Abstract
Hepatocellular carcinoma (HCC) is a rapidly rising cause of liver-related death worldwide. Most patients are diagnosed at an advanced stage of disease, when systemic therapy is the only viable option for treatment. Significant strides have been made in the molecular understanding of HCC development and growth stimulation. The c-Met pathway has been found to be an important pathway in half of all patients with HCC. HCC tumors with high c-Met activation are associated with an aggressive phenotype and poor prognosis. Tivantinib is a MET receptor tyrosine kinase inhibitor with a broad spectrum of anti-tumor effects currently being studied for the treatment of HCC. Phase I and II data are available for tivantinib in the treatment of solid tumors, including HCC. There appears to be an adequate safety profile, with the main side-effect being neutropenia. In HCC patients with elevated c-Met activity, tivantinib results in an improved time to progression of 2.7 months, compared with 1.4 months in placebo-treated patients. Further studies are ongoing, but early data suggest that tivantinib is a therapy that deserves close attention in the coming years for patients with HCC.
Keywords: Hepatocellular carcinoma, c-Met, Tivantinib, Tyrosine kinase inhibitor, Liver cancer
Introduction
Hepatocellular carcinoma (HCC) is the third most common cancer in the world, with over 700,000 new cases being diagnosed each year. HCC is the fifth most common cancer in men and seventh most common in women as well as the second and sixth leading cause of cancer death in males and females, respectively.1 Among those diagnosed with HCC, only those with early-stage disease are candidates for curative therapy, which accounts for approximately 15% of patients.2 Curative therapy consists of liver resection, radiofrequency ablation, or transplant. The large majority of HCC patients have a poor prognosis, with a less than 50% survival rate at 1 year.3
The aim of this review is to provide the background, rationale, and strategies involved in the development of tivantinib as a MET receptor tyrosine kinase inhibitor for use against HCC.
Dysregulation of various cellular pathways has been implicated in the pathogenesis of HCC. These pathways include the Wnt/β-catenin, Raf/mitogen activated protein (MAP)/extracellular signal-regulated kinase (ERK), p53, transforming growth factor-β (TGF-β), and human mesenchymal-epithelial transition (c-Met) pathways.
The Wnt/β-catenin pathway controls cellular proliferation, motility, and embryonic development. In HCC, genetic mutations involving proteins in the Wnt/β-catenin pathway lead to increased activity and tumor development. Beta-catenin mutations have been found in 13–34% of HCCs. These mutations lead to β-catenin stabilization and accumulation within the nucleus, which then leads to the upregulation of target genes and proto-oncogenes such as c-Myc and cyclin D1.4
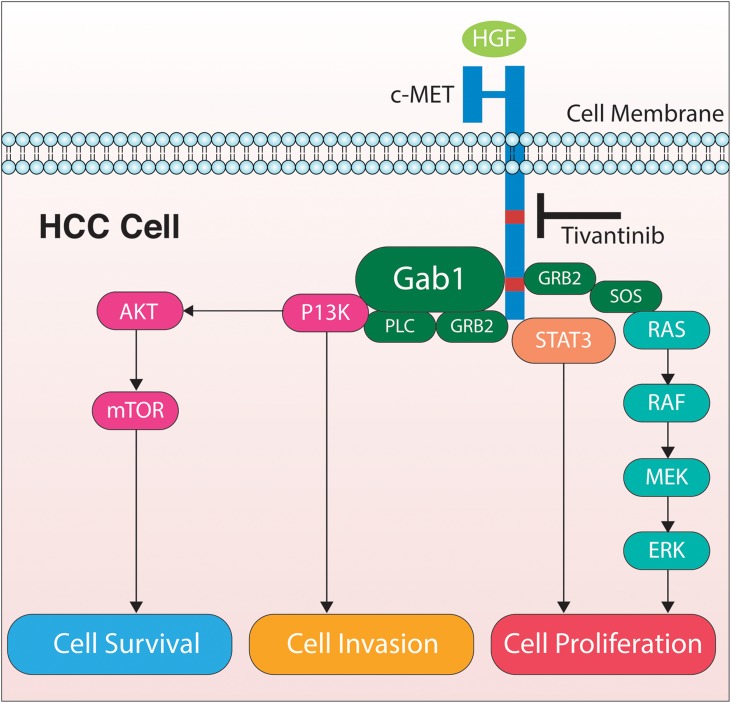
The Raf/MEK/ERK signaling pathway is a MAP kinase cascade, with cellular functions ranging from proliferation, to cell cycle arrest, angiogenesis, terminal differentiation and apoptosis. It can be stimulated by the Ras family of proteins, which are also known to be dysregulated in HCC. Ras proteins are GTPases and well-characterized human proto-oncogenes. Ras signaling can activate the MAP/MEK/ERK pathway in addition to other effect or proteins, which can lead to cell growth, survival and migration. Mutations in Ras genes are rare. However, overexpression of Ras proteins has been shown in HCC and cirrhotic livers. High levels of MAP kinase and decreased levels of Ras/Raf/MEK/ERK inhibitors have also been shown in HCC cells compared with cells from normal liver.5 The human c-Met pathway is integral to embryonic development and tissue repair. Hepatocyte growth factor (HGF) is the ligand that binds to the transmembrane tyrosine kinase receptor, cellular MET (cMet), to alter epithelial-mesenchymal interactions.6,7 The MET proto-oncogene encodes c-Met, which is commonly expressed in cells of epithelial-endothelial origin. When activated, the c-Met pathway leads to increased cell growth, invasion, motility, protection from apoptosis and angiogenesis, and drug resistance (Fig. 1).8 The c-Met pathway is one of the most commonly dysregulated pathways in human cancers.9 Both HGF and c-Met are frequently over-expressed in human sarcomas and carcinomas, including HCC.10
Figure 1. The mechanism of action of tivantinib is to block the HGF-cMET pathway, which stimulates multiple intracellular pathways that result in cell survival, invasion, and proliferation.
In the liver, HGF enhances hepatic regeneration and suppresses hepatocyte apoptosis. Decreased activity of HGF results in delayed hepatic regeneration and fibrosis. In murine models, ectopic HGF and c-Met overexpression drive tumor growth and metastasis. HCC tumors with activation of the HGF-MET pathway have been associated with an aggressive phenotype and poor prognosis. Such tumors are often found to have vascular invasion, metastases, increased microvessel density, poor cellular differentiation, and decreased survival time.11 High c-MET tumors are defined as those with ≥50% of tumor cells with moderate or strong MET staining. HGF overexpression is seen in 33% and c-Met overexpression in 20–48% of patients with HCC.12
Currently, sorafenib is the only systemic therapy available with proven efficacy in improving survival when compared with placebo in patients with unresectable HCC. Sorafenib is an oral multi-tyrosine kinase and angiogenesis inhibitor, which has become the standard treatment in advanced HCC. In Child-Pugh class A patients with advanced HCC who were previously untreated, sorafenib increased time to progression of disease from 2.8 mo to 5.5 mo, and there was an increase in median survival from 7.9 mo to 10.7 mo compared with placebo. There are currently no alternative treatments for patients with advanced HCC who do not respond to sorafenib.13 Tivantinib is a MET receptor tyrosine kinase inhibitor with a broad spectrum of anti-tumor effects currently being studied for the treatment of HCC. Also known as ARQ197, it is a small, orally bioavailable, highly selective c-Met inhibitor. It acts in a non-ATP competitive manner to disrupt constitutive and HGF-mediated c-Met phosphorylation as well as downstream c-Met signaling pathways (Fig. 1). The selectivity of tivantinib was determined when the compound was profiled against a panel of 230 human kinases. Tivantinib inhibited five kinases in total, including c-Met. Ron kinase, which belongs to the same kinase family as c-Met, was only inhibited at higher concentrations of the drug.14 The non-ATP competitive mechanism of tivantinib sets it apart from other available c-Met kinase inhibitors, which are ATP dependent. Tivantinib has an inhibitory constant (Ki) of 355nmol/L which suggests that tivantinib is not as potent as other c-Met inhibitors; however, cellular assays determining anti-proliferative and inhibitory effects of the compound indicate a biochemical potency similar to ATP-dependent c-Met inhibitors.14
In a phase I open-label study by Yap et al,14 the safety and tolerability, as well as identification of the dose-limiting toxicities and maximum tolerated dose of tivantinib, were evaluated. In this single-center study, tivantinib was administered to 51 patients with solid tumors twice daily (BID) for 28 days in a dose-escalating manner, with a starting dose of 100mg BID and a maximum of 400mg BID. The most prevalent cancer was prostate cancer (25.5%); no patients with HCC were included. The most common adverse events (AEs) were grade 1 and 2 nausea (13.7%), vomiting (11.8%) and fatigue (15.7%). Dose-limiting toxicities included grade 3 fatigue at 200mg BID in one patient. Mucositis, palmar-plantar erythrodysesthesia and hypokalemia was found in one patient, and febrile neutropenia in another, both at 400mg BID. The HGF/c-Met pathway is known to be an integral part of hematopoiesis which suggests that neutropenia is an on-target effect.
Based on the results, a dose of 360mg BID of the crystalline A formulation of tivantinib was recommended for a phase 2 study. Of note, one patient was found to have significantly higher drug levels and AEs than the remainder of the cohort. Genetic analysis identified the single-nucleotide polymorphism (SNP) CYP2C19*2. Tivantinib is metabolized by the cytochrome P450 CYP2C19 enzyme, and the above SNP results in enzyme deficiency. It is most commonly seen among Asians (35%) followed by those of African descent (17%) and Caucasians (15%).15
Paired tumor biopsies before and after treatment were obtained in 15 patients. ARQ197 decreased total c-Met and the phosphorylated form as well as phosphorylated focal adhesion kinase, and increased terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling staining on tumor tissue. Tumor stabilization, as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) model, was observed for ≥4 mo in 14 patients, 2 of whom had minor tumor regression. In 43 patients found to have circulating epithelial cells (CECs) in peripheral blood samples prior to treatment, 58% had a reduction in the number of CECs. Among 15 patients with detectable circulating tumor cells (CTCs), 53% had a ≥30% decline in the number of CTCs after treatment.15
A phase I trial by Rosen et al enrolled 74 patients with advanced solid tumors, but no HCC.15 Patients were treated with tivantinib in doses ranging from 120mg to 360mg BID for an average of 6.5 weeks. They determined the maximum tolerated dose of tivantinib to be 360mg BID, with two patients experiencing dose-limiting toxicity at 360mg BID. The predominant AEs included fatigue (16%), nausea (12%) diarrhea (6%) and vomiting (7%). A partial response to tivantinib was seen in 4.9% and stabilization of disease was seen in 38 patients. This resulted in a 67% disease control rate.16
Both phase I studies recommended a phase II study dose of 360mg BID; however few patients with hepatic impairment or HCC were included in the studies. Therefore, a phase IB study was conducted to evaluate the safety of tivantinib in patients with HCC and cirrhosis of whom twenty-one patients had advanced HCC, Eastern Cooperative Oncology Group (ECOG) status 0 or 1, and Child-Pugh class A or B cirrhosis without clinically evident ascites. Tivantinib was administered at 360mg BID with dose reductions if grade 3 or 4 AEs were encountered. All patients received previous systemic therapy, of which 91% received prior sorafenib therapy, and 7% received more than one previous therapy.
The median duration of treatment was 1.8 mo, ranging from 0.1 to 15.9 mo. Seventeen patients (81%) terminated treatment due to progression of disease, and the remaining four (19%) terminated as a result of AEs. Five patients had dose reductions as a result of AEs, and eight (38%) required dose interruptions (<2 weeks) after which tivantinib was resumed. The most common AEs were neutropenia, anemia, leucopenia, asthenia, anorexia, diarrhea and fatigue. Neutropenia was the primary reason for dose reduction, interruption or discontinuation. Eleven (52%) patients experienced grade 3 or higher AEs. Serious AEs occurred in four patients (19%) and included grade 3 anemia, grade 3 anemia with grade 4 neutropenia, grade 4 neutropenia and leukopenia, and grade 4 neutropenia, and leukopenia with grade 5 septic shock. Seventy-seven myelosuppressive events were related to tivantinib. There was no drug-related worsening of liver function.
Pharmacokinetic data revealed a plasma concentration approximately two times higher in patients with HCC versus those with other tumors. However, there was a wide range of variability and no relationship was seen between drug level and tivantinib exposure, Child-Pugh class, or baseline demographic variables.
Of the 21 patients enrolled in this Phase Ib study, 16 were evaluated for tumor response to tivantinib. Stabilization of disease was seen in 9 out of the 16 patients (56%) with a median time to progression of 3.3 mo. In the intent-to-treat population, the time to progression of disease was 1.8 mo.17
A phase II multicenter, randomized, double-blind, placebo-controlled study was subsequently undertaken to evaluate the efficacy of tivantinib in treating patients with advanced HCC and Child-Pugh class A cirrhosis who progressed on, or were intolerant of, first-line systemic therapy. First-line therapies utilized were sorafenib in 103 patients and sunitinib in 4 patients. The primary endpoint was time to progression, which was defined as the time from randomization to radiologic progression of disease. Secondary objectives included determination of progression-free survival, overall survival, proportion of patients with an objective response, disease control and objective response within the crossover group. One hundred and seven adults were enrolled who had histologically or cytologically confirmed, advanced, unresectable disease previously treated for at least 3 weeks with a systemic therapy and who were found to have radiologic progression of disease or intolerance to therapy. Patients were allowed to have received locoregional therapy up to 4 weeks prior to randomization and needed a radiologically measurable tumor for assessment. All were ECOG performance status 1 or less with Child-Pugh class A cirrhosis or without cirrhosis. Patients also had to have acceptable baseline bone marrow, liver and renal function as defined by lab criteria (platelet count ≥ 60 × 109cells/L, hemoglobin ≥ 90 mg/L, absolute neutrophil count ≥ 1.5 × 109 cells/L, total bilirubin ≤ 34.2 μmol/L, alanine and aspartate aminotransferases ≤ 5 times the upper limit of normal, international normalized ratio of 0.8–1.4 if not on anticoagulation, or ≤ 3 times the upper limit if anticoagulated, and an albumin ≥ 28mg/L). A biopsy specimen was also required at baseline for biomarker analysis.
Patients were randomized to oral tivantinib at a starting dose of 360mg BID, 240mg BID or placebo until significant toxicity or radiologic or clinical progression of disease was seen. Patients were grouped based on ECOG performance status and vascular invasion. Once initiated on tivantinib, a large number of patients receiving 360mg BID experienced ≥ grade 3 neutropenia; therefore all patients were dose reduced to 240mg BID. Up to two dose reductions were allowed due to significant AEs, defined as any grade 3–4 events, grade 2 neutropenia, thrombocytopenia or anemia that did not resolve within 7 days. If more than two dose reductions were required, patients were withdrawn from the study.
Tumor burden was evaluated every 6 weeks with CT or MRI and, at the end of the study, assessed using a modified RECIST model, allowing measurement of up to five liver lesions and two lesions outside the liver. Those in the placebo arm who had tumor progression were then allowed to receive open-label tivantinib in a crossover study. Those who progressed within the tivantinib group were allowed to withdraw from the study and receive other therapies.
Median patient follow up was 5.5 mo. At the time of analysis, disease progressed in 65% of patients treated with tivantinib and 72% in the placebo arm. Time to progression of disease was 1.6 months in the tivantinib group versus 1.4 mo in the placebo group (p=0.04). Median progression-free survival and median overall survival were not statistically significant between the tivantinib and placebo arms. Disease control was attained in 31 (44%) of patients on tivantinib and in 11 (31%) of placebo patients.
Seventy-seven patients had tumor tissue available for immunohistochemical analysis. Of the 77 tumors analyzed, 37 (48%) of patients had tumors with elevated MET activity. High MET activity was defined as ≥50% of tumor cells with moderate or strong (2+ or 3+) staining intensity. A high MET activity subgroup analysis revealed that time to progression was 2.7 mo in the group treated with tivantinib versus 1.4 mo in the group given placebo (p=0.03). The median overall survival in the high Met activity group was 7.2 mo in the group that received tivantinib versus 3.8 mo in the placebo group (p=0.02).
AEs occurred in 69 patients who received tivantinib, with 46% being grade 1–2 AEs. Serious AEs were seen in 34% of tivantinib patients and 39% of placebo patients. Grade 3 or worse neutropenia was observed in 8 (11%) of patients treated with tivantinib 360mg BID and 2 (6%) of patients on a 240mg BID dose, and none in the placebo group. Four deaths from severe neutropenia occurred in the tivantinib group: in three patients who received 360mg BID and in one patient who received 240mg BID.
Dose reductions has a result of AEs occurred in 17 (24%) patients on tivantinib, with neutropenia being the most common AE. Discontinuation of medication due to AEs occurred in 13 (18%) of the treatment group and 8 (22%) of the placebo group patients. The most commonly encountered AE resulting in discontinuation was disease progression, which occurred in 3 (4%) of the tivantinib patients and 4 (11%) of the placebo patients.18
A phase III trial on the efficacy of tivantinib in treating patients with MET- high, locally advanced HCC is currently enrolling participants. It is a double-blind, placebo-controlled, randomized study whose primary outcomes are overall survival, progression-free survival and safety. Patients who have histologically confirmed MET-high tumors, and a tumor non-response or progression after 4 or more weeks of a sorafenib-containing systemic therapy, and an ECOG performance status ≤ 1, are eligible. Excluded are those patients who are post-transplant or have Child-Pugh class B or C cirrhosis. Patients will either be treated with tivantinib 240mg BID or with placebo. The study began enrollment in December 2012 and is estimated to be completed in December 2015.19
Conclusions
Tivantinib is an orally bioavailable MET kinase inhibitor that acts through an ATP-dependent pathway to disrupt activation of the c-Met pathway. It has been used in the treatment of human carcinomas. Phase I studies recommend a treatment dose of 360 mg BID; however, the recommended dose resulted in a significant number of patients with ≥ grade 3 neutropenia. The 240 mg BID dose of tivantinib has an improved safety profile and still provides survival benefit in patients with HCC. Those HCC patients who appear to have maximal benefit from tivantinib are those with high MET activity. Based on the currently available data, tivantinib shows promising data in the treatment of advanced HCC where first-line therapy has been unsuccessful. It seems to be well tolerated, with the main side effects related to neutropenia. Further research is needed, but attention should be paid to this important new therapeutic option.
Abbreviations
- AE
adverse event
- ARQ197
tivantinib
- ATP
adenosine triphosphate
- CEC
circulating epithelial cells
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- HGF
hepatocyte growth factor
- MET/c-Met
mesenchymal-epithelial transition
- RECIST
Response Evaluation Criteria in Solid Tumors
- RTK
receptor tyrosine kinase
- SNP
single-nucleotide polymorphism
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Finn RS. Development of molecularly targeted therapies in hepatocellular carcinoma: Where do we go now? Clin Cancer Res. 2010;16:390–397. doi: 10.1158/1078-0432.CCR-09-2084. [DOI] [PubMed] [Google Scholar]
- 3.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Digest Liver Dis. 2010;42(Suppl 3):S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CM, Ng IO. Molecular pathogenesis of hepatocellular carcinoma. Liver Int. 2008;28:160–174. doi: 10.1111/j.1478-3231.2007.01637.x. [DOI] [PubMed] [Google Scholar]
- 5.Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 6.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 7.Borowiak M, Garratt AN, Wustefelt T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 9.Christensen JG, Burrows J, Salgia R. C-Met as a target for human cancer and characterization of the inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Venepalli NK, Goff L. Targeting the HGF-cMET axis in hepatocellular carcinoma. Int J Hepatol. 2013:341636. doi: 10.1155/2013/341636. Epub31 Mar 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 12.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Munshi N, Jeay S, Li Y, Chen C, France DS, Ashwell MA, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010;9:1544–1553. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 15.Yap TA, Olmos D, Brunetto AT, Tunariu N, Barriuso J, Riisnaes R, et al. Phase I trial of a selective c-Met inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–1279. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 16.Rosen LS, Senzer N, Mekhail T, Ganapathi R, Chai F, Savage RE, et al. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res. 2011;17:7754–7764. doi: 10.1158/1078-0432.CCR-11-1002. [DOI] [PubMed] [Google Scholar]
- 17.Santoro A, Simonelli M, Rodriguez-Lope C, Zucali P, Camacho LH, Granito A, et al. A Phase-1b study of Tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosis. Br J Cancer. 2013;108:21–24. doi: 10.1038/bjc.2012.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethern JL, et al. Tivantinib for second line treatment of advanced hepatocellular carcinoma: a randomized, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 19. www.clinicaltrials.gov, accessed June 2013. [Google Scholar]