Abstract
Objective
Vascular endothelial growth factor (VEGF) signaling induces Notch signaling during angiogenesis. Flt-1/VEGF receptor-1 (VEGFR-1) negatively modulates VEGF signaling. Therefore, we tested the hypothesis that disrupted Flt-1 regulation of VEGF signaling causes Notch pathway defects that contribute to dysmorphogenesis of Flt-1 mutant vessels.
Approach and Results
Wild-type (WT) and flt-1−/− mouse embryonic stem (ES) cell-derived vessels were exposed to pharmacological and protein-based Notch inhibitors with and without added VEGF. Vessel morphology, endothelial cell proliferation, and Notch target gene expression levels were assessed. Similar pathway manipulations were performed in developing vessels of zebrafish embryos. Notch inhibition reduced flt-1−/− ES cell-derived vessel branching dysmorphogenesis and endothelial hyper-proliferation, and rescue of flt-1−/− vessels was accompanied by a reduction of elevated Notch targets. Surprisingly, WT vessel morphogenesis and proliferation were unaffected by Notch suppression, Notch targets in WT endothelium were unchanged, and Notch suppression perturbed zebrafish intersegmental vessels (ISVs) but not caudal vein plexuses (CVPs). In contrast, exogenous VEGF caused WT ES cell-derived vessel and zebrafish ISV dysmorphogenesis that was rescued by Notch blockade.
Conclusions
Elevated Notch signaling downstream of perturbed VEGF signaling contributes to aberrant flt-1−/− blood vessel formation. Notch signaling may be dispensable for blood vessel formation when VEGF signaling is below a critical threshold.
Keywords: vessel branching, VEGF, Flt-1, Notch, angiogenesis, ES cells, zebrafish
INTRODUCTION
Oxygen and nutrient delivery in developing embryos depends on the formation of vascular networks, and many pathologies, including solid tumor growth, also involve the development and remodeling of blood vessels.1 Growth factors released from nutrient-deprived tissues initiate angiogenic sprouting from pre-existing vessels. Endothelial cells emerge from parent vessels and begin migrating outward using local guidance cues to ensure proper extension.2 As the sprout lengthens, extrinsic patterning cues provided by other cell types and the extracellular matrix guide the sprout toward other vessels or sprouts.3,4 A connection forms between the nascent sprout and its target, and this newly-formed branch acquires a patent lumen for blood flow.5 A range of molecular mechanisms, including the VEGF and Notch pathways, regulate these cellular processes for vascular network expansion.
Vascular endothelial growth factor (VEGF)-A induces and directs endothelial cell sprouting. Binding of VEGF-A to the tyrosine kinase receptor Flk-1 (VEGFR-2) initiates signaling in endothelial cells to promote migration, proliferation, and survival.6 Flt-1 (VEGFR-1) binds VEGF-A with 10-fold higher affinity than Flk-1 but acts primarily as a ligand sink, limiting the amount of VEGF-A that can access the Flk-1 receptors on the endothelial cell surface.7 Both membrane-bound Flt-1 (mFlt-1) and soluble Flt-1 (sFlt-1) modulate endothelial cell proliferation,8 but sFlt-1 uniquely regulates vessel branching by contributing to a local sprout guidance mechanism.2 Expression of both VEGF receptors is regulated during sprouting angiogenesis as part of a dynamic competition among endothelial cells to lead the extending sprout,9 and the Notch pathway is important in the competition for tip cell position.
The Notch pathway facilitates cell-cell communication in many contexts, and it is important for lateral inhibition.10 As one cell acquires a particular role or fate, the Notch pathway is utilized to restrict neighboring cells from acquiring the same fate or phenotype, as seen in Drosophila trachea development,11 and epidermal differentiation.12 Endothelial cells express the Notch1 and Notch4 receptors, as well as the ligands Delta-like 1 (Dll1), Dll4, Jagged1 and Jagged2.13 Ligand-binding of Notch receptors leads to a series of enzymatic cleavages that result in release of the intracellular domain. The Notch intracellular domain (NICD) translocates into the nucleus and forms a complex that activates the transcription of target genes such as Hes and Hey. Notch coordinates vessel sprouting such that suppression of Notch signaling yields increased vessel sprouting.9,14 The Notch pathway also negatively modulates endothelial cell division, and reduced Notch signaling promotes endothelial cell proliferation.15
Crosstalk between the VEGF and Notch pathways is important for orchestrating endothelial cell behaviors during angiogenesis.16,17 In response to VEGF stimulation, some endothelial cells initiate new sprouts and emerge as “tip” cells, while other cells follow as stalk cells and contribute to vessel expansion through proliferation.18 To accomplish this coordination, VEGF signals through Flk-1 to increase Dll4 expression on emerging tip cells. Tip cell Dll4 ligands engage Notch receptors on adjacent stalk cells to reduce their sensitivity to VEGF through increased expression of Flt-119,20 and reduced expression of Flk-1 and Flt-4.21-24 Here we directly test the hypothesis that Flt-1 is critical to VEGF-Notch crosstalk in developing blood vessels. We show that Flt-1 is upstream of Notch signaling through regulation of VEGF signaling, and thus mediates an important feedback loop in VEGF-Notch pathway crosstalk during blood vessel formation.
RESULTS
Notch Inhibition Rescues Branching and Proliferation Defects in flt-1−/− Vessels
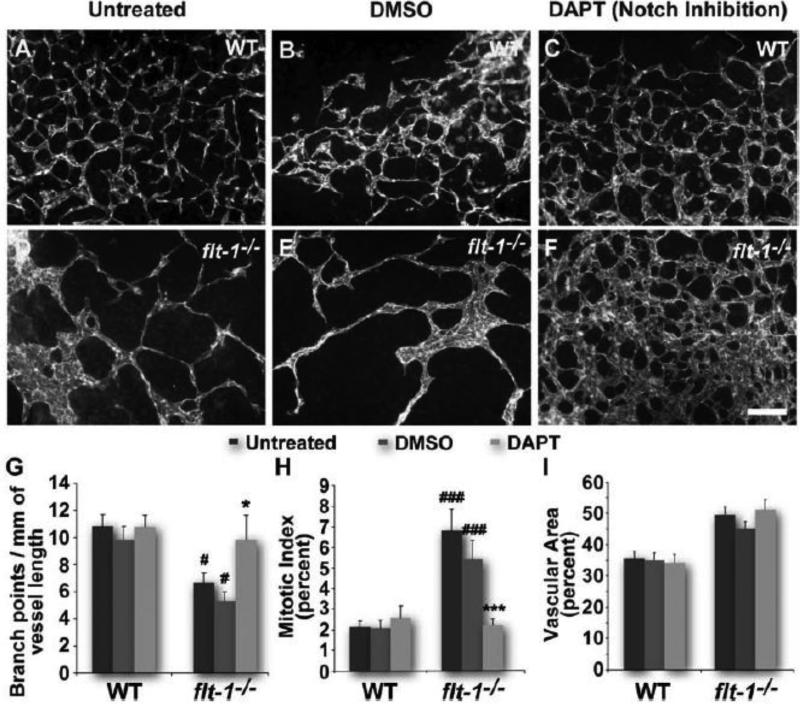
Loss of Flt-1 leads to vessel overgrowth and branching dysmorphogenesis through elevated VEGF signaling.7,8,25 Because Notch signaling is activated by VEGF signaling,17 we hypothesized that elevated VEGF signaling due to genetic loss of flt-1 increases Notch signaling and contributes to vessel branching defects. To test this hypothesis, we utilized differentiation of mouse ES cells in vitro to form primitive lumenized vessel networks in the context of other embryonic cell types.26 Although these vessels lack blood flow, their development in vitro mimics in vivo development of primitive vessel networks.27 First, we manipulated Notch signaling during ES cell differentiation by incubation with the Notch inhibitor DAPT during the angiogenic phase (days 6-8). Although WT tip cell numbers increased with Notch inhibition (Figure I in online-only Data Supplement), vessel branching and proliferation, as well as vessel area and diameter, were not significantly different from controls (Figure 1A-C, G-I; Figure IIA in online-only Data Supplement). Interestingly, loss of flt-1 (flt-1−/−) also led to increased tip cell numbers despite an overall reduction in vessel branching (Figure I in online-only Data Supplement, Figure 1D-F, G), suggesting the existence of multiple control points for successful branch formation. In contrast to Notch-inhibited WT vessels, the reduced vessel branching of ES cell-derived vessels lacking Flt-1 was rescued with Notch inhibition (Figure 1D-F, G), despite no change in tip cell numbers with DAPT treatment (Figure I in online-only Data Supplement). Notch blockade also unexpectedly reduced the excessive endothelial proliferation characteristic of flt-1−/− ES cell-derived vessels (Figure 1H). However, the increased vessel area and diameter of flt-1−/− vessels was not rescued by Notch blockade (Figure 1I; Figure IIA in online-only Data Supplement).
Figure 1. Notch inhibition by DAPT rescues the dysmorphogenesis of flt-1−/− blood vessels.
Wild-type (A-C) and flt-1−/− (D-F) day 8 ES cell-derived vessels stained for PECAM-1. Scale bar, 100 μm. Dy 8 vessel networks assessed for branch points per vessel length (G). #, p≤0.05 vs. WT of same treatment group. *, p≤0.05 vs. flt-1−/−/untreated or flt-1−/−/DMSO. Dy 7 vessel mitotic indicies were quantified by counting PH3+/PECAM-1+ cells and normalizing to total PECAM-1+ cells (H). ###, p≤0.0005 vs. WT of same treatment group. ***, p≤0.0005 vs. flt-1−/−/untreated or flt-1−/−/DMSO. Vessel area relative to total area for dy 8 ES cell-derived blood vessels (I). Values are averages +/− standard error of the mean (SEM).
To further investigate Flt-1 interactions with Notch, we disrupted Notch signaling with Dll4-Fc, a competitive inhibitor of Notch-Dll4 interactions.28 Similar to Notch inhibition with DAPT, WT ES cell-derived vessel branching, area, and endothelial cell mitotic index were unaffected by Dll4-Fc treatment (Figure 2A-C, G-I). However, the reduced vessel branching and elevated endothelial cell mitotic index of flt-1−/− mutant vessels was normalized by Dll4-Fc exposure (Figure 2D-F, G-H). Similar to DAPT-mediated Notch reduction, the vascular area of flt-1−/− ES cell-derived vessels was unchanged by Dll4-Fc (Figure 2D-F, I). Taken together, these results indicate that although reduced Notch signaling increased WT tip cells, this did not affect WT vessel branching; in contrast, vessels lacking flt-1 function were phenotypically rescued by Notch blockade.
Figure 2. Flt-1−/− blood vessel dysmorphogenesis is rescued by Dll4-Fc treatment.
Wild-type (A-C) and flt-1−/− mutant (D-F) day 8 ES cell-derived vessels stained for PECAM-1. Scale bar, 100 μm. Dy 8 branch points were counted and normalized to vessel length (G). #, p≤0.05 vs. WT of same treatment group. *, p≤0.05 vs. flt-1−/−/untreated or flt-1−/−/BSA. Mitotic indicies calculated for dy 7 vessels (H). ##, p≤0.005 vs. WT of same treatment group. **, p≤0.005 vs. flt-1−/−/untreated or flt-1−/−/BSA. Dy 8 ES cell-derived vessels assessed for vascular area (I). Values are averages +/− SEM.
Because WT ES cell-derived vessels were unexpectedly phenotypically unaffected by Notch blockade, we asked whether this was a model-specific effect or evidence that Notch effects are also context-dependent in vivo. To test this idea, we analyzed the developing vessels in the zebrafish embryo, an established model of blood vessel formation that occurs in the context of blood flow.29 Notch manipulations in zebrafish are reported to affect vessel formation in certain scenarios,30,31 but not all situations of vessel growth.32 Moreover, the caudal vein plexus (CVP) does not exhibit detectable Notch activation via Notch reporter readout (Wiley et al, in revision). Therefore, we subjected zebrafish embryos to Notch inhibition via DAPT treatment and analyzed them for vascular defects. We found perturbed intersegmental vessel (ISV) development in Notch-inhibited embryos (Figure 3A-C), similar to previous reports.30,31 However, in these same embryos, the CVPs were unaffected, as determined by the presence of multiple lumenized vessels conducting blood flow. (Figure 3A-C). These observations demonstrate that effects of Notch inhibition on blood vessel formation in in vivo are also context-dependent.
Figure 3. Notch inhibition by DAPT disrupts zebrafish intersegmental vessel (ISV) formation but has no effect on the developing caudal vein plexus (CVP).
DMSO-treated (A) and DAPT-treated (B) 48 hpf Tg(kdrl:GFP) zebrafish embryos. Scale bars, 100 μm. Embryos with normal (top inset, A) and defective ISVs (top inset, B), as well as normal (bottom inset, A and B) and defective CVPs, were quantified (C). ###, p≤0.0001 vs. ISV/DMSO. ***, p≤0.0001 vs. ISV/DAPT. Values are percentages.
VEGF-A-Disrupted Vessel Morphology is Affected by Notch Blockade
Since loss of flt-1 elevates VEGF-A-mediated signaling,8 we reasoned that the differences in response to Notch blockade between WT and flt-1−/− ES cell-derived vessels might result from the amount of VEGF signaling experienced by the vessels. Thus we hypothesized that Notch inhibition would elicit changes in WT vessels exposed to ectopic VEGF-A. To test this idea, we inhibited Notch signaling in WT and flt-1−/− vessels with and without addition of exogenous VEGF-A. Added VEGF-A caused a significant decrease in WT vessel branching, and an increase in endothelial proliferation and vessel area, suggesting that added VEGF-A recapitulates, though not fully, the loss of flt-1 (Figure 4A-C, G-I). Notch inhibition of VEGF-A-treated WT vessels partially normalized these changes (Figure 4A-C, G-I). VEGF-A treatment of flt-1−/− ES cell-derived vessels had no effect on vessel branching, area, or endothelial mitotic index, consistent with the idea that loss of Flt-1 elevates VEGF signaling independent of additional ligand (Figure 4D-I). Exposure to ectopic VEGF-A and Notch blockade rescued flt-1−/− vessel branching dysmorphogenesis and endothelial mitotic index without vessel area rescue, similar to Notch blockade alone (Figure 4D-I). These results indicate that WT vessels are not intrinsically defective in Notch-mediated responses, but rather that Notch responsiveness depends on the level of VEGF signaling.
Figure 4. Notch blockade rescues vessel defects induced by added VEGF.
VEGF-treated WT (A-C) and flt-1−/− (D-F) day 8 ES cell-derived vessels stained for PECAM-1. Scale bar, 100 μm. Dy 8 vessels evaluated for branch points per vessel length (G). *, p≤0.05 vs. WT/untreated or WT/VEGF+DAPT. ##, p≤0.002 vs. WT/untreated. ***, p≤0.008 vs. flt-1−/−/untreated, flt-1−/−/VEGF, or flt-1−/−/VEGF+DMSO. Mitotic indicies of dy 7 ES cell-derived vessels (H). *, p≤0.05 vs. WT/VEGF. **, p≤0.01 vs. WT/VEGF or WT/VEGF+DMSO. #, p≤0.05 vs. WT/untreated. ***, p≤0.006 vs. flt-1−/−/untreated, flt-1−/−/VEGF, or flt-1−/−/VEGF+DMSO. Dy 8 vascular area (I). *, p≤0.05 vs. WT/VEGF or WT/VEGF+DMSO. #, p≤0.002 vs WT/untreated. Values are averages +/− SEM.
We next manipulated VEGF and Notch signaling in zebrafish embryos to further explore the influence of VEGF signaling levels on the Notch responsiveness of developing blood vessels. Zebrafish ISVs are more sensitive to Vegf manipulations than the CVP.33 For this reason, we focused on ISV defects in Notch-inhibited embryos with and without the over-expression of Vegfaa via heat-shock-induction of the Tg(hsp70l:vegfaa) transgene. Increased Vegfaa induced significant morphological perturbations in the ISVs of developing zebrafish (Figure 5A, C, E). Notch blockade in embryos over-expressing Vegfaa led to an additional and significant increase in ISV defects (Figure 5, B, D-F). Although zebrafish vessels exposed to Notch blockade in conjunction with increased VEGF signaling exhibited a distinct phenotypic outcome from ES cell-derived vessels, the interaction between the VEGF and Notch pathways was consistent between the two models as seen by the increase in defective zebrafish ISVs. Taken together, these observations indicate that endothelial cells vary in their responsiveness to Notch, depending upon VEGF signaling levels.
Figure 5. Notch inhibition by DAPT exacerbates VEGF-A-mediated zebrafish intersegmental vessel (ISV) defects.
ISVs from DMSO- and DAPT-treated WT (A-B) and Tg(hsp70l:vegfaa) (C-D) zebrafish embryos at 48 hpf visualized by endothelial expression of GFP [Tg(kdrl:GFP)]. Scale bar, 50 μm. Embryos with affected ISVs (B-D) were quantified, and penetrance was determined as the percent of embryos with an ISV phenotype (E). **, p≤0.005 vs. WT/DMSO. ##, p≤0.007 vs. WT/DMSO. *, p≤0.016 vs. Tg(hsp70l:vegfaa)/DMSO. Values are averages +/− SEM. Of the Tg(hsp70l:vegfaa) embryos with an ISV phenotype, the percent of somites with affected ISVs was determined (F). *, p≤0.0001 for DMSO vs. DAPT. Severities for individual zebrafish are shown as diamonds, with bars representing averages +/− SEM.
Elevated Notch Target Gene Expression in flt-1−/− Vessels is Rescued by Notch Blockade
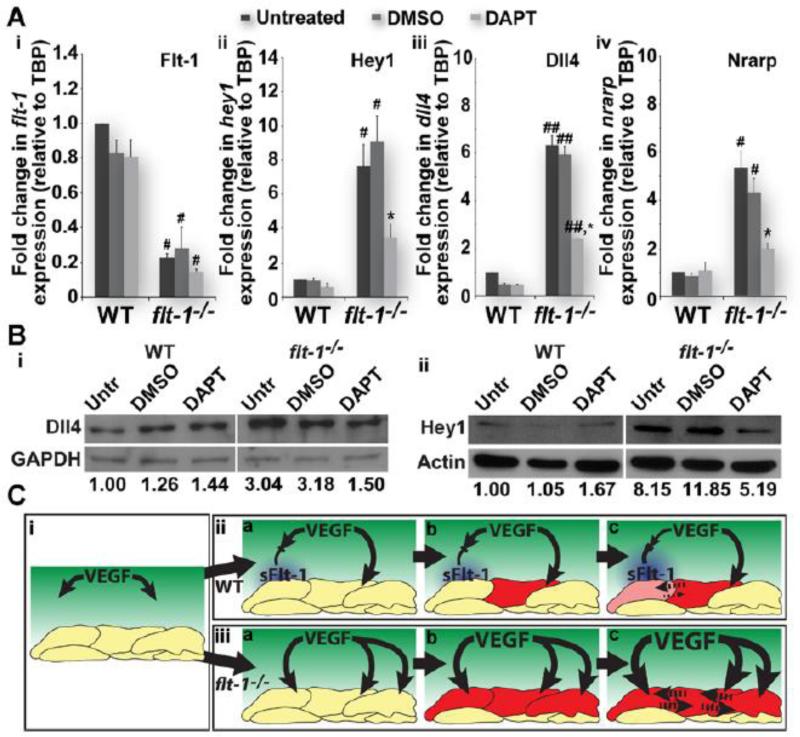
To determine if Notch pathway transcriptional targets are elevated in flt-1−/− mutant vessels, we dissociated WT and flt-1−/− ES cell cultures and used magnetic-bead assisted cell sorting (MACS) to enrich for endothelial cells. Real-time quantitative PCR was used to assess RNA levels of the Notch targets hey1, dll4, and nrarp. As expected, Flt-1 RNA levels were reduced in enriched endothelial cell preps from flt-1−/− vessels, while all three Notch targets were increased at least 5-fold (Figure 6A). Interestingly, Notch target gene RNA levels in WT enriched endothelial cell preps showed no significant changes with Notch blockade (Figure 6Aii-iv). In contrast, the elevated expression of Notch targets in flt-1−/− mutant preps was rescued back down toward WT levels with Notch blockade (Figure 6Aii-iv).
Figure 6. Loss of endothelial flt-1 up-regulates the Notch pathway.
Flt-1−/− endothelial cell-enriched preps increases Notch target RNAs (A). Real-time quantitative PCR of Flt-1 (Ai) and Notch pathway components Hey1 (Aii), Dll4 (Aiii), and Nrarp (Aiv) from untreated, vehicle control-treated, and DAPT-treated WT and flt-1−/− endothelial cell-enriched preps. (Ai), #, p≤0.05 vs. WT of the same treatment group. (Aii), #, p≤0.05 vs. WT of the same treatment group. *, p≤0.05 vs. flt-1−/−/untreated or flt-1−/−/DMSO. (Aiii), ##, p≤0.008 vs. WT of the same treatment group. *, p≤0.01 vs. flt-1−/−/untreated or flt-1−/−/DMSO. (Aiv), #, p≤0.05 vs. WT of the same treatment group. *, p≤0.05 vs. flt-1−/−/untreated or flt-1−/−/DMSO. Values are averages + SEM. Flt-1−/− endothelial cell-enriched preps have elevated Notch target proteins (B). Representative Western blots for Dll4 (75 kD) and Hey1 (34 kD), as well as GAPDH (36 kD) and actin (45 kD) (for normalization), from untreated, vehicle control-treated, and DAPT-treated WT and flt-1−/− ES cell-derived endothelial cell-enriched preps. Dll4 signal intensities were normalized to those for corresponding GAPDH control bands, and untreated WT levels were set to 1 for comparison (Bi). Hey1 levels were also compared across treatment groups and cell types using actin control bands, just as described for Dll4 and GAPDH (Bii). Model of Flt-1-mediated crosstalk between the VEGF and Notch pathways (C). The model illustrates how Flt-1 (blue), and soluble Flt-1 (sFlt-1) in particular (iia-c), modulates the concentration of available VEGF (green, i-iii) that induces Dll4 expression in endothelial cells (red and pink cells, iia-c). Notch signaling between adjacent cells (dotted lines in iic) then reinforces competition dynamics for sprouting (iic), which completes the Flt-1-mediated feedback loop between VEGF and Notch signaling pathways (iic). In the absence of Flt-1 activity (iiia-c), VEGF induces widespread activation of Dll4 (red cells, iiia-c), and thus Notch signaling is elevated, and normal competition dynamics among endothelial cells are disrupted (dotted lines in iiic). In addition, without Flt-1-mediated feedback, VEGF signaling is unchecked (iiic), exacerbating the excessive Notch signaling and further undermining normal sprouting and proliferation.
We next evaluated protein levels of Notch pathway components in WT and flt-1−/− endothelial cell-enriched preps exposed to Notch blockade. Protein levels for the transcription factor Hey1 and the Notch1 ligand Dll4, which are also Notch targets, were also highly elevated in the flt-1−/− EC-enriched preps (Figure 6B). These elevated levels of Notch targets were partially rescued with Notch blockade. However, Notch targets were unchanged in WT EC-enriched preps exposed to Notch blockade (Figure 6B). The lack of change in Notch target gene expression in the WT scenario supports the finding that Notch blockade does not affect the overall morphology of WT ES cell-derived vessels, while the elevation with loss of flt-1 and partial rescue with Notch blockade suggests that Notch is a required effector downstream of elevated VEGF signaling.
DISCUSSION
The rescue of flt-1−/− ES cell-derived vessel branching dysmorphogenesis by Notch blockade demonstrates that Flt-1 regulation of VEGF signaling upstream of the Notch pathway is critical for normal vascular development. In addition, VEGF over-expression in zebrafish impaired the ability of Flt-1 to modulate VEGF activity and induced ISV defects that were further affected by Notch suppression. Previous studies showed that Flt-1 expression was up-regulated downstream of Notch signaling, but did not critically test flt-1 function in the cross-talk.9,20,22,34,35 Our data support an additional requirement for flt-1 upstream of Notch via modulation of VEGF signaling. Thus Flt-1 mediates a critical component of the feedback loop that governs coordination of endothelial cell behavior during vascular development (Figure 6C).
We propose that Flt-1 mediates crosstalk between the VEGF and Notch pathways by keeping VEGF signaling at appropriate levels to effectively use Notch for lateral inhibition (Figure 6Cii). Moreover, Flt-1 completes the VEGF-Notch feedback loop by further reinforcing the differential responsiveness of endothelial cells to the oncoming VEGF. Loss of Flt-1 modulation of VEGF signaling results in excessively high Notch signaling, undermining the VEGF-Notch feedback loop and disrupting coordination of endothelial cell phenotypes (Figure 6Ciii). Thus, flt-1−/− endothelial cells are predicted to experience excessive lateral inhibition via Notch signaling. Consistent with this model, we found that the reduced branching and elevated endothelial proliferation in flt-1−/− blood vessel networks25,36 was rescued by lowering elevated levels of Notch signaling through Notch blockade. Notch blockade in zebrafish ISVs exposed to ectopic VEGF elicited additional changes in vessel morphology, suggesting that VEGF-mediated effects on vessel formation are influenced by Notch manipulation. RNA and protein levels of Notch targets in ES cell-derived endothelial cells are consistent with the idea that loss of Flt-1 modulation of VEGF signaling leads to Notch hyper-activation. In this way, Notch signaling downstream of VEGF is required for the defects in flt-1−/− blood vessel formation. Bentley et al. developed a computational model of VEGF and Notch signaling interactions during vessel branching, and their simulation results suggested a need for Notch signaling (i.e. lateral inhibition) to be “turned down” in situations of high VEGF signaling.37 The current study provides experimental evidence that Flt-1 regulates the feedback loop between VEGF and Notch signaling to effectively “turn down” signaling levels of both pathways, and thus supports proper coordination of endothelial cell behaviors.
Excessive flt-1−/− endothelial cell proliferation is reduced with Notch inhibition, suggesting a unique relationship between upstream Flt-1 regulation of VEGF signaling and the downstream Notch pathway in modulating endothelial proliferation. Increased Notch signaling causes endothelial cells to adopt a stalk cell phenotype14 but is also known to suppress endothelial cell proliferation.17,19,38-40 However, stalk cells are presumed to undergo division more frequently than tip cells for sprout elongation,18 which is seemingly incongruent with stalk cells experiencing elevated Notch signaling.14 Interestingly, flt-1 mutant endothelial cells over-proliferate despite having elevated levels of Notch signaling, and both elevated Notch target levels and elevated endothelial cell division were rescued by Notch blockade. In one model consistent with these observations, flt-1−/− endothelial cells have elevated lateral inhibition (Figure 6Ciii), and Notch blockade releases some endothelial cells from this lateral inhibition, allowing them to contribute more to branching and less to vessel expansion via proliferation. Nevertheless, further investigation will be required to elucidate how Flt-1 integrates VEGF and Notch signals to regulate endothelial cell division.
Wild-type ES cell-derived vessels and zebrafish embryo CVP exposed to Notch blockade showed no obvious changes in overall vessel morphology or endothelial cell proliferation despite an increase in tip cell numbers, and Notch blockade did not affect Notch target gene expression levels in WT endothelial cells. In contrast, Notch blockade in the postnatal retina, in tumors, and in wound healing models increases vessel density and branching, although these increases do not necessarily result in more lumenized conduits.14,22,41-43 Thus an increase in tip cells may not inherently result in more patent vessel branches, as seen in the current study. Furthermore, not all Notch perturbations affect vessel branching, as previous observations of embryonic and yolk sac vessels in Notch-manipulated mice revealed defects in network remodeling and arterio-venous specification rather than plexus formation.44-46 These data and our results suggest that non-Notch pathways may act in parallel or in place of Notch to regulate vessel branching in certain situations. We hypothesized that the level of VEGF signaling might determine the involvement of Notch signaling in endothelial cells and thus their response to Notch blockade. Indeed, we found that adding VEGF ligand to ES cell-derived vessels or developing zebrafish ISVs affected vessel formation, and Notch blockade had additional effects on these vessels. These results are consistent with previous studies showing that endothelial cells respond to Notch inhibition more strongly with added VEGF.32,47,48 Thus, Notch-based therapies will need to be developed with consideration of the treatment context.
Pathological conditions such as cancer and diabetes have as hallmarks mis-regulated angiogenesis associated with aberrant VEGF signaling. Anti-angiogenic therapies, particularly those targeting the VEGF pathway, have had limited success due to acquired resistance and suboptimal efficacy.49 Notch perturbations in mouse tumor and hind-limb ischemia models increase the formation of poorly-perfused vessels.41-43 This undermines recovery following ischemia,41 but for solid tumors it reduces tumor burden,42,43 supporting the potential for Notch-based cancer therapies. Thus, understanding the systemic effects of disrupted Notch signaling50 and how Notch intersects with other pathways will be essential for development of effective treatments. In the present study, we found that Flt-1 is important in VEGF-Notch signaling crosstalk, and that loss of flt-1 disrupts VEGF signaling which in turn perturbs the Notch pathway and contributes to flt-1−/− vessel dysmorphogenesis.
Supplementary Material
SIGNIFICANCE.
In the current study, we have shown that the VEGF receptor Flt-1 plays an important role in the crosstalk between VEGF and Notch signaling to coordinate endothelial cell dynamics during blood vessel formation. Previous studies showed that Notch signaling up-regulates Flt-1 expression. Here we have found evidence for an additional requirement for Flt-1 in regulating VEGF signaling upstream of the Notch pathway. Thus, disrupted Flt-1 activity undermines this critical VEGF-Notch feedback loop and perturbs the coordination of endothelial cells during angiogenesis. Because therapeutic strategies, particularly those treating solid tumors, are being developed to target these pathways, we believe our study addresses the important need for understanding how these pathways intersect and possible systemic effects of disrupted signaling.
ACKNOWLEDGMENTS
The authors thank Dr. Erich Kushner for critical reading of the manuscript and technical assistance with the endothelial cell isolations; and Catherine Wright for technical assistance with quantitative PCR assays.
Sources of Funding.
This work was supported by NIH grants R01HL43174 (to VLB), F32HL95359 (to JCC) and T32-CA009156-35 (to KPM).
Abbreviations
- VEGF
Vascular Endothelial Growth Factor
- mFlt-1
membrane-bound Flt-1
- sFlt-1
soluble Flt-1
- Dll4
Delta-like 4
- NICD
Notch intracellular domain
- ISV
Intersegmental vessel
- CVP
Caudal vein plexus
Footnotes
Disclosures.
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- 4.Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 7.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol. 2004;164:1531–1535. doi: 10.1016/S0002-9440(10)63711-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol. 2008;181:847–858. doi: 10.1083/jcb.200709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, Schulte-Merker S, Gerhardt H. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 10.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 11.Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- 12.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 14.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 15.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 16.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 17.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2008;75:144–154. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Funahashi Y, Shawber CJ, Vorontchikhina M, Sharma A, Outtz HH, Kitajewski J. Notch regulates the angiogenic response via induction of VEGFR-1. J Angiogenes Res. 2010;2:3. doi: 10.1186/2040-2384-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 22.Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shawber CJ, Funahashi Y, Francisco E, et al. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest. 2007;117:3369–3382. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 25.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 26.Kearney JB, Bautch VL. In vitro differentiation of mouse ES cells: hematopoietic and vascular development. Methods Enzymol. 2003;365:83–98. doi: 10.1016/s0076-6879(03)65006-8. [DOI] [PubMed] [Google Scholar]
- 27.Larina IV, Shen W, Kelly OG, Hadjantonakis AK, Baron MH, Dickinson ME. A membrane associated mCherry fluorescent reporter line for studying vascular remodeling and cardiac function during murine embryonic development. Anat Rec (Hoboken) 2009;292:333–341. doi: 10.1002/ar.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerfield M. The Zebrafish Book, a Guide for the Laboratory Use of Zebrafish (Danio Rerio) University of Oregon Press; 2000. [Google Scholar]
- 30.Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, le Noble F. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 32.Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- 33.Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol. 2011;13:686–692. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 35.Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood. 2002;99:2397–2407. doi: 10.1182/blood.v99.7.2397. [DOI] [PubMed] [Google Scholar]
- 37.Bentley K, Gerhardt H, Bates PA. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2008;250:25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Liu ZJ, Xiao M, Balint K, Soma A, Pinnix CC, Capobianco AJ, Velazquez OC, Herlyn M. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J. 2006;20:1009–1011. doi: 10.1096/fj.05-4880fje. [DOI] [PubMed] [Google Scholar]
- 39.Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol. 2004;24:8813–8822. doi: 10.1128/MCB.24.20.8813-8822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trindade A, Kumar SR, Scehnet JS, Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS, Duarte A. Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos. Blood. 2008;112:1720–1729. doi: 10.1182/blood-2007-09-112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Haj Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ Res. 2010;107:283–293. doi: 10.1161/CIRCRESAHA.110.221663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 43.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 44.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–1832. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs LT, Starling C, Chervonsky AV, Gridley T. Notch1 activation in mice causes arteriovenous malformations phenocopied by ephrinB2 and EphB4 mutants. Genesis. 2010;48:146–150. doi: 10.1002/dvg.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Copeland JN, Feng Y, Neradugomma NK, Fields PE, Vivian JL. Notch signaling regulates remodeling and vessel diameter in the extraembryonic yolk sac. BMC Dev Biol. 2011;11:12. doi: 10.1186/1471-213X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao L, Arany PR, Wang YS, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials. 2009;30:4085–4093. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell. 2012;22:489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 50.Yan M, Callahan CA, Beyer JC, Allamneni KP, Zhang G, Ridgway JB, Niessen K, Plowman GD. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6–7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.