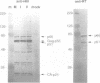
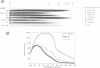
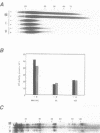
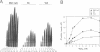Abstract
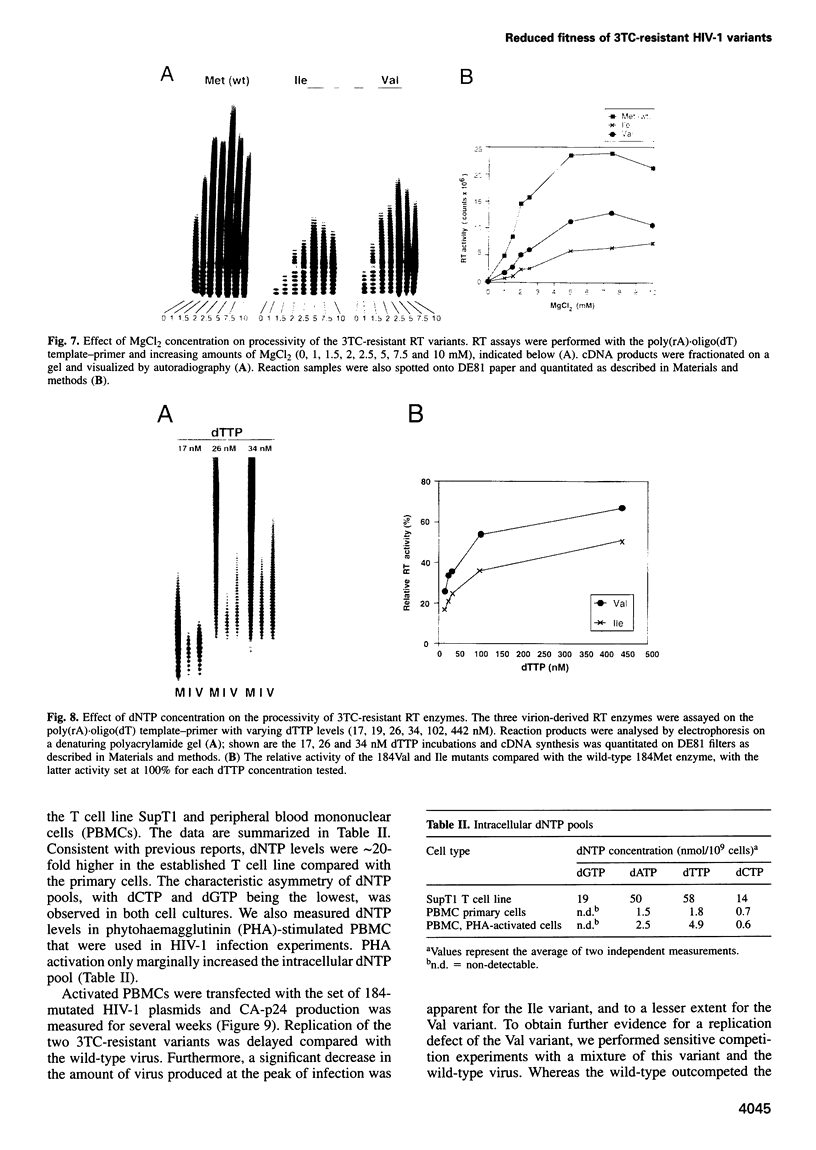
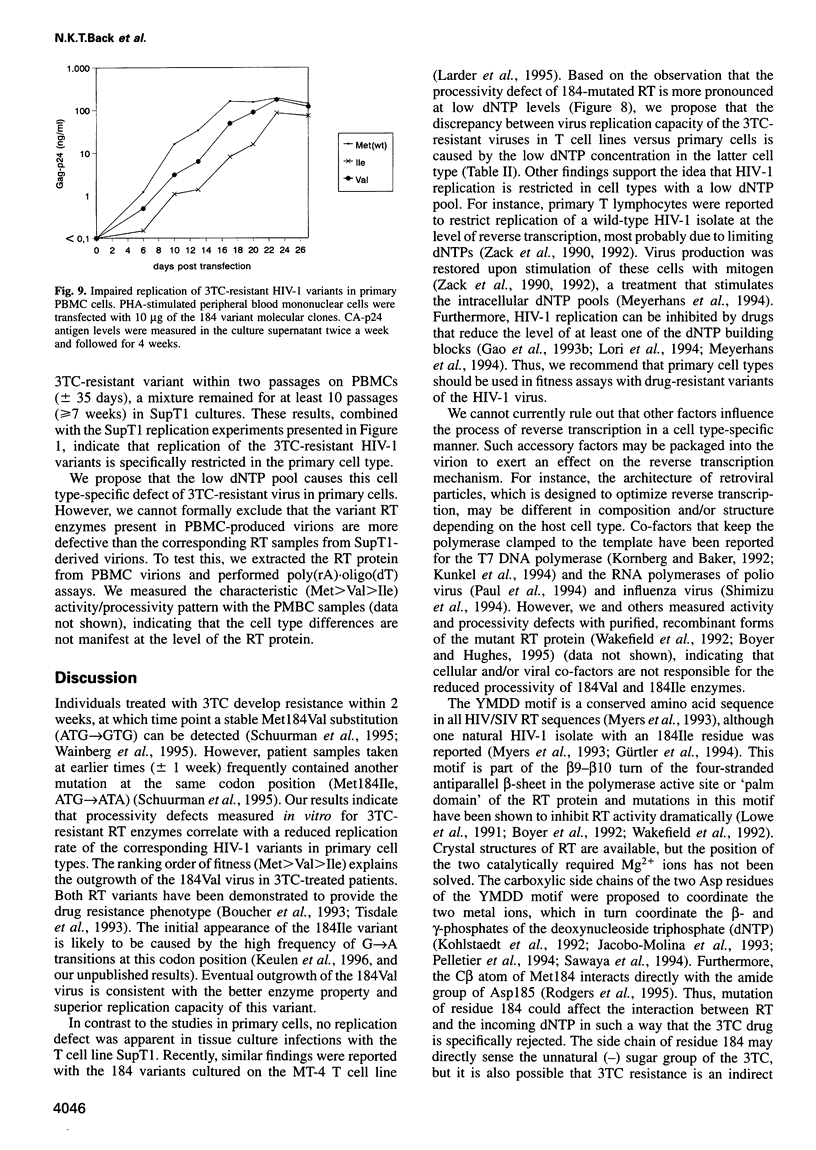
Human immunodeficiency virus type 1 (HIV-1) variants with resistance mutations in the reverse transcriptase (RT) gene appear during drug therapy with the nucleoside analogue 2',3'-dideoxy-3'-thiacytidine (3TC). These resistance mutations alter the methionine (Met) residue of the conserved YMDD motif, which is part of the catalytic core of the RT enzyme. Isoleucine (Ile) variants are initially observed, followed by the appearance and eventual outgrowth of viruses encoding valine (Val). Similar replication kinetics were measured for wild-type and 3TC-resistant HIV-1 viruses in tissue culture infections of a T cell line, but we measured reduced polymerase activity for the two mutant RT enzymes compared with the wild-type enzyme (Ile = 43% and Val = 67%). Gel analysis of the reverse transcription products revealed that both 3TC-resistant RT mutants produce significantly shorter cDNA molecules than the wild-type enzyme [Met (wt)>Val>Ile], indicating that 3TC-resistant RT polymerases are less processive enzymes. Interestingly, these enzyme defects were more pronounced under limiting dNTP concentrations and we therefore assayed virus replication in primary cells that contain relatively low dNTP levels. Under these conditions, we measured significantly reduced replication kinetics for the 3TC-resistant HIV-1 variants [Met (wt)>Val>Ile]. If the level of virus replication can be similarly reduced in 3TC-treated patients that develop drug-resistant HIV-1 variants, this may be of considerable clinical benefit.
Full text
PDF
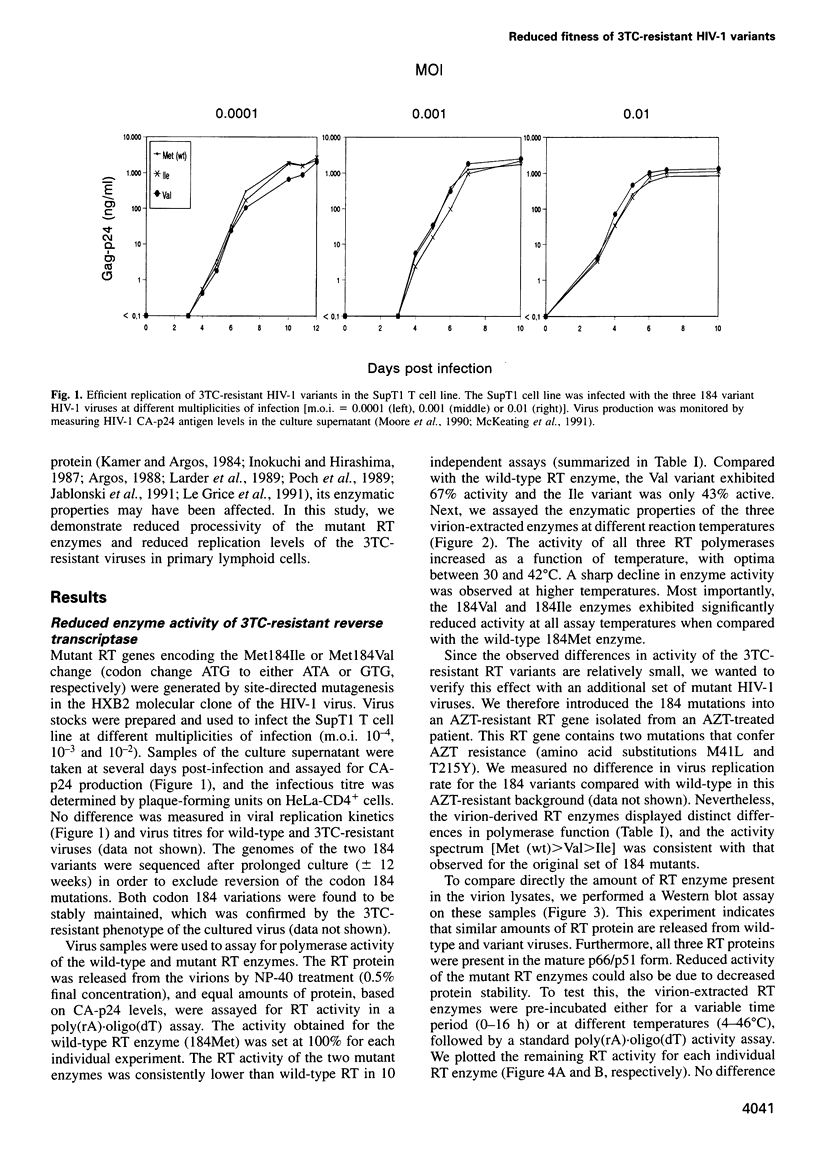
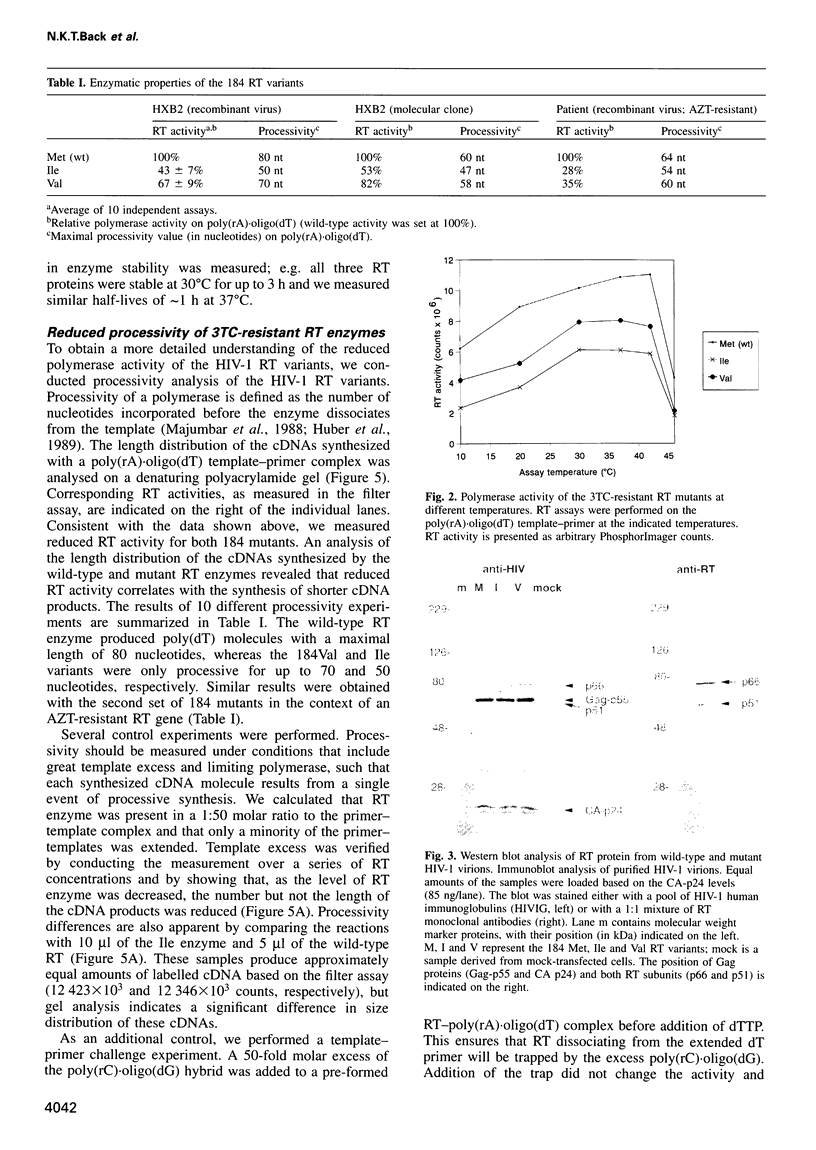
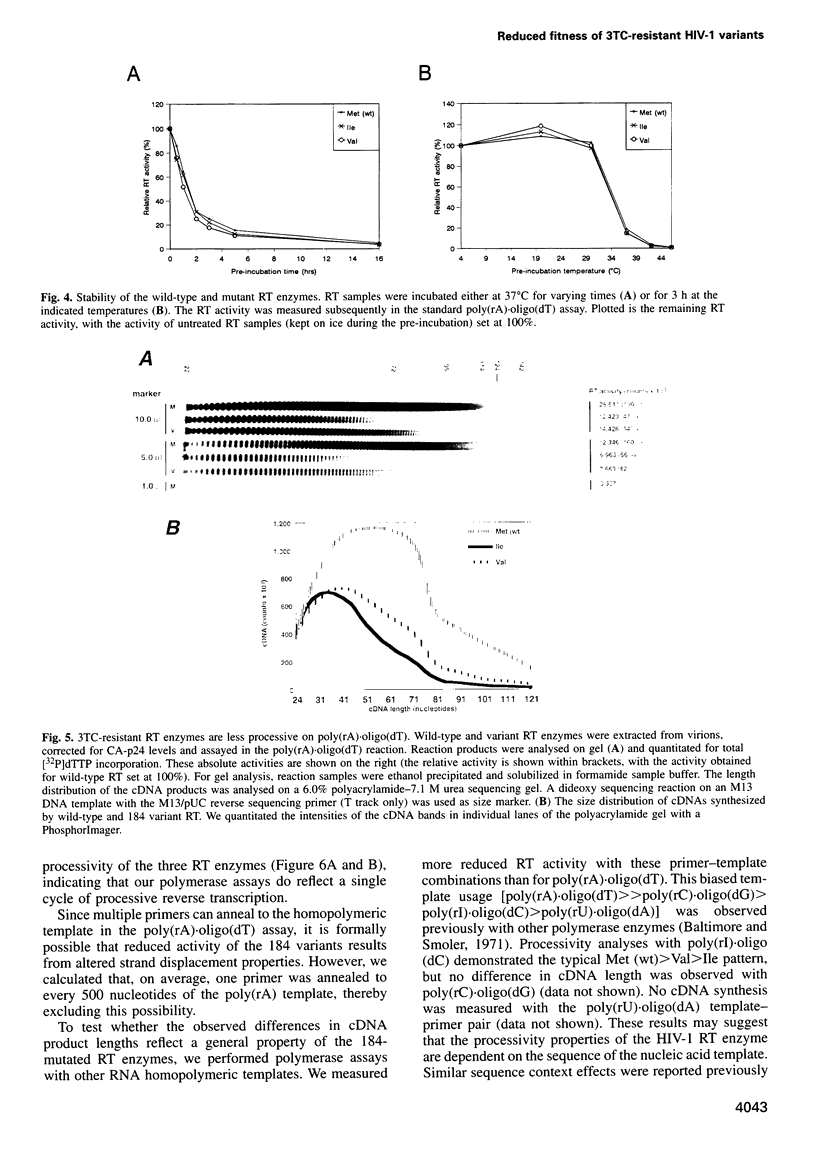
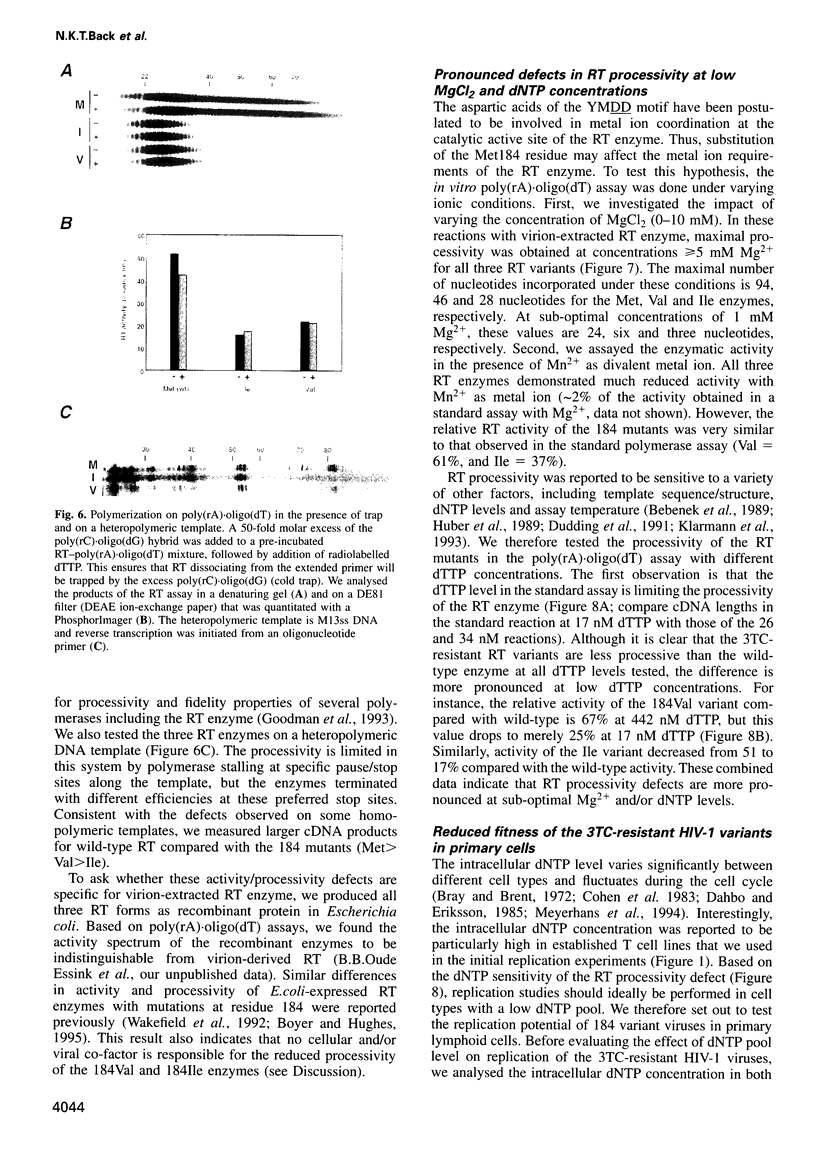



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebenek K., Abbotts J., Roberts J. D., Wilson S. H., Kunkel T. A. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J Biol Chem. 1989 Oct 5;264(28):16948–16956. [PubMed] [Google Scholar]
- Boucher C. A., Cammack N., Schipper P., Schuurman R., Rouse P., Wainberg M. A., Cameron J. M. High-level resistance to (-) enantiomeric 2'-deoxy-3'-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993 Oct;37(10):2231–2234. doi: 10.1128/aac.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. L., Ferris A. L., Hughes S. H. Cassette mutagenesis of the reverse transcriptase of human immunodeficiency virus type 1. J Virol. 1992 Feb;66(2):1031–1039. doi: 10.1128/jvi.66.2.1031-1039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. L., Hughes S. H. Analysis of mutations at position 184 in reverse transcriptase of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1995 Jul;39(7):1624–1628. doi: 10.1128/aac.39.7.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G., Brent T. P. Deoxyribonucleoside 5'-triphosphate pool fluctuations during the mammalian cell cycle. Biochim Biophys Acta. 1972 May 10;269(2):184–191. doi: 10.1016/0005-2787(72)90425-x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Coates J. A., Cammack N., Jenkinson H. J., Jowett A. J., Jowett M. I., Pearson B. A., Penn C. R., Rouse P. L., Viner K. C., Cameron J. M. (-)-2'-deoxy-3'-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992 Apr;36(4):733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. A., Cammack N., Jenkinson H. J., Mutton I. M., Pearson B. A., Storer R., Cameron J. M., Penn C. R. The separated enantiomers of 2'-deoxy-3'-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992 Jan;36(1):202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995 Jan 27;267(5197):483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- Cohen A., Barankiewicz J., Lederman H. M., Gelfand E. W. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J Biol Chem. 1983 Oct 25;258(20):12334–12340. [PubMed] [Google Scholar]
- Dahbo Y., Eriksson S. On the mechanism of deoxyribonucleoside toxicity in human T-lymphoblastoid cells. Reversal of growth inhibition by addition of cytidine. Eur J Biochem. 1985 Aug 1;150(3):429–434. doi: 10.1111/j.1432-1033.1985.tb09038.x. [DOI] [PubMed] [Google Scholar]
- Dudding L. R., Nkabinde N. C., Mizrahi V. Analysis of the RNA- and DNA-dependent DNA polymerase activities of point mutants of HIV-1 reverse transcriptase lacking ribonuclease H activity. Biochemistry. 1991 Oct 29;30(43):10498–10506. doi: 10.1021/bi00107a019. [DOI] [PubMed] [Google Scholar]
- Erice A., Balfour H. H., Jr Resistance of human immunodeficiency virus type 1 to antiretroviral agents: a review. Clin Infect Dis. 1994 Feb;18(2):149–156. doi: 10.1093/clinids/18.2.149. [DOI] [PubMed] [Google Scholar]
- Gao Q., Gu Z., Parniak M. A., Cameron J., Cammack N., Boucher C., Wainberg M. A. The same mutation that encodes low-level human immunodeficiency virus type 1 resistance to 2',3'-dideoxyinosine and 2',3'-dideoxycytidine confers high-level resistance to the (-) enantiomer of 2',3'-dideoxy-3'-thiacytidine. Antimicrob Agents Chemother. 1993 Jun;37(6):1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Cara A., Gallo R. C., Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Goodman M. F., Creighton S., Bloom L. B., Petruska J. Biochemical basis of DNA replication fidelity. Crit Rev Biochem Mol Biol. 1993;28(2):83–126. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- Gürtler L. G., Hauser P. H., Eberle J., von Brunn A., Knapp S., Zekeng L., Tsague J. M., Kaptue L. A new subtype of human immunodeficiency virus type 1 (MVP-5180) from Cameroon. J Virol. 1994 Mar;68(3):1581–1585. doi: 10.1128/jvi.68.3.1581-1585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Huber H. E., McCoy J. M., Seehra J. S., Richardson C. C. Human immunodeficiency virus 1 reverse transcriptase. Template binding, processivity, strand displacement synthesis, and template switching. J Biol Chem. 1989 Mar 15;264(8):4669–4678. [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A. Interference with viral infection by defective RNA replicase. J Virol. 1987 Dec;61(12):3946–3949. doi: 10.1128/jvi.61.12.3946-3949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski S. A., Luo M., Morrow C. D. Enzymatic activity of poliovirus RNA polymerase mutants with single amino acid changes in the conserved YGDD amino acid motif. J Virol. 1991 Sep;65(9):4565–4572. doi: 10.1128/jvi.65.9.4565-4572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Molina A., Ding J., Nanni R. G., Clark A. D., Jr, Lu X., Tantillo C., Williams R. L., Kamer G., Ferris A. L., Clark P. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam P., Larder B. A. Recombinant virus assay: a rapid, phenotypic assay for assessment of drug susceptibility of human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1994 Jan;38(1):23–30. doi: 10.1128/aac.38.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarmann G. J., Schauber C. A., Preston B. D. Template-directed pausing of DNA synthesis by HIV-1 reverse transcriptase during polymerization of HIV-1 sequences in vitro. J Biol Chem. 1993 May 5;268(13):9793–9802. [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Patel S. S., Johnson K. A. Error-prone replication of repeated DNA sequences by T7 DNA polymerase in the absence of its processivity subunit. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6830–6834. doi: 10.1073/pnas.91.15.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Chesebro B., Richman D. D. Susceptibilities of zidovudine-susceptible and -resistant human immunodeficiency virus isolates to antiviral agents determined by using a quantitative plaque reduction assay. Antimicrob Agents Chemother. 1990 Mar;34(3):436–441. doi: 10.1128/aac.34.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A. Interactions between drug resistance mutations in human immunodeficiency virus type 1 reverse transcriptase. J Gen Virol. 1994 May;75(Pt 5):951–957. doi: 10.1099/0022-1317-75-5-951. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Harrigan P. R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995 Aug 4;269(5224):696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grice S. F., Naas T., Wohlgensinger B., Schatz O. Subunit-selective mutagenesis indicates minimal polymerase activity in heterodimer-associated p51 HIV-1 reverse transcriptase. EMBO J. 1991 Dec;10(12):3905–3911. doi: 10.1002/j.1460-2075.1991.tb04960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lori F., Gallo R. C. Hydroxyurea and AIDS: an old drug finds a new application? AIDS Res Hum Retroviruses. 1995 Oct;11(10):1149–1151. doi: 10.1089/aid.1995.11.1149. [DOI] [PubMed] [Google Scholar]
- Lori F., Malykh A., Cara A., Sun D., Weinstein J. N., Lisziewicz J., Gallo R. C. Hydroxyurea as an inhibitor of human immunodeficiency virus-type 1 replication. Science. 1994 Nov 4;266(5186):801–805. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- Lowe D. M., Parmar V., Kemp S. D., Larder B. A. Mutational analysis of two conserved sequence motifs in HIV-1 reverse transcriptase. FEBS Lett. 1991 May 6;282(2):231–234. doi: 10.1016/0014-5793(91)80484-k. [DOI] [PubMed] [Google Scholar]
- Majumdar C., Abbotts J., Broder S., Wilson S. H. Studies on the mechanism of human immunodeficiency virus reverse transcriptase. Steady-state kinetics, processivity, and polynucleotide inhibition. J Biol Chem. 1988 Oct 25;263(30):15657–15665. [PubMed] [Google Scholar]
- McKeating J. A., McKnight A., Moore J. P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991 Feb;65(2):852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Vartanian J. P., Hultgren C., Plikat U., Karlsson A., Wang L., Eriksson S., Wain-Hobson S. Restriction and enhancement of human immunodeficiency virus type 1 replication by modulation of intracellular deoxynucleoside triphosphate pools. J Virol. 1994 Jan;68(1):535–540. doi: 10.1128/jvi.68.1.535-540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Paul A. V., Cao X., Harris K. S., Lama J., Wimmer E. Studies with poliovirus polymerase 3Dpol. Stimulation of poly(U) synthesis in vitro by purified poliovirus protein 3AB. J Biol Chem. 1994 Nov 18;269(46):29173–29181. [PubMed] [Google Scholar]
- Pelletier H., Sawaya M. R., Kumar A., Wilson S. H., Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994 Jun 24;264(5167):1891–1903. [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocancourt D., Bonnerot C., Jouin H., Emerman M., Nicolas J. F. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990 Jun;64(6):2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers D. W., Gamblin S. J., Harris B. A., Ray S., Culp J. S., Hellmig B., Woolf D. J., Debouck C., Harrison S. C. The structure of unliganded reverse transcriptase from the human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1222–1226. doi: 10.1073/pnas.92.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya M. R., Pelletier H., Kumar A., Wilson S. H., Kraut J. Crystal structure of rat DNA polymerase beta: evidence for a common polymerase mechanism. Science. 1994 Jun 24;264(5167):1930–1935. doi: 10.1126/science.7516581. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Chu C. K., Peck A., McMillan A., Mathis R., Cannon D., Jeong L. S., Beach J. W., Choi W. B., Yeola S. Activities of the four optical isomers of 2',3'-dideoxy-3'-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob Agents Chemother. 1992 Mar;36(3):672–676. doi: 10.1128/aac.36.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Lloyd R. M., Jr, Nguyen M. H., Cannon D. L., McMillan A., Ilksoy N., Chu C. K., Liotta D. C., Bazmi H. Z., Mellors J. W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993 Apr;37(4):875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurman R., Nijhuis M., van Leeuwen R., Schipper P., de Jong D., Collis P., Danner S. A., Mulder J., Loveday C., Christopherson C. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J Infect Dis. 1995 Jun;171(6):1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Handa H., Nakada S., Nagata K. Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res. 1994 Nov 25;22(23):5047–5053. doi: 10.1093/nar/22.23.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tisdale M., Kemp S. D., Parry N. R., Larder B. A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3'-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Drosopoulos W. C., Salomon H., Hsu M., Borkow G., Parniak M., Gu Z., Song Q., Manne J., Islam S. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996 Mar 1;271(5253):1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Salomon H., Gu Z., Montaner J. S., Cooley T. P., McCaffrey R., Ruedy J., Hirst H. M., Cammack N., Cameron J. Development of HIV-1 resistance to (-)2'-deoxy-3'-thiacytidine in patients with AIDS or advanced AIDS-related complex. AIDS. 1995 Apr;9(4):351–357. [PubMed] [Google Scholar]
- Wakefield J. K., Jablonski S. A., Morrow C. D. In vitro enzymatic activity of human immunodeficiency virus type 1 reverse transcriptase mutants in the highly conserved YMDD amino acid motif correlates with the infectious potential of the proviral genome. J Virol. 1992 Nov;66(11):6806–6812. doi: 10.1128/jvi.66.11.6806-6812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong J. J., Goudsmit J., Keulen W., Klaver B., Krone W., Tersmette M., de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992 Feb;66(2):757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Korte D., Haverkort W. A., van Gennip A. H., Roos D. Nucleotide profiles of normal human blood cells determined by high-performance liquid chromatography. Anal Biochem. 1985 May 15;147(1):197–209. doi: 10.1016/0003-2697(85)90028-4. [DOI] [PubMed] [Google Scholar]
- van Leeuwen R., Katlama C., Kitchen V., Boucher C. A., Tubiana R., McBride M., Ingrand D., Weber J., Hill A., McDade H. Evaluation of safety and efficacy of 3TC (lamivudine) in patients with asymptomatic or mildly symptomatic human immunodeficiency virus infection: a phase I/II study. J Infect Dis. 1995 May;171(5):1166–1171. doi: 10.1093/infdis/171.5.1166. [DOI] [PubMed] [Google Scholar]