interactions with KLRG1+ and CD103+ cells in vitro. Upon ligation, KLRG1 inhibits TCR signaling and NK cytotoxicity which could be a way for E-cadherin+ cells to impair inflammatory immune responses10. Interestingly, CD103 is found on major mediators of the immune response, such as DC and T cell subsets11,12. Hence, E-cadherin might serve to bring these cells in closer contact with macrophages, thereby potentially influencing their retention and phenotype during polarized Th2 responses.
Besides their function in cell adhesion, E-cadherin and its associated catenins may modulate intracellular signaling molecules, including β-catenin/Wnt13, phosphatidylinositol 3-kinase (PI3K)14, Rho-family GTPases15 and NFκB16,17,18. As such, E-cadherin reduces the inflammatory response in keratinocytes and epithelial cells. Furthermore, KLRG1 engagement of E-cadherin on DCs lowers their secretion of inflammatory cytokines, thereby exerting immunosuppressive effects19. Hence, it is conceivable that the E-cadherin/catenin complex might exert similar activities in macrophages and could contribute to the anti-inflammatory character and immunoregulatory capacity of alternatively activated M(IL-4/IL-13) during Th2 responses.
We demonstrate here that E-cadherin overexpression indeed suppresses the secretion of inflammatory mediators. Yet, while E-cadherin is a valuable marker for polarized Th2 responses and M(IL-4/IL-13), its macrophage-specific deletion has no major in vivo effects on macrophage activity during Th2 responses, nor on cell fusion during Schistosoma mansoni granuloma formation.
Results
E-cadherin expression in Raw264.7 macrophages reduces their inflammatory phenotype upon stimulation
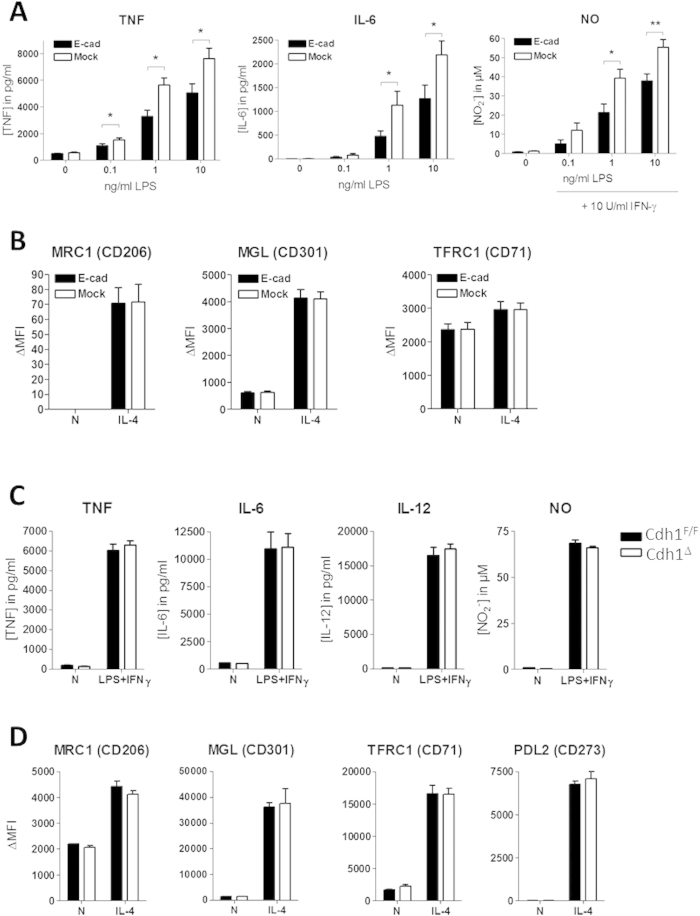
Besides its well-described role as an adhesive molecule, E-cadherin influences inflammatory signaling pathways such as NF-κB and thereby inhibits the inflammatory activation of various cell types16,17,18. As such, E-cadherin is now emerging as a potentially important immunological regulator20,21. To test the effect of E-cadherin expression on inflammatory cues in macrophages, we generated four independent E-cadherin over-expressing Raw264.7 transfectants and four E-cadherin-negative mock transfectants (Figure S1A). LPS dose-dependently induced TNF and IL-6 secretion by Raw264.7 macrophages after 24 h stimulation and this response was significantly lowered in E-cadherin-overexpressing transfectants (Fig. 1A). Even at the highest LPS concentration, E-cadherin was still able to inhibit LPS-induced production of inflammatory cytokines. Moreover, NO induction by IFNγ and increasing concentrations of LPS was reduced in macrophages that express E-cadherin. Conversely, E-cadherin overexpression did not affect M(IL-4) polarization as illustrated by the unaltered IL-4-induced surface expression of Macrophage Mannose Receptor (MMR, CD206), Macrophage Galactose-type C-type Lectin (MGL, CD301), and Transferrin Receptor 1 (CD71) (Fig. 1B). Together, these data show that E-cadherin actively participates in down-tuning the macrophage inflammatory responsiveness when overexpressed, a feature that would be consistent with its predominant expression in anti-inflammatory M(IL-4) macrophages.
Figure 1. E-cadherin-overexpressing Raw264.7 macrophages display reduced inflammatory responses.
Raw-E-cadherin and Raw-Mock transfectants (n = 4) were left untreated or were stimulated for 24 h with (A) indicated concentrations LPS (+ IFNγ) or (B) IL-4. Similarly, BMDM from naive Cdh1Δ and Cdh1F/F mice (n = 3) were treated with (C) 10 ng/ml LPS + 10 U/ml IFNγ or (D) with IL-4. MLPS(+IFNγ) and MIL-4 polarization was assessed by measuring the secretion of IL-6, IL-12, TNF and NO or by determining the IL-4-induced surface expression MRC1, MGL, TFRC1 and PDL2, respectively. IL-12 secretion was not detected in Raw264.7 cells and these cells did not show IL-4-induced PDL2 upregulation. Data represent the mean ± SEM of 4 individual Raw264.7 clones (A,B) or BMDM from 3 individual mice (C,D). ΔMFI = [median fluorescence intensity]positive staining − [median fluorescence intensity]isotype control. *P < .05; **P < .01; ***P < .001
In naive conditions, primary macrophages express very low levels of E-cadherin6. To assess whether E-cadherin deletion at baseline would affect subsequent M(LPS+IFNγ) polarization in primary macrophages, E-cadherin-deficient bone marrow-derived macrophages (LysM-cre x Cdh1F/F, termed Cdh1Δ) were generated and treated with LPS+IFNγ. These cells displayed a similar M(LPS+IFNγ) polarization as their WT (Cdh1F/F) counterparts (Fig. 1C) in vitro. Accordingly, we did not observe differences in survival between Cdh1Δ and Cdh1F/F mice upon LPS-induced sepsis as typical M1 response in vivo (Figure S1B). Moreover, bone marrow-derived macrophages from Cdh1Δ and Cdh1F/F mice displayed similar M(IL-4) polarization (Fig. 1D), phagocytosis and autophagy (Figure S1C,D). Hence, only high levels of E-cadherin at baseline alter the responsiveness to a subsequent inflammatory insult.
Cdh1Δ and control mice are equally susceptible to Taenia crassiceps infection
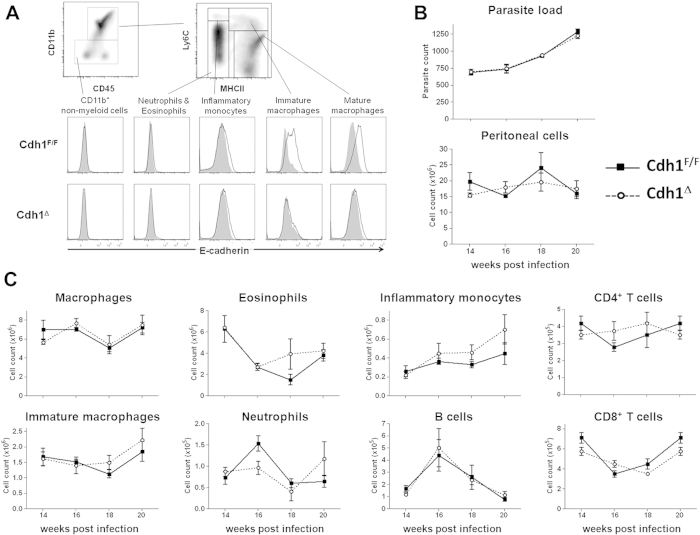
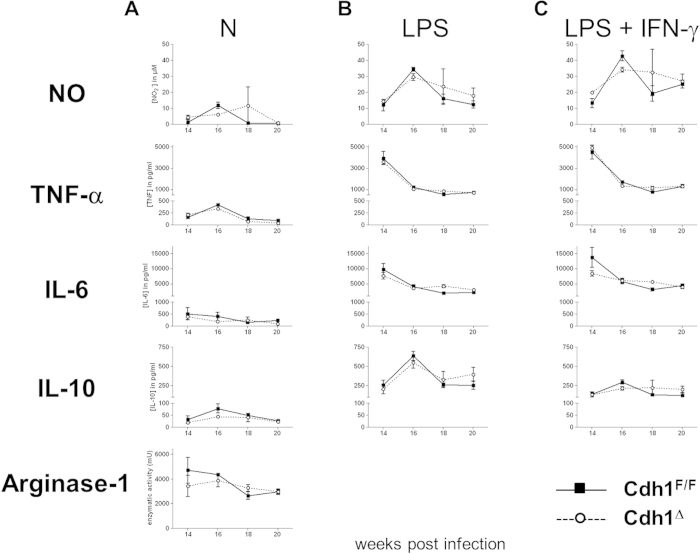
Next, we set out to investigate the immunoregulatory role of E-cadherin in M(IL-4/IL-13) macrophages in vivo by assessing the course of Th2- and M(IL-4/IL-13)-associated diseases in Cdh1Δ versus Cdh1F/F mice. Taenia crassiceps helminths initially induce a Th1 response which becomes highly Th2 polarized after 4 weeks of infection, resulting in the accumulation of M(IL-4/IL-13) macrophages that are essential for the in vivo maintenance of the parasite22. Hence, an alteration of the macrophage activation state, possibly triggered by the absence of E-cadherin, might impact the course of this infectious disease. In the peritoneal exudate cells from 14 weeks infected WT mice, surface expression of E-cadherin was confined to CD11bhiLy6ChiMHC-IIhi immature macrophages and CD11bhiLy6CloMHC-IIhi mature macrophages. This expression is blunted in Cdh1Δ mice, illustrating the efficiency of the gene deletion in macrophages (Fig. 2A). However, parasite load as well as the total cell counts were similar in the peritoneal cavities of Cdh1Δ and Cdh1F/F infected mice at all time points measured (Fig. 2B). In addition, the peritoneal cellular composition, gated as shown in Figure S2, is largely comparable between both mouse strains (Fig. 2C), excluding a major impact of E-cadherin expression in macrophages on the course of this disease. We next focussed specifically on the activation state of peritoneal macrophages from late-stage infected Cdh1F/F and Cdh1Δ mice. Macrophages from both strains had a similar arginase activity and spontaneously secreted comparable levels of NO, TNF, IL-6 and IL-10 upon in vitro culture (Fig. 3A), suggesting no major differences in the inflammatory status of these in vivo-elicited populations. Finally, we assessed the LPS responsiveness of the E-cadherin+ Cdh1F/F macrophages versus the E-cadherin− Cdh1Δ macrophages from infected mice. As illustrated in Fig. 3B,C, these in vivo-elicited cells produced similar amounts of NO, TNF, IL-6 and IL-10, both spontaneously and upon LPS or LPS+IFN-γ stimulation in vitro. In conclusion, during a helminth infection that strongly polarizes the macrophages towards M(IL-4/IL-13), the lack of E-cadherin in these cells is not sufficient to alter their activation state and to have an impact on the outcome of the disease.
Figure 2. Cdh1Δ mice are equally susceptible to Taenia crassiceps infection.
(A) Freshly isolated peritoneal cells from T. crassiceps infected Cdh1F/F and Cdh1Δ mice (14 weeks p.i.) were subjected to multicolour FACS analysis. Myeloid and non-myeloid (G1) cells were first gated based on CD11b expression. Within the CD11b+ myeloid cell gate, Ly6C-MHC II staining discriminates between Ly6Cint/MHC IIneg eosinophils and neutrophils, Ly6Chigh/MHC IIneg inflammatory monocytes, Ly6Chi/MHC IIpos immature macrophages and Ly6Clow/MHC IIhigh mature macrophages. The histograms show an overlay of isotype staining (grey, filled) and anti-E-cadherin staining (bold line) on these gated peritoneal cell types from T. crassiceps infected Cdh1F/F (top) and Cdh1Δ (bottom) mice. (B) Parasite load (top) and total leukocyte cell count (bottom) is similar in the peritoneal cavities of Cdh1Δ and Cdh1F/F infected mice at all indicated time points. (C) Peritoneal cells from T. crassiceps infected Cdh1F/F and Cdh1Δ mice were isolated at indicated time point and subjected to multicolour FACS analysis and all leukocyte populations were gated as shown in Figure S2. The absolute number of macrophages, eosinophils, inflammatory monocytes, immature macrophages, neutrophils, B cells and CD4+ and CD8+ T cells in the peritoneal cavities of T. crassiceps infected Cdh1F/F and Cdh1Δ mice (average ± SEM of 5 mice per group per time point) is shown.
Figure 3. Peritoneal macrophages from Cdh1F/F and Cdh1Δ T. crassiceps-infected mice display a similar activation status.
(A) Peritoneal macrophages obtained from Cdh1F/F and Cdh1Δ mice at different time points after infection display similar arginase activity and spontaneously secrete equal levels of NO, TNF, IL-6 and IL-10 upon 24 h in vitro culture. Additionally, these in vivo-elicited cells secrete similar amounts of NO, TNF, IL-6 and IL-10 upon (B) LPS and (C) LPS+IFN-γ stimulation. Values are the mean ± SEM of 5 mice per group per time point.
Cdh1Δ and control mice are equally susceptible to allergic airway inflammation, but Cdh1Δ mice display lower B cell counts in their BAL
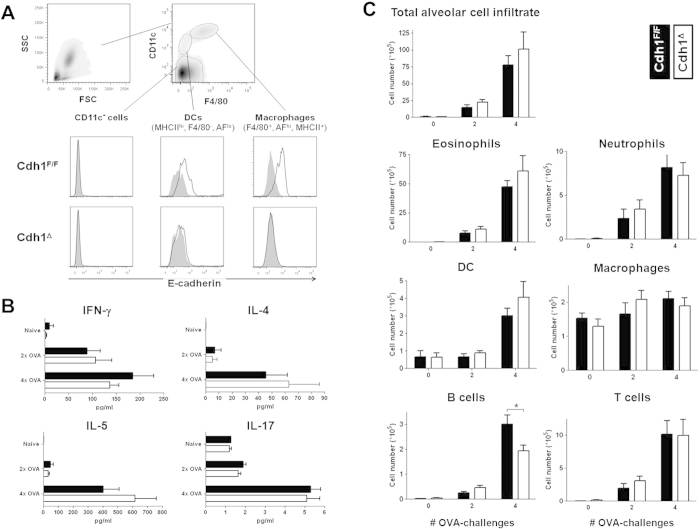
To further assess a potential role for macrophage E-cadherin during strongly polarized Th2-associated pathologies, we turned to a model of ovalbumine (OVA)-induced allergic airway inflammation. Sensitized mice were either challenged twice (short term protocol 2× OVA) or four times (long term protocol 4× OVA) with OVA allergen, during which E-cadherin-expressing alveolar macrophages (CD11chigh F4/80+ MHC-II+ autofluorescence (AF)high cells) and DCs (CD11chigh F4/80− MHC-IIhi AFlow cells) are induced (Fig. 4A for 4× OVA, data not shown for 2× OVA). Again, the E-cadherin expression was absent in Cdh1Δ mice. While two ovalbumin challenges (2× OVA) were sufficient to induce an initial modest inflammatory response with faintly increased IL-4 and IL-5 levels in the bronchoalveolar lavage (BAL), the inflammation and associated secretion of IL-4, IL-5 and IL-17 were further increased by two additional ovalbumin challenges (4× OVA). However, no significant differences in BAL cytokine concentrations were observed between Cdh1Δ and Cdh1F/F control mice (Fig. 4B). In agreement with the similar cytokine profiles, Cdh1Δ and Cdh1F/F mice had comparable total BAL cell numbers (gated as shown in Figure S3) and a comparable recruitment of eosinophils, neutrophils and T cells to the alveolar space. Surprisingly, B cell counts were consistently lower in the BAL of Cdh1Δ mice (Fig. 4C). Overall, deletion of macrophage (and DC) E-cadherin expression does not seem to affect the severity of ovalbumine-induced allergic airway responses.
Figure 4. Cdh1Δ mice are equally susceptible to ovalbumine-induced allergic airway inflammation.
Allergic asthma was induced in Cdh1F/F and Cdh1Δ animals by ovalbumin sensitization with Alum adjuvant, followed by 2 (2× OVA, short term protocol) or 4 (4× OVA, long term protocol) ovalbumin aerosols. (A) Cells in the bronchoalveolar lavage (BAL) were stained with anti-CD11c, anti-F4/80, anti-MHC II and anti-E-cadherin or isotype control and analysed via FACS. Within gated CD11chigh BAL, a distinction was made between autofluorescence (AF)low MHC IIhigh DC and AFhigh F4/80+ alveolar macrophages and histogram overlays of isotype staining (grey, filled) and anti-E-cadherin staining (bold line) are shown for 4× OVA-challenged Cdh1F/F (top) and Cdh1Δ (bottom) mice. (B) IFN-γ , IL-4, IL-5 and IL-17 concentrations were measured in the BAL fluid of Cdh1F/F (black bars) and Cdh1Δ (white bars) mice. (C) Different leukocyte populations in the BAL of 2× and 4× ovalbumin-challenged Cdh1F/F and Cdh1Δ mice were gated as in Figure S3 and absolute cell counts are shown (average ± SEM of 5 mice per group per time point). *P < .05.
Cdh1Δ mice display normal granuloma formation during schistosomiasis
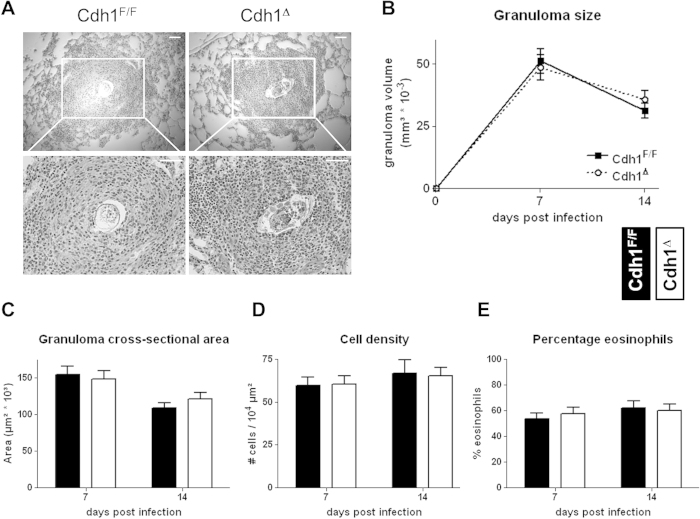
Earlier findings from our lab and others showed an involvement of E-cadherin in IL-4-mediated macrophage fusion leading to multinucleated giant cell formation in vitro6,23, but the in vivo relevance of these findings remained unaddressed. As a model system, we intravenously challenged Cdh1Δ and Cdh1F/F mice with Schistosoma mansoni eggs24, resulting in the induction of a polarized Th2 immune response and the formation of large pulmonary granulomas consisting mainly of eosinophils and macrophages (Fig. 5A). IL-4/IL-13-dependent formation of multinucleated giant cells is a hallmark of such granulomatous responses, suggesting a potential role for macrophage E-cadherin in this process25. However, no significant differences were found in granuloma size (Fig. 5B) nor cross-sectional area (Fig. 5C) between Cdh1Δ and Cdh1F/F mice at 7 and 14 days post challenge. In both mouse strains the granuloma size and area equally decreased from day 7 to day 14 post challenge. Moreover, the cell density within the granuloma (Fig. 5D) and its composition (as measured by the percentage eosinophils, Fig. 5E) was the same in both mouse strains. Overall, we have no evidence to conclude that macrophage E-cadherin expression contributes to granuloma formation in the lungs after intravenous S. mansoni egg challenge.
Figure 5. Cdh1Δ mice display normal granuloma formation during schistosomiasis.
(A) H&E stained lung sections from Schistosoma mansoni egg-challenged Cdh1F/F and Cdh1Δ mice containing a representative granuloma are shown at 10× (top) and 20× (bottom) magnification. (B) The granuloma size (calculated as V = 4/3*Π*r3), (C) the granuloma cross-sectional area, (D) the granuloma density (calculated as the amount of cells per 104 μm2) and (E) the percentage eosinophils within each granuloma is shown after 7 and 14 days post egg challenge (average ± SEM, n = 5 mice per group per time point).
Discussion
Parasitic helminths and allergens induce polarized Th2 responses, supporting alternative macrophage activation. While M(IL-4/IL-13) macrophages are key regulators of these diseases26,27, discriminative surface markers allowing their identification and contributing to their function remained limited. Previously, we identified E-cadherin as a protein that associates with IL-4/IL-13-exposed mouse and human macrophages that can be employed as useful reporter of polarized Th2 responses and M(IL-4/IL-13) macrophages6.
While E-cadherin clearly contributes to IL-4-driven macrophage fusion and the formation of multinucleated giant cells in vitro6,23, the in vivo significance of this observation remained unstudied so far. In this context, we here assessed E-cadherin’s contribution to granuloma formation during schistosomiasis in vivo. Schistosoma mansoni eggs are potent inducers of M(IL-4/IL-13) macrophages, and granuloma formation around these ova is an important characteristic of schistosomiasis28,29. ‘Granuloma closure’, preventing the diffusion of parasitic substances to the surroundings, is an important event and cadherins are documented to be expressed during this process30. As such, macrophage E-cadherin could serve to bring granulomatous cells into close contact, allowing the formation a compactly packed cell mass around the schistosome egg. Nevertheless, an experimental intravenous challenge with Schistosoma mansoni eggs equally induced granuloma formation in the lungs of Cdh1Δ and Cdh1F/F mice. In addition, no differences in granuloma parameters could be observed between these mouse strains. Probably a wide range of fusiogenic molecules is involved during the compaction of granulomas, and deleting one (in this case E-cadherin) in one of the participating granulomatous cell types (here macrophages) is not enough to impair this process. Overall, we have no data to conclude that macrophage E-cadherin expression is fundamental for granuloma formation. Together our findings support a model whereby a complex collection of molecules interact to form multinucleated giant cells and granulomas, as suggested earlier31,32. Within this collection of participating molecules, redundancy might exist, as is demonstrated here for E-cadherin.
Besides its potential contribution to macrophage fusion, E-cadherin enables M(IL-4/IL-13) macrophages to interact with KLRG1+ cells and to trap αEβ7 integrin (CD103)-expressing cells in vitro6. Both KLRG1 and CD103 are E-cadherin ligands and are detected on major mediators of the immune system, including DC, NK and T cell subpopulations11,12,21,33,34. As such, E-cadherin on M(IL-4/IL-13) macrophages might influence the retention of these cells in order to instruct their phenotype during Th2 immune responses. In parallel to its role during cell/cell interactions, the E-cadherin/catenin complex regulates inflammatory cascades in epithelial cells16,18 and thus might also modulate the phenotype of macrophages. In this context, possible effects of enhanced macrophage E-cadherin expression on NFκB, PI3K and β-catenin/Wnt signaling might modulate the macrophage activation status in a subtle manner. Indeed, these pathways are crucial to instruct the inflammatory phenotype of macrophages and are known to be modulated by E-cadherin and its different catenins in epithelial cells, keratinocytes, but also in tolerogenic DC13,14,15,16,17,21. Supporting this hypothesis, we showed here that E-cadherin over-expression in Raw 264.7 macrophage cell lines blunted the secretion of inflammatory mediators upon TLR engagement in vitro.
Doing so in vivo, macrophage E-cadherin could help to determine the outcome of typical Th2 cytokine-driven diseases like Taenia crassiceps helminth infection and allergic asthma, during which E-cadherin-expressing M(IL-4/IL-13) macrophages are present. During the experimental mouse model of cysticercosis, infection with Taenia crassiceps evokes a Th1 response in the early phase of infection, which gradually switches to a Th2 response35,36. While Th2 responses are widely accepted to mediate protection against most helminths, a polarized STAT4-dependent, IL-12-mediated Th1 response and MIF-expressing inflammatory M1 macrophages are required to control Taenia crassiceps infections37,38,39. In accordance, STAT6-deficient mice, which lack AAMs and fail to induce Th2 responses, control Taenia crassiceps infections22,40. Based on the observations that E-cadherin has mainly inhibitory effects on pro-inflammatory signaling cascades in macrophages and other cell types, one would expect that Cdh1Δ mice have more M1-polarized macrophages and thus clear Taenia crassiceps helminths more efficiently. However, the course of parasitemia as well as the leukocyte count and cellular composition was similar in the peritoneum of Cdh1Δ and Cdh1F/F infected mice, ruling out an important role of E-cadherin expression in macrophages during cysticercosis (Fig. 2). Additionally, the lack of macrophage E-cadherin expression during this strongly Th2 polarizing disease did not affect the basal macrophage activation state, nor the LPS(+IFNγ)-induced secretion of inflammatory factors (Fig. 3). In the latter case, an LPS+IFNγ-mediated downregulation of E-cadherin6 could explain why no differences were observed between in vivo-elicited Cdh1Δ and Cdh1F/F macrophages.
Allergic asthma is another prototypical Th2 cytokine-driven disease which strongly instructs E-cadherin expression in M(IL-4/IL-13) macrophages. Yet, E-cadherin deletion in alveolar macrophages of Cdh1Δ mice does not affect BAL cytokine levels and its cellular composition (Fig. 4). Based on the fact that E-cadherin is a cell adhesion molecule, one could hypothesize that E-cadherin+ alveolar macrophages could interact with E-cadherin+ epithelial cells in the lung, enabling their trapping and retention. However, since BAL macrophage counts were similar in Cdh1Δ and Cdh1F/F asthmatic mice, we have no evidence to support this hypothesis. Remarkably, B cell numbers were always lower in the BAL of ovalbumine-challenged Cdh1Δ mice. While some B cell subsets are reported to express a nonclassical cadherin during B cell development41, we did not detect any E-cadherin-expressing CD11b-negative cells (including B cells) in the BAL of asthmatic mice (Fig. 2A). In addition, these B cells did not express the other E-cadherin ligands CD103 and KLRG1 (data not shown) and therefore it seems unlikely that the reduced B cell counts in the BAL of allergic Cdh1Δ mice can be explained by a reduced trapping of those cells by E-cadherin-deficient alveolar macrophages. Hence, why B cell numbers are reduced during ovalbumin-induced allergic asthma in macrophage-specific E-cadherin-deficient mice remains unknown. Of note, while LysM-Cre x Cdh1F/F mice are considered to be macrophage -specific E-cadherin-deficient mice, the DCs in the BAL of ovalbumine-challenged mice also displayed abrogated E-cadherin expression (Fig. 4A). Hence, alveolar DCs during allergic asthma display active lysozyme M promoters, which is in agreement with previous publications reporting lysozyme expression by some DC subsets42,43. In any case, deletion of E-cadherin in alveolar macrophages and DCs does not affect the degree of ovalbumin-induced experimental airway inflammation. Thus, while E-cadherin supresses inflammatory signaling in macrophages in vitro, these effects are clearly not strong enough to alter the overall macrophage activation status during polarized Th2 responses in vivo.
Overall, employing macrophage-specific E-cadherin-deficient mice, we demonstrate that E-cadherin in macrophages is largely unnecessary for in vivo granuloma formation, and for the regulation of Th2 responses. Irrespective of its in vivo redundancy, the E-cadherin/catenin complex offers a valuable tool to detect M(IL-4/IL-13) macrophages in vivo.
Methods
Ethics Statement
The study was carried out in strict accordance with the recommendations in ‘Guidelines for the Use of Laboratory Animals in Research, Teaching and Testing’ of the International Council for Laboratory Animal Science. The permission of the local authorities has been given (accreditation N° LA1210220) and all animal work was approved by the appropriate committee (‘Ethische commisie voor dierproeven’) at the Vrije Universiteit Brussel (ethics committee protocol number 07-220-03).
E-cadherin-overexpressing Raw264.7 cell lines
The generation of Raw264.7-E-cadherin transfectants were described previously6.
Mice
To generate mice in which the E-cadherin gene was disrupted in macrophages, floxed Cdh1F/F C57BL/6 mice (kind gift of Dr. J Jonkers, The Netherlands Cancer Institute, Amsterdam, The Netherlands44) were crossed with LysM-Cre C57BL/6 mice (Jackson Laboratory, Bar Harbour, Maine, USA45). Homozygous LysM-Cre+/+-Cdh1F/F conditional KO (hereafter referred to as Cdh1Δ) mice were compared to LysM-Cre−/−-Cdh1F/F littermate controls (hereafter referred to as Cdh1F/F). In Cdh1Δ mice the IL-4-induced E-cadherin expression, either upon in vitro stimulation or during in vivo pathologies is ablated in >90% of the macrophages6.
Disease models
To study cysticercosis, mice were inoculated intraperitoneally (ip) with 10 Taenia crassiceps cestodes, and peritoneal cells and helminths were collected at different time intervals post infection for further analysis35. To study the involvement of macrophage E-cadherin expression during granuloma formation in the lung, mice were injected intravenously (iv) with 5000 Schistosoma mansoni ova, were sacrificed 7 or 14 days later and the lungs were fixed and removed for further analysis (adapted from24).
To sensitize mice for allergic asthma, animals were injected ip at day 0 and 7 with 10 μg grade V chicken egg ovalbumin (OVA; Sigma) adsorbed on 1 mg Alum (Pierce, Rockford, IL) in PBS. Next, mice were challenged at day 14 and 15 (2× OVA, short term protocol) or at day 14, 15, 21 and 22 (4× OVA, long term protocol) for 30 min with aerosols, consisting of 1% grade III OVA in PBS46. 20 h after the last challenge, mice were sacrificed and broncho-alveolar lavage (BAL) was performed by PBS rinsing of the lungs. The BAL fluid (BALF) was centrifuged and supernatant and cell pellets were collected for further analysis.
In vitro stimulation of macrophages
Bone marrow-derived macrophages (BMDM) were generated from naïve mice as detailed earlier6. Peritoneal macrophages from Taenia crassiceps infected mice were obtained by rinsing the peritoneum with PBS/10% sucrose. After 3 h culture, non-adherent cells were washed away and plastic-adherent macrophages were used for analysis. Macrophages were cultured for 24 h in RPMI1640 medium supplemented with 10% heat-inactivated FCS, 0.03% L-glutamine, 100 mg/mL streptomycin and 100 mg/mL penicillin, 1 mM nonessential amino acids, 1 mM sodium pyruvate (all from Invitrogen, Carlsbad, CA) and 0.02 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) in the presence of 0.1, 1 or 10 ng/ml E. coli LPS with or without 10 U/ml recombinant mouse IFN-γ or in the presence of 20 ng/ml recombinant mouse IL-4 (BD Bioscience).
Cytokine, NO and arginase measurement
Cytokines in the macrophage culture supernatants were quantified with sandwich ELISAs for TNF (R&D Systems, Minneapolis, MN), IL-6, IL-12 and IL-10 (BD Pharmingen), in accordance to the suppliers’ protocols. Cytokine levels in BALF were measured by Bio-Plex (Bio-Rad, Hercules, CA). NO2− in culture supernatants was quantified by a standard Griess reaction47. Arginase activity was measured as described earlier48.
Flow cytometry and analysis of E-cadherin expression
Quantitative real-time PCR, Western blot and flow cytometry for E-cadherin mRNA and protein expression was performed as described earlier6. Gating on distinct immune cell types during the different disease models is shown in Figures S2 and S3. All antibodies are listed in Table 1. Data were acquired with a FACSCantoII (BD Biosciences) and analyzed using FlowJo (TreeStar, Ashland, OR).
Table 1. List of used antibodies.
| Marker/fluorophore | Clone | Isotype | Supplier |
|---|---|---|---|
| rat IgG2a/pure isotype ctrl | NA/LE | rat IgG2a | BD Bioscience |
| E-cadherin/pure | ECCD2 | rat IgG2a | Dr. M. Takeichi (University of Kyoto, Japan) |
| anti-rat Ig/PE/APC | polyclonal | goat Ig | BD Bioscience |
| CD16/CD32/pure Fc-Block | 2.4G2 | rat IgG2b | BD Bioscience |
| E-cadherin/pure (Western Blot) | 36 | mouse IgG2a | BD Bioscience |
| β-actin/pure (Western Blot) | AC-15 | mouse IgG1 | Abcam (Cambridge, UK) |
| CD11c/PerCp-Cy5.5 | N418 | hamster IgG | eBioscience (San Diego, CA) |
| Ly6c/AF647 | ER-MP20 | rat IgG2a | Serotec (Raleigh, NC) |
| F4/80/APC-Alexa Fluor 750 | BM8 | rat IgG2a | eBioscience |
| CD45.2/APC | 104 | mouse IgG2a | BD Bioscience |
| IA/IE/FITC | M5/114.15.2 | rat IgG2b | eBioscience |
| Ly6G/FITC/PE | 1A8 | rat IgG2a | BD Bioscience |
| CCR3/FITC | 83101 | rat IgG2a | R&D Systems (San Jose, CA) |
| CD4/FITC | RM4-5 | mouse IgG2a | eBioscience |
| CD8b/PE | Ly-3 | rat IgG2b | BD Bioscience |
| CD19/PE | 1D3 | rat IgG2a | BD Bioscience |
| Siglec-F/PE | E50-2440 | Rat IgG2a | BD Bioscience |
| CD11b-PE-Cy7 | M1/70 | rat IgG2b | BD Bioscience |
| CD3e/FITC | 145-2C11 | hamster IgG1 | BD Bioscience |
| B220/PE | RA3-6B2 | rat IgG2a | BD Bioscience |
| CD71/PE | C2 | rat IgG1 | BD Bioscience |
| CD273/PE | TY25 | rat IgG2a | BBD Bioscience |
| CD206/APC | C068C2 | rat IgG2a | Biolegend (San Diego, CA) |
| CD301/APC | ER-MP23 | Rat IgG2a | Serotec |
Schistosoma mansoni granuloma histopathology
For measurement of Schistosoma mansoni granulomas, lungs (5 mice per group) were inflated with a 1:1 PBS/Tissue-Tek OCT compound (Gentaur, Kampenhout, Belgium) mixture and stored at −20 °C. Next, 7 μm sections were prepared with a Leica CM1950 cryostat, stained with Hematoxilin-Eosin (HE) and acquired on Nikon Elipse 600 microscope using a Digital Sight DS-U2 and a 10×/0.25 (Ph1 DL WD 10.5), 20×/0.4 (Ph1 DL WD1.3) or a 40×/0.65 (Ph2 DL WD 0.57) objective lense (all from Nikon Instruments Inc., Lewisville, TX). At least 20 granulomas per mouse were analysed using ImageJ (National Institutes of Health). The volume of each granuloma was calculated as 4/3*Π*r3, the cross-sectional area was determined by ImageJ, the cell density was counted as the amount of cells per area of 10 μm2 and the percentage eosinophils in each cross-sectional area was evaluated manually.
Statistics
Statistical significance was tested via the unpaired t test using GraphPad Prism 4 (GraphPad Software, San Diego, CA).
Additional Information
How to cite this article: Van den Bossche, J. et al. E-cadherin expression in macrophages dampens their inflammatory responsiveness in vitro, but does not modulate M2-regulated pathologies in vivo. Sci. Rep. 5, 12599; doi: 10.1038/srep12599 (2015).
Supplementary Material
Acknowledgments
The authors thank Ella Omasta, Lea Brys, Marie-Thérèse Detobel, Nadia Abou, Eddy Vercauteren, Angela van Diepen and Muriel Smet, for their technical aid. This work was supported by a doctoral grant from “FWO-Vlaanderen” (Jan Van den Bossche/ www.fwo.be), “IWT-Vlaanderen” (Damya Laoui/ www.iwt.be) and a postdoctoral grant from “Stichting tegen Kanker” (Jo A Van Ginderachter/ www.kanker.be).
Footnotes
Author Contributions J.V.d.B. designed research, performed experiments, interpreted results, prepared all figures, and wrote the manuscript. D.L., T.N. and B.S. performed experiments. H.H.S., C.H.H., J.G. and P.D.B. designed research and interpreted results. J.A.V.G. designed research, interpreted results and wrote the manuscript. All authors reviewed the manuscript.
References
- Murray P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J. et al. Pivotal Advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol 91, 685–699 (2012). [DOI] [PubMed] [Google Scholar]
- Mantovani A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677–686 (2004). [DOI] [PubMed] [Google Scholar]
- Martinez F. O. & Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime. Rep 6, 13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. & Martinez F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010). [DOI] [PubMed] [Google Scholar]
- Van den Bossche J. et al. Alternatively activated macrophages engage in homotypic and heterotypic interactions through IL-4 and polyamine-induced E-cadherin/catenin complexes. Blood 114, 4664–4674 (2009). [DOI] [PubMed] [Google Scholar]
- Hassanzadeh Ghassabeh G. et al. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood 108, 575–583 (2006). [DOI] [PubMed] [Google Scholar]
- Helming L. et al. Essential role of DAP12 signaling in macrophage programming into a fusion-competent state. Sci Signal 1, ra11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M. et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202, 345–351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmer M. S. et al. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int Immunol 19, 391–400 (2007). [DOI] [PubMed] [Google Scholar]
- Suffia I., Reckling S. K., Salay G. & Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol 174, 5444–5455 (2005). [DOI] [PubMed] [Google Scholar]
- Coombes J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204, 1757–1764 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. & Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science (New York, NY) 303, 1483–1487 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis G., Miotti S., Mazzi M., Canevari S. & Tomassetti A. E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene 28, 1206–1217 (2009). [DOI] [PubMed] [Google Scholar]
- Asnaghi L. et al. E-cadherin negatively regulates neoplastic growth in non-small cell lung cancer: role of Rho GTPases. Oncogene 29, 2760–2771 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S., Poser I., Jobin C., Hellerbrand C. & Bosserhoff A. K. Loss of E-cadherin leads to upregulation of NFkappaB activity in malignant melanoma. Oncogene 23, 8509–8519 (2004). [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Song W., Pasolli H. A., Williams S. E. & Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci USA 105, 15399–15404 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M. et al. p120-catenin mediates inflammatory responses in the skin. Cell 124, 631–644 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh C., Fugere C. & Brossay L. Immunoregulatory functions of KLRG1 cadherin interactions are dependent on forward and reverse signaling. Blood 114, 5299–5306 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche J. & van Ginderachter J. A. E-cadherin: from epithelial glue to immunological regulator. Eur J Immunol 43, 34–37 (2013). [DOI] [PubMed] [Google Scholar]
- Van den Bossche J., Malissen B., Mantovani A., De B. P. & van Ginderachter J. A. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood 119, 1623–1633 (2012). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa M., David J. R., Bojalil R., Satoskar A. R. & Terrazas L. I. Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling. J Immunol 168, 3135–3139 (2002). [DOI] [PubMed] [Google Scholar]
- Moreno J. L., Mikhailenko I., Tondravi M. M. & Keegan A. D. IL-4 promotes the formation of multinucleated giant cells from macrophage precursors by a STAT6-dependent, homotypic mechanism: contribution of E-cadherin. J Leukoc Biol 82, 1542–1553 (2007). [DOI] [PubMed] [Google Scholar]
- Nair M. G. et al. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med 206, 937–952 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D. et al. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol 163, 337–342 (1999). [PubMed] [Google Scholar]
- Kreider T., Anthony R. M., Urban J. F. Jr. & Gause W. C. Alternatively activated macrophages in helminth infections. Curr Opin Immunol 19, 448–453 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol 181, 6117–6124 (2008). [DOI] [PubMed] [Google Scholar]
- Herbert D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004). [DOI] [PubMed] [Google Scholar]
- Pesce J. T. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5, e1000371 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi H. L. et al. Four whole-istic aspects of schistosome granuloma biology: fractal arrangement, internal regulation, autopoietic component and closure. Mem Inst Oswaldo Cruz 101 Suppl 1, 219–231 (2006). [DOI] [PubMed] [Google Scholar]
- Helming L. & Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol 37, 33–42 (2007). [DOI] [PubMed] [Google Scholar]
- Oursler M. J. Recent advances in understanding the mechanisms of osteoclast precursor fusion. J Cell Biochem 110, 1058–1062 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersdorf N., Ding X., Tietze J. K. & Hanke T. Characterization of mouse CD4 T cell subsets defined by expression of KLRG1. Eur J Immunol 37, 3445–3454 (2007). [DOI] [PubMed] [Google Scholar]
- Ito M. et al. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med 203, 289–295 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys L. et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol 174, 6095–6104 (2005). [DOI] [PubMed] [Google Scholar]
- Raes G. et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol 77, 321–327 (2005). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa M., Satoskar A. R., David J. R. & Terrazas L. I. Altered T helper responses in CD40 and interleukin-12 deficient mice reveal a critical role for Th1 responses in eliminating the helminth parasite Taenia crassiceps. Int J Parasitol 33, 703–711 (2003). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa M. et al. Macrophage migration inhibitory factor plays a critical role in mediating protection against the helminth parasite Taenia crassiceps. Infect Immun 71, 1247–1254 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sosa M. et al. A STAT4-dependent Th1 response is required for resistance to the helminth parasite Taenia crassiceps. Infect Immun 72, 4552–4560 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J. L. et al. Early removal of alternatively activated macrophages leads to Taenia crassiceps cysticercosis clearance in vivo. Int J Parasitol 40, 731–742 (2010). [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Shimizu T., Karasuyama H., & Melchers F. The identification of a nonclassical cadherin expressed during B cell development and its interaction with surrogate light chain. J Biol Chem 275, 31134–31144 (2000). [DOI] [PubMed] [Google Scholar]
- Jakubzick C. et al. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med 205, 2839–2850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelouard H. et al. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer’s patch dendritic cells that express lysozyme. Gastroenterology 138, 173–184 (2009). [DOI] [PubMed] [Google Scholar]
- Derksen P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006). [DOI] [PubMed] [Google Scholar]
- Clausen B. E., Burkhardt C., Reith W., Renkawitz R. & Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- Bogaert P. et al. Inflammatory signatures for eosinophilic vs. neutrophilic allergic pulmonary inflammation reveal critical regulatory checkpoints. Am J Physiol Lung Cell Mol Physiol 300, L679–L690 (2011). [DOI] [PubMed] [Google Scholar]
- Namangala B., Noel W., De Baetselier P., Brys L. & Beschin A. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomosis. J Infect Dis 183, 1794–1800 (2001). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol 170, 5064–5074 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.