Abstract
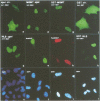
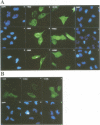
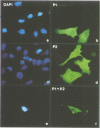
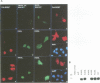
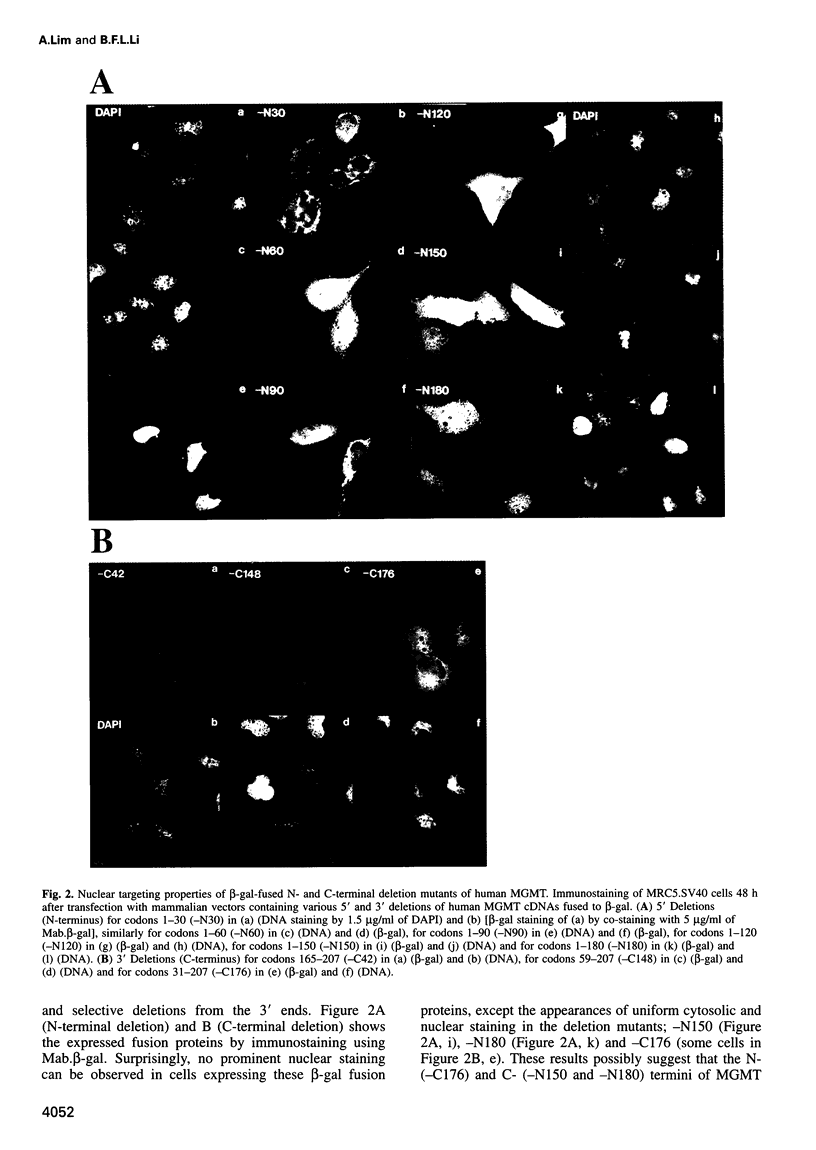
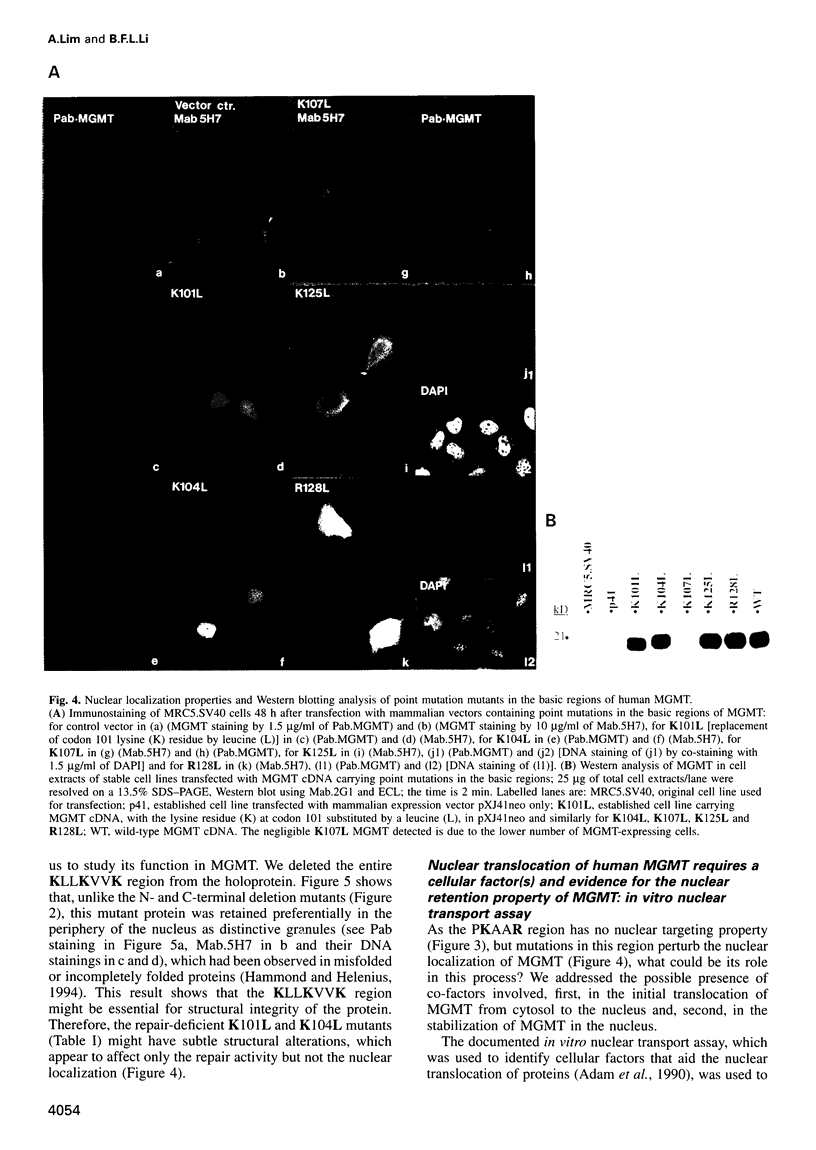
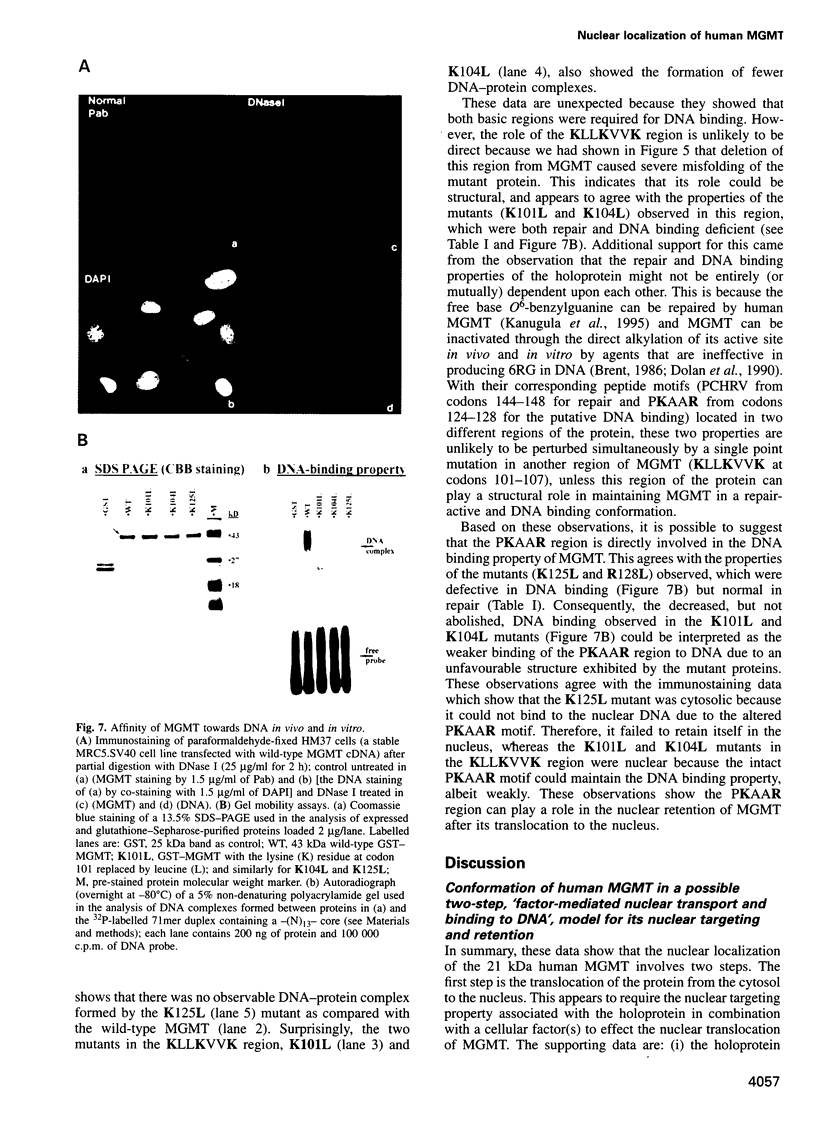
Human O6-methylguanine-DNA methyltransferase (MGMT) protects human cells from the mutagenic effects of alkylating agents by repairing the O6-alkylguanine residues formed by these agents in the nuclear DNA. We report here a study showing a possible two-step model for the nuclear localization of the 21 kDa human protein. The first step is the translocation of the protein from the cytosol to the nucleus. This appears to require the nuclear targeting property associated with the holoprotein in combination with a cellular factor(s) to effect the nuclear translocation of MGMT. The second step involves the nuclear retention of MGMT (to prevent its export from the nucleus). This requires a basic region (PKAAR, codons 124-128) that can bind to the non-diffusible DNA elements in the nucleus. Supporting data for such mechanisms are: (i) the holoprotein can target the cytosolic 110 kDa beta-galactosidase into the nucleus; (ii) purified recombinant MGMT requires a cellular factor for transport across the nuclear membrane; (iii) nuclear MGMT can be removed selectively by DNase I; (iv) the repair-positive K125L mutant, which alters the PKAAR motif, remains in the cytosol and fails to bind DNA in vitro; and (v) polypeptide containing the PKAAR motif has no nuclear targeting property. Interestingly, mutants in another basic region, KLLKVVK (codons 101-107) are DNA binding and repair deficient but entirely nuclear. As these substitutions affect the functional properties of human MGMT, they are potential targets for genetic screening of individuals for risk assessment to alkylating agents.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Marr R. S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990 Sep;111(3):807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayi T. C., Loh K. C., Ali R. B., Li B. F. Intracellular localization of human DNA repair enzyme methylguanine-DNA methyltransferase by antibodies and its importance. Cancer Res. 1992 Dec 1;52(23):6423–6430. [PubMed] [Google Scholar]
- Ayi T. C., Oh H. K., Lee T. K., Li B. F. A method for simultaneous identification of human active and active-site alkylated O6-methylguanine-DNA methyltransferase and its possible application for monitoring human exposure to alkylating carcinogens. Cancer Res. 1994 Jul 15;54(14):3726–3731. [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Inactivation of purified human O6-alkylguanine-DNA alkyltransferase by alkylating agents or alkylated DNA. Cancer Res. 1986 May;46(5):2320–2323. [PubMed] [Google Scholar]
- Brent T. P., von Wronski M. A., Edwards C. C., Bromley M., Margison G. P., Rafferty J. A., Pegram C. N., Bigner D. D. Identification of nitrosourea-resistant human rhabdomyosarcomas by in situ immunostaining of O6-methylguanine-DNA methyltransferase. Oncol Res. 1993;5(2):83–86. [PubMed] [Google Scholar]
- Chan C. L., Wu Z., Ciardelli T., Eastman A., Bresnick E. Kinetic and DNA-binding properties of recombinant human O6-methylguanine-DNA methyltransferase. Arch Biochem Biophys. 1993 Jan;300(1):193–200. doi: 10.1006/abbi.1993.1027. [DOI] [PubMed] [Google Scholar]
- Chueh L. L., Nakamura T., Nakatsu Y., Sakumi K., Hayakawa H., Sekiguchi M. Specific amino acid sequences required for O6-methylguanine-DNA methyltransferase activity: analyses of three residues at or near the methyl acceptor site. Carcinogenesis. 1992 May;13(5):837–843. doi: 10.1093/carcin/13.5.837. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Moschel R. C., Pegg A. E. Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5368–5372. doi: 10.1073/pnas.87.14.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumenco L. L., Allay E., Norton K., Gerson S. L. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science. 1993 Jan 8;259(5092):219–222. doi: 10.1126/science.8421782. [DOI] [PubMed] [Google Scholar]
- Dumenco L. L., Warman B., Hatzoglou M., Lim I. K., Abboud S. L., Gerson S. L. Increase in nitrosourea resistance in mammalian cells by retrovirally mediated gene transfer of bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1989 Nov 1;49(21):6044–6051. [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Gerace L. Nuclear export signals and the fast track to the cytoplasm. Cell. 1995 Aug 11;82(3):341–344. doi: 10.1016/0092-8674(95)90420-4. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Laskey R. A., Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994 Dec 2;79(5):767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994 Jul;126(1):41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel T., Zabel U., van Zee K., Müller J. M., Fanning E., Baeuerle P. A. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992 Mar 20;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Nakabeppu Y., Kawate H., Sakumi K., Hayakawa H., Sekiguchi M. Intracellular localization and function of DNA repair methyltransferase in human cells. Mutat Res. 1994 Nov;315(3):199–212. doi: 10.1016/0921-8777(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Nakabeppu Y., Sekiguchi M. Artificial control of nuclear translocation of DNA repair methyltransferase. J Biol Chem. 1994 Mar 11;269(10):7645–7650. [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kanugula S., Goodtzova K., Edara S., Pegg A. E. Alteration of arginine-128 to alanine abolishes the ability of human O6-alkylguanine-DNA alkyltransferase to repair methylated DNA but has no effect on its reaction with O6-benzylguanine. Biochemistry. 1995 May 30;34(21):7113–7119. doi: 10.1021/bi00021a024. [DOI] [PubMed] [Google Scholar]
- LaCasse E. C., Lefebvre Y. A. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995 May 25;23(10):1647–1656. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., White R. G., Dunham R. G., Kanda P. Effect of basic and nonbasic amino acid substitutions on transport induced by simian virus 40 T-antigen synthetic peptide nuclear transport signals. Mol Cell Biol. 1988 Jul;8(7):2722–2729. doi: 10.1128/mcb.8.7.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Rafferty J. A., Elder R. H., Fan C. Y., Bromley M., Harris M., Thatcher N., Potter P. M., Altermatt H. J., Perinat-Frey T. Immunohistological examination of the inter- and intracellular distribution of O6-alkylguanine DNA-alkyltransferase in human liver and melanoma. Br J Cancer. 1992 Aug;66(2):355–360. doi: 10.1038/bjc.1992.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem L. K., Wong C. W., Lim A., Li B. F. Factors influencing the repair of the mutagenic lesion O6-methylguanine in DNA by human O6-methylguanine-DNA methyltransferase. J Mol Biol. 1993 Jun 20;231(4):950–959. doi: 10.1006/jmbi.1993.1344. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mitra G., Pauly G. T., Kumar R., Pei G. K., Hughes S. H., Moschel R. C., Barbacid M. Molecular analysis of O6-substituted guanine-induced mutagenesis of ras oncogenes. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8650–8654. doi: 10.1073/pnas.86.22.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990 Oct 1;50(19):6119–6129. [PubMed] [Google Scholar]
- Pollock R., Treisman R. A sensitive method for the determination of protein-DNA binding specificities. Nucleic Acids Res. 1990 Nov 11;18(21):6197–6204. doi: 10.1093/nar/18.21.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty J. A., Tumelty J., Skorvaga M., Elder R. H., Margison G. P., Douglas K. T. Site-directed mutagenesis of glutamic acid 172 to glutamine completely inactivated human O6-alkylguanine-DNA-alkyltransferase. Biochem Biophys Res Commun. 1994 Feb 28;199(1):285–291. doi: 10.1006/bbrc.1994.1226. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Dargemont C., Kühn L. C., Nigg E. A. Nuclear export of proteins: the role of nuclear retention. Cell. 1993 Aug 13;74(3):493–504. doi: 10.1016/0092-8674(93)80051-f. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tano K., Shiota S., Collier J., Foote R. S., Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci U S A. 1990 Jan;87(2):686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh D. B. The role of O6-methylguanine-DNA methyltransferase in cell survival, mutagenesis and carcinogenesis. Mutat Res. 1985 Jan-Mar;145(1-2):1–16. doi: 10.1016/0167-8817(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]