Abstract
The relationship between the neutrophil-to-lymphocyte ratio (NLR) and tumours as a prognostic factor has been reported in many studies. In this meta-analysis, we evaluated the prognostic role of the NLR in pancreatic cancer (PC). A systematic search was performed in PubMed and Embase for relevant studies. Data from and characteristics of each study were extracted. A meta-analysis was performed to analyse the prognostic role of the NLR using the hazard ratio (HR) and 95% confidence intervals (95% CI). As a result, a total of 2035 patients in 9 cohorts were included in this meta-analysis. The pooled HR of 1.587 (95% CI: 1.411–1.785, p < 0.01) showed that patients with an elevated NLR were expected to have shorter overall survival (OS) after treatment. This meta-analysis suggests that an elevated NLR can be used as a predictor of survival in patients with pancreatic cancer.
Pancreatic cancer (PC) results in approximately 227,000 deaths per year1 and ranks as the fifth most common cancer and the fourth leading cause of cancer-related mortality worldwide2. The prognosis of pancreatic cancer is poor, and the majority of patients survive less than 1 year after diagnosis. Virchow in 1863 first reported the association between inflammation and cancer3. Recent investigations revealed inflammatory markers as a predictor of prognosis of different types of cancers. Pretreatment serum-based inflammatory markers, such as NLR, platelet to lymphocyte ratio (PLR) and C-reactive protein (CRP), have been linked to the prognosis of different types of cancer4,5. As a marker of systemic inflammatory response, the NLR has been investigated as an effective prognostic indicator for several types of cancer. Some studies have revealed that an elevated NLR in patients with pancreatic cancer was linked to a poor prognosis, but results contradicting this connection have been presented by some studies, such as that by Hamed, Sanjay and Clark6,7,8. The purpose of this meta-analysis was to examine the link between the NLR and the prognosis in pancreatic cancer.
Results
Selection and characteristics of included studies
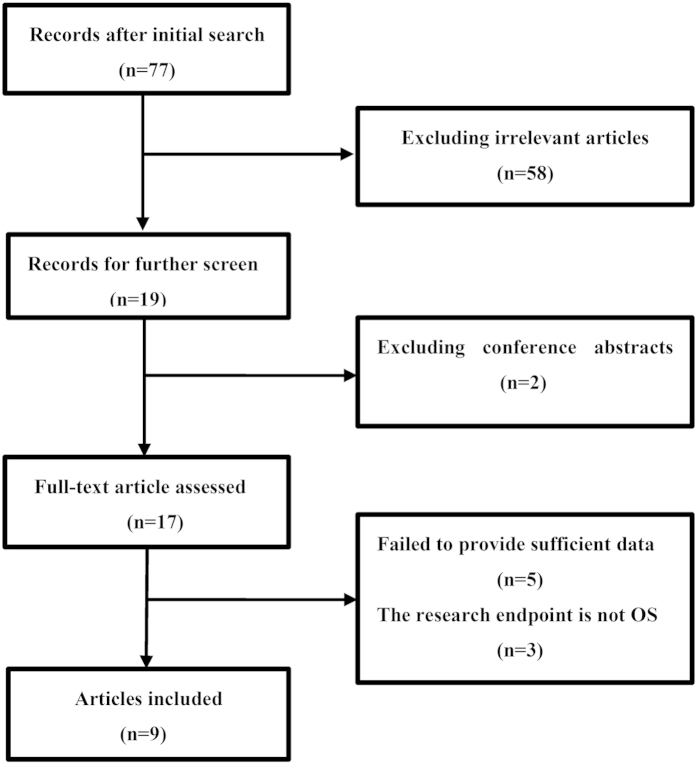
A flow chart of the literature search is shown in Fig. 1. The initial search algorithm retrieved a total of 77 studies. After the first review, only 19 studies related to the NLR and the prognosis of PC were further evaluated. Of those studies, 10 reports were excluded for the following reasons: the endpoint of 3 studies was cancer-specific survival (CSS) or disease-free survival (DFS); 5 articles did not provide sufficient data for estimating the HR and 95% CI; and 2 articles were conference abstracts without detailed data. Thus, 9 studies9,10,11,12,13,14,15,16,17 published between 2007 and 2014 were included in our meta-analysis. The characteristics of the included studies are summarized in Table 1. A total of 2035 patients were included. The studies came from the UK (n = 1), China (n = 4), Japan (n = 2), Ireland (n = 1) and Australia (n = 1). The NLR was calculated on the basis of pretreatment laboratory data using the white blood cell (WBC) counts from the included studies. Eight of the 9 studies used multivariate analysis13.
Figure 1. A flow chart outlining study selection.
Table 1. Characteristics of all identified studies.
| Study | Year | Country | Treatment | HR(95%CI) | Multivariate analysis | Result | Endpoint |
|---|---|---|---|---|---|---|---|
| Xue14 | 2014 | Japan | nonsurgery | 1.92(1.27 – 2.9) | Yes | Positive | OS |
| Wang11 | 2012 | China | Surgery & nonsurgery | 2.537(1.313 – 4.902) | Yes | Positive | OS |
| Luo10 | 2014 | China | nonsurgery | 1.42(1.15 – 1.74) | Yes | Positive | OS |
| Martin15 | 2014 | Australia | nonsurgery | 1.60(1.07 – 2.40) | Yes | Positive | OS |
| An12 | 2010 | China | surgery & nonsurgery | 4.489(1.372 – 14.692) | Yes | Positive | OS |
| Bhatti13 | 2010 | UK | surgery | 1.78(1.09 – 2.93) | No | Positive | OS |
| Teo9 | 2013 | Ireland | nonsurgery | 2.93(1.59 – 5.64) | Yes | Positive | OS |
| Ben17 | 2014 | China | Surgery | 1.51(1.15 – 1.99) | Yes | Positive | OS |
| Dai16 | 2014 | China | Surgery & nonsurgery | 1.404(1.078 – 1.830) | Yes | Positive | OS |
| Study | sample | sample of elevated NLR | NLR cut-off | sample of distant metastasis | stage | sampling time |
|---|---|---|---|---|---|---|
| Xue14 | 252 | 36 | 4 | 83 | Advanced | pretreatment |
| Wang11 | 177 | 12 | 3, 4 | 0 | Ib-IV | pretreatment |
| Luo10 | 403 | 194 | 3.1 | 309 | Advanced | pretreatment |
| Martin15 | 124 | 60 | 5 | 84 | Advanced | pretreatment |
| An12 | 89 | 18 | 5 | – | III- IV | pretreatment |
| Bhatti13 | 84 | 13 | 4 | 0 | Early | pretreatment |
| Teo9 | 85 | 58 | 3 | 47 | Advanced | pretreatment |
| Ben17 | 381 | 267 | 2 | 0 | I-III | pretreatment |
| Dai13 | 440 | 300 | 2 | 143 | I-IV | pretreatment |
“-”: nor reported; “OS”: overall survival.
Meta-analysis results
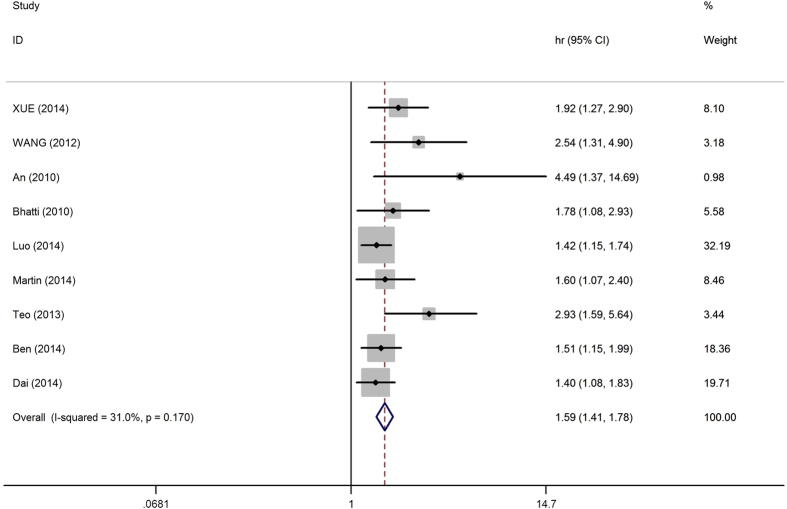
Because the heterogeneity test showed that minor heterogeneity exists (I2 = 31.0%, p = 0.170) between the studies, a fixed-effects model was used for the analysis. A pooled HR of 1.587 (95%CI: 1.411–1.785, p < 0.01) showed that patients with an elevated NLR were expected to have shorter OS after treatment (Fig. 2). Because of relatively minor heterogeneity, we did not conduct further meta-regression or subgroup analysis.
Figure 2. Forest plot of HR and 95%CI for each study.
Fixed-effects pooled HR = 1.59, 95% CI = 1.41 − 1.78, P < 0.01; I2 = 31.0%, Pheterogeneity = 0.170.
Publication bias
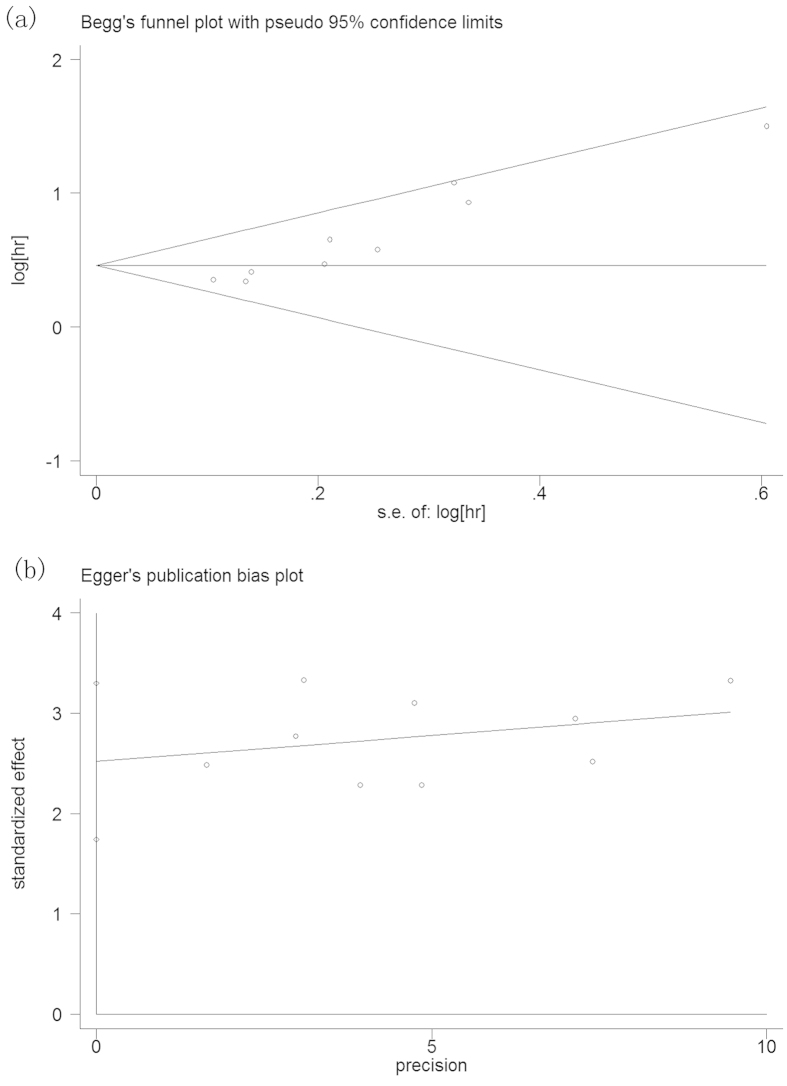
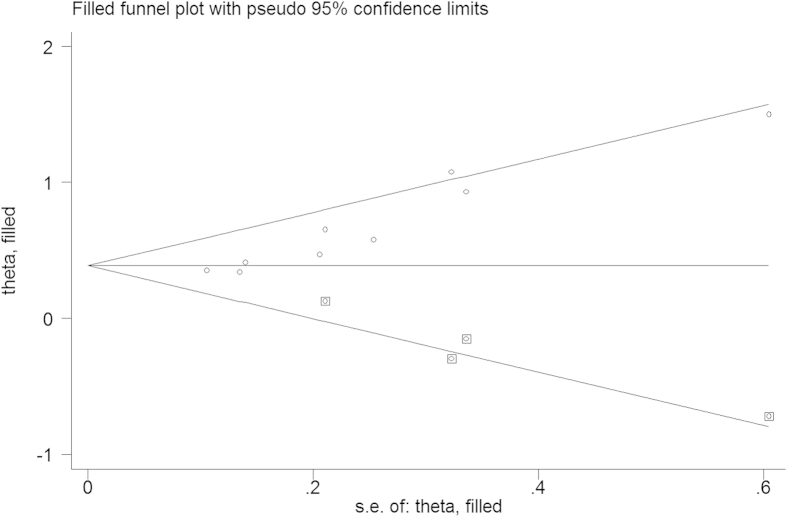
A publication bias estimate was used to evaluate the reliability of the meta-analysis results. A funnel plot (Fig. 3) was constructed, and the Begg’s test and Egger’s test showed that Pr > |z| = 0.002 and P > |t| = 0.000, respectively. The results revealed publication bias in this meta-analysis. To identify the source of publication bias, we used the trim-and-fill method (Fig. 4). As a result, there were four studies hypothetically remained unpublished, and the recalculated pooled HR of 1.475 (95%CI: 1.322-1.645, p < 0.01) indicated a positive outcome even though publication bias still exists.
Figure 3.
Begg’s (a) and Egger’s (b) funnel plot for the assessment of potential publication bias.
Figure 4. Trim-and-fill funnel plot for identifying the source of publication bias.
Discussion
The relationship between inflammation and tumours has been well established. Tumours not only can develop under the stimuli of inflammation, such as in HBV—induced hepatic cancer, but also can induce systemic and local inflammatory responses that may provide a favourable microenvironment for tumour invasion and metastasis3,18,19. Growing evidence has demonstrated that the association between inflammation and tumours could be applied to the prevention and treatment of cancer, such as anti-inflammation therapy for bladder cancer20,21. The NLR, which can be readily determined using a widely available peripheral blood test, is drawing increasing attention. An elevated NLR is usually caused by neutrophilia and lymphopenia. Lymphopenia indicates disease severity22,23 and is linked to the immune escape of tumour cells from tumour-infiltrating lymphocytes (TIL)24,25. It has been shown that elevated levels of tumour-infiltrating lymphocytes in the primary tumour site are associated with good prognosis26. It has also been reported that tumour cells can inhibit cytotoxic T lymphocyte infiltration by producing immunosuppressive cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor–β (TGF—β), or IL—10, and by reducing IL—2, a cytokine that can maintain cytotoxic T lymphocyte function27. Conversely, neutrophils have been reported to be the primary source of circulating VEGF, which has been shown to have a critical role in tumour-related angiogenesis and therefore has a close relationship with vascular invasion and metastasis in cancers28. Neutrophils from healthy donors were found to inhibit the cytolytic ability of lymphocytes when cocultured in vitro, and the magnitude of suppression was related to the number of neutrophils29. An elevated NLR generates a favourable immune microenvironment that promotes vascular invasion and suppression of the host immune system. Therefore, an elevated NLR is associated with poor prognoses.
To our knowledge, this meta-analysis, which included 9 cohorts and 2035 patients, is the first to identify the prognostic role of a pretreatment peripheral blood NLR in pancreatic cancer. According to the results, an elevated NLR predicts a poor prognosis and shorter OS in pancreatic cancer patients.
The meta-analysis had some limitations that call for cautious interpretation of the results. First, only 9 studies were included, and, because of insufficient data, studies6,7,8 with negative results were excluded. Second, the cut-off value for defining high NLR in each included study was not the same (Table 1), which may have contributed to heterogeneity. Third, some studies provided only a Kaplan-Meier curve and did not report HR or a 95% CI. Theoretically, these factors could be obtained through estimation from the curve, but that approach can be inaccurate. Therefore, we removed articles that did not meet our criteria from the analysis6,30,31. Fourth, besides articles with OS as an endpoint, we also searched for articles with CSS or DFS as an endpoint that focused on the relationship between NLR and PC prognosis, but we found only three articles32,33,34 (CSS: n = 2, DFS: n = 1). Given the small number, we did not include these articles in the meta-analysis.
In conclusion, this meta-analysis suggests that an elevated NLR can be used as a predictor of poor survival in patients with pancreatic cancer that is easily accessible and has a low cost. However, because of the study’s limitations, more multi-centre prospective cohorts need to be conducted to validate the role of the NLR in cancer.
Methods
Search strategy
We systematically searched PubMed and Embase with a search strategy based on the terms “NLR,” “neutrophil-to-lymphocyte ratio,” “neutrophil to lymphocyte ratio,” or “neutrophil lymphocyte ratio” and “pancreatic cancer,” “pancreatic carcinoma,” “pancreatic tumor,” or “pancreatic adenocarcinoma”. The last search was performed on January 16, 2015. To retrieve additional potentially eligible studies, the rences of articles and reviews were also examined.
Inclusion and exclusion criteria
Studies fulfilling the following criteria were included in the meta-analysis: (1) the NLR was measured with pretreatment serum-based methods; (2) the association between NLR and OS in patients with PC was reported; (3) hazard ratio (HR) and 95% CI were reported; and (4) all included patients were pathologically diagnosed and did not have any tumours besides pancreatic tumours. The following publications were excluded: (1) studies that did not report HR or 95% CI; (2) letters, reviews, expert opinions, or case reports; and (3) studies that had a sample size less than 50.
Data extraction
The following items were collected from each study: first author’s name, year of publication, country of the study population, sample size, sampling time, predominant treatment (surgical or nonsurgical), cut-off value for the NLR, PC stage and HRs with 95% CI.
Statistical analysis
HRs with 95% CI from each study were extracted to generate a pooled HR. If both univariate and multivariate analyses were reported in the same study, multivariate analysis was chosen for the meta-analysis. We performed a test of heterogeneity using Cochran’s Q test and Higgins I-squared statistic. P ≥ 0.10 and I2 ≤ 50% were considered the values that indicated homogeneity, and a fixed-effects model was subsequently applied. P < 0.10 and I2 > 50% were considered the values that indicated severe heterogeneity, and the pooled HR was subsequently calculated by applying the random-effects model. Begg’s funnel plot and Egger’s linear regression test were used to evaluate publication bias. All statistical analyses were carried out using STATA 12.0 (STATA Corporation, College Station, TX, USA).
Additional Information
How to cite this article: Cheng, H. et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. Sci. Rep. 5, 11026; doi: 10.1038/srep11026 (2015).
Footnotes
Author Contributions Conceived and designed the analyses: H.C. Reference collection and data management: H.C., F.L. Performed the analyses: H.C., F.L. and M.J. Wrote and reviewed the manuscript: H.C., F.L. M.J. L.Y., C.W. and Z.Z. All authors reviewed the manuscript.
References
- Raimondi S., Maisonneuve P. & Lowenfels A. B. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 6, 699–708 (2009). [DOI] [PubMed] [Google Scholar]
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- Balkwill F. & Mantovani A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001). [DOI] [PubMed] [Google Scholar]
- Roxburgh C. S. & McMillan D. C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6, 149–163 (2010). [DOI] [PubMed] [Google Scholar]
- McMillan D. C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12, 223–226 (2009). [DOI] [PubMed] [Google Scholar]
- Hamed M. O., Roberts K. J., Smith A. M. & Morris Stiff G. Elevated pre-operative neutrophil to lymphocyte ratio predicts disease free survival following pancreatic resection for periampullary carcinomas. Pancreatology 13, 534–538 (2013). [DOI] [PubMed] [Google Scholar]
- Sanjay P. et al. Preoperative serum C-reactive protein levels and post-operative lymph node ratio are important predictors of survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. JOP 13, 199–204 (2012). [PubMed] [Google Scholar]
- Clark E. J. et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB(Oxford) 9, 456–460 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo M. et al. Prognostic role of neutrophil-to-lymphocyte ratio in advanced pancreatic ductal adenocarcinoma: impact of baseline fluctuation and changes during chemotherapy. Tumori 99, 516–522 (2013). [DOI] [PubMed] [Google Scholar]
- Luo G. et al. Blood Neutrophil-Lymphocyte Ratio Predicts Survival in Patients with Advanced Pancreatic Cancer Treated with Chemotherapy. Ann Surg Oncol 22, 670–676 (2014). [DOI] [PubMed] [Google Scholar]
- Wang D. S. et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 29, 3092–3100 (2012). [DOI] [PubMed] [Google Scholar]
- An X. et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 15, 516–522 (2010). [DOI] [PubMed] [Google Scholar]
- Bhatti I., Peacock O., Lloyd G., Larvin M. & Hall R. I. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 200, 197–203 (2010). [DOI] [PubMed] [Google Scholar]
- Xue P. et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 3, 406–415 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. L. et al. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J 44, 676–682 (2014). [DOI] [PubMed] [Google Scholar]
- Inoue D. et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol 45, 61–66 (2014). [DOI] [PubMed] [Google Scholar]
- Ben Q. et al. Validation of the Pretreatment Neutrophil-Lymphocyte Ratio as a Predictor of Overall Survival in a Cohort of Patients With Pancreatic Ductal Adenocarcinoma. Pancreas 44, 471–477 (2014). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A. & Balkwill F. Cancer-related inflammation. Nature 454, 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R. & Karin M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelao J. E., Yuan J. M., Gago-Dominguez M., Yu M. C. & Ross R. K. Non-steroidal anti-inflammatory drugs and bladder cancer prevention. Bri J Cancer 82, 1364–1369 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty S. E. et al. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am J Epidemiol 173, 721–730 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calman K. C. Tumour immunology and the gut. Gut 16, 490–499 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nind A. P., Nairn R. C., Rolland J. M., Guli E. P. & Hughes E. S. Lymphocyte anergy in patients with carcinoma. Bri J Cancer 28, 108–117 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino S. et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 15, 6412–6420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner M., Schimanski C. C. & Neurath M. F. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol 12, 7233–7238 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropponen K. M., Eskelinen M. J., Lipponen P. K., Alhava E. & Kosma V. M. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 182, 318–324 (1997). [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Old L. J. & Smyth M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011). [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J. & Bjorgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Bri J Cancer 49, 375–377 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie H. T., Klassen L. W. & Kay H. D. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. J Immunol 134, 230–234 (1985). [PubMed] [Google Scholar]
- Aliustaoglu M. et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology 57, 640–645 (2010). [PubMed] [Google Scholar]
- Sugiura T., Uesaka K., Kanemoto H., Mizuno T. & Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol 20, 4330–4337 (2013). [DOI] [PubMed] [Google Scholar]
- Szkandera J. et al. External validation of the derived neutrophil to lymphocyte ratio as a prognostic marker on a large cohort of pancreatic cancer patients. PloS One 8, e78225, 10.1371/journal.pone.0078225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz M. et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Bri J Cancer 109, 416–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea G. et al. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg 35, 868–872 (2011). [DOI] [PubMed] [Google Scholar]