Abstract
Liver involvement was one of the first extraglandular manifestations to be reported in patients with primary Sjögren syndrome (SS). In the 1990s, a study of liver involvement in patients with primary SS integrated the evaluation of clinical signs of liver disease, liver function and a complete panel of autoantibodies. Recent developments in the field of hepatic and viral diseases have significantly changed the diagnostic approach to liver involvement in SS. The most recent studies have shown that, after eliminating hepatotoxic drugs and fatty liver disease, the two main causes of liver disease in primary SS are chronic viral infections and autoimmune liver diseases. The differential diagnosis of liver disease in primary SS (viral vs autoimmune) is clinically important, since the two processes require different therapeutic approaches and have different prognoses. With respect to viral infections, chronic HCV infection is the main cause of liver involvement in SS patients from the Mediterranean area, while chronic HBV infection may be the main cause of liver involvement in SS patients from Asian countries. After eliminating viral hepatitis, primary biliary cirrhosis (PBC) should be considered the main cause of liver disease in primary SS. PBC-related SS patients may have a broad spectrum of abnormalities of the liver, including having no clinical or analytical data suggestive of liver disease. Autoimmune hepatitis (AIH) is the second most frequently found autoimmune liver disease to be associated with SS (all reported cases are type I), and nearly 10% of these patients have an AIH-PBC overlap. Finally, IgG4-related disease must be investigated in patients with SS presenting with sclerosing cholangitis, especially when autoimmune pancreatitis or retroperitoneal fibrosis are also present.
Keywords: Sjögren syndrome, Liver disease, Hepatitis B virus, Hepatitis C virus, Primary biliary cirrhosis, Autoimmune hepatitis, Sclerosing cholangitis
Introduction
Sjögren syndrome (SS) is a systemic autoimmune disease in which immune-mediated inflammation causes secretory gland dysfunction, leading to dryness of the main mucosal surfaces.1 Although xerophthalmia and xerostomia are the most frequent sicca symptoms, nearly 30% of patients present with extraglandular manifestations, and 5% may develop a hematological neoplasia. The cause of SS is unknown, but genetic and environmental factors seem to play a role. The disease may be more frequent than was previously thought, affecting an estimated 2–4 million people in the United States,2 and with a prevalence of 0.1–3.3% in European countries.3
SS primarily affects white perimenopausal women, with a female:male ratio ranging from 14:14 to 24:15 in the largest reported series. The disease may occur at all ages, but typically has its onset in the fourth to sixth decades of life, although some cases are detected in younger female patients, especially in mothers of babies with congenital heart block.6 When sicca symptoms appear in a previously healthy person, the syndrome is classified as primary Sjögren syndrome. When sicca features are found in association with another systemic autoimmune disease, most commonly rheumatoid arthritis (RA), systemic sclerosis (SSc) or systemic lupus erythematosus (SLE), it is classified as associated Sjögren syndrome.
The variability in the presentation of SS may partially explain delays in diagnosis of up to 9 years from the onset of symptoms.1 Although most patients present with sicca symptoms, various clinical and analytical features may indicate an undiagnosed SS. In addition, SS is a disease that may be expressed in many guises, depending on the specific epidemiological, clinical or immunological features. Clinically, two main patterns of disease expression are observed: patients with only glandular involvement (sicca-limited disease), who have a low frequency of immunological abnormalities and extraglandular features, and patients with a predominant “systemic” expression in addition to the sicca involvement.1 Patients with positive immunological features need a closer follow-up, with special attention to the development of extraglandular manifestations. The therapeutic management of SS is mainly centered on the control of sicca features, using substitutive and oral muscarinic agents, while corticosteroids and immunosuppressive agents play a key role in the treatment of extraglandular features.
Gastrointestinal involvement has been little studied in primary SS, and may include altered esophageal motility, gastroesophageal reflux, chronic gastritis and, less frequently, malabsorption. In contrast, liver involvement was one of the first reported extraglandular manifestations included in the systemic expression of SS, and new developments in the field of hepatic and viral diseases have significantly changed the diagnostic approach to patients with SS presenting with altered liver profiles.
Historical overview
Results of evaluation of liver involvement in primary SS have varied substantially across reported studies owing to the heterogeneity of the definition of hepatic disease. In the first studies published in the 1960s, liver involvement was evaluated exclusively by the presence of hepatomegaly, with a prevalence of 20%. In 1965, Bloch et al7 found a prevalence of 27% of liver involvement diagnosed by the presence of hepatomegaly and/or raised alkaline phosphatase in the first well-reported series of patients with SS. In contrast, Golding et al8 reported, in 1970, a close association between SS and liver diseases. The authors found a high frequency of sicca syndrome in patients with various liver diseases, including chronic active hepatitis, primary biliary cirrhosis (PBC) and cryptogenetic cirrhosis. In the 1970s, anti-mitochondrial antibodies(AMA) were included as a marker of liver disease in SS patients. Subsequent studies found a closer association between SS and PBC in comparison with other types of autoimmune liver disease.9,10 However, it was not until 1994 when the spectrum of liver diseases in patients with primary SS was fully investigated, including the evaluation of clinical signs of liver disease, liver function tests, and a complete series of autoantibodies.11,12
Recent studies have shown that liver function tests may be altered in 10–20% of patients with primary SS.13 After eliminating potentially hepatotoxic drugs, the main causes of liver involvement described in SS are chronic viral infections (especially in geographic areas with a high prevalence) and autoimmune liver diseases.14–16 The objective of this article is to comprehensively review the main causes of liver involvement in SS.
Chronic viral hepatitis
Chronic viral hepatitis has emerged as a significant cause of liver involvement in patients with SS, especially in certain geographical areas, broadening the spectrum of liver diseases classically reported in these patients (Table 1).17–19 In fact, the geoepidemiology of chronic viral infections is essential in order to evaluate liver involvement in patients with systemic autoimmune diseases.
Table 1. Main clinical, analytical, immunological and histopathological differences among HCV infection, primary biliary cirrhosis, autoimmune hepatitis and sclerosing cholangitis17–19 .
| Hepatitis C virus infection | Primary biliary cirrhosis | Autoimmune hepatitis | Sclerosing cholangitis (SC) | |
| Female:male ratio | 1:2.5 | 9:1 | 3.6:1 | 1:2 |
| Mean age (years) | 30–49 | 35–60 | 15–40 | 25–45 |
| Incidence | Not well known because acute infection is generally asymptomatic | 0.33–5.8 per 100,000 inhabitants/year | 0.08–3 per 100,000 inhabitants/year | 0–1.3 per 100,000 inhabitants/year |
| Prevalence | 2.8% worldwide (predominance in Africa) | 1.91–40.2 per 100,000 inhabitants | 11.6–35.9 per 100,000 inhabitants | 0–16.2 per 100,000 inhabitants |
| Clinical symptoms/signs | Jaundice, non-specific* | Jaundice, non-specific* | Non-specific* | Jaundice, non-specific* |
| Liver profile | Cytolysis/cholestatic pattern | Cholestatic pattern predominance but raised aminotransferases may be present | Cytolysis pattern predominance but cholestatic pattern may also be present | Cholestatic predominance but cytolysis pattern may also be present |
| Hypergammaglobulinemia | + | ++ | +++ | − |
| Other biochemical tests | − | Hyperlipidemia, hypercholesterolemia, ↑IgM | ↑IgG | ↑IgG (IgG4 if associated with IgG4-related disease) |
| Liver biopsy |
Acute hepatitis: hepatocyte ballooning degeneration, Kupffer cell hyperplasia, lobular and sinusoid inflammatory cell infiltrates, acidophil bodies Chronic hepatitis: lymphoid aggregate or follicle in the portal tract (according to severity: necroinflammatory activity or fibrosis) |
Stage I: Portal inflammation; formation of granulomas Stage II: periportal inflammation Stage III: fibrous bridges between portal tracts Stage IV: regenerative nodules |
Portal monocytic infiltrate with scattered eosinophils and “interface hepatitis” | Periductal concentric (“onion-skin”) fibrosis of intra- and extra-hepatic bile ducts |
| Pathognomonic autoantibodies | − | anti-AMA-M2 (others: AMA-M4, -M5, -M8, -M9) | anti-SMA, anti-LKM-1, (others: anti-LC-1, anti-SLA/LPA) | − |
| Other autoantibodies | ANA, RF | ANA, thyroid antibodies, SMA | ANA, ANCA | ANCA, ANA, SMA, ACA, RF |
| Cryoglobulins | + | − | − | − |
| Hypocomplementemia | + | − | − | − |
| Treatment |
Genotypes 2,3,4: Pegylated interferon alpha and ribavirin Genotype 1: Pegylated interferon alpha and ribavirin together with boceprevir or telaprevir |
Ursodeoxycholic acid | Corticoids +/− azathioprine | Ursodeoxycholic acid. Percutaneous or endoscopic placement of biliary stent in dominant biliary strictures. Orthotopic liver transplantation for advanced disease |
| related diseases | Cryoglobulinemia, SS, porphyria cutanea tarda | SS, SSc, RA, SLE, PM, autoimmune thyroiditis | Hemolytic anemia, type-1diabetes, RA, autoimmune thyroiditis | IgG4-related disease, inflammatory bowel disease |
Affected patients may be completely asymptomatic, and may be diagnosed after incidental discovery of abnormal liver function tests. Non-specific symptoms: fatigue, nausea, anorexia, weight loss, pruritus, upper abdominal discomfort.
AMA, anti-mitochondrial antibodies; ANA, anti-nuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; LC-1, liver cytosol type 1; LKM-1, liver kidney microsome type 1; PM, polymyositis; RA, rheumatoid arthritis; RF, rheumatoid factor; SLA/LPA, soluble liver antigen/liver-pancreas antigen; SS, Sjögren syndrome; SLE, systemic lupus erythematosus; SMA, smooth muscle antibodies; SSc, systemic sclerosis
Hepatitis C virus infection
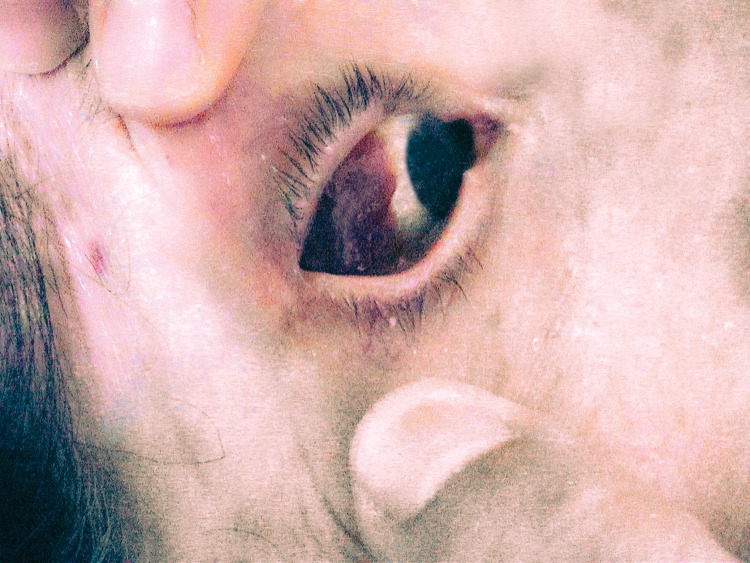
The discovery of the hepatitis C virus (HCV) in 1989 radically changed the predominant etiology of liver involvement in SS patients. Further experimental,20,21 virological22,23 and clinical studies24–26 revealed a close association between HCV and SS. In addition, a large multicenter study17 showed that HCV-related SS was indistinguishable in most cases from the primary SS form using the main sets of classification criteria. Two thirds of HCV-related SS patients presented with cryoglobulinemia, which may be considered the key immunological marker of SS associated with HCV, and the main cause of vasculitis in these patients.27 In primary SS, cryoglobulinemia is associated with extraglandular involvement, an enhanced risk of B-cell lymphoma (Fig. 1), and poor survival,1 and in HCV-related SS patients the prevalence of cryoglobulinemia is five-fold higher than those without HCV.27
Fig. 1. Ocular B-cell lymphoma in a patient with chronic hepatitis C virus and Sjögren syndrome.
The current classification criteria include chronic HCV infection as an exclusion criterion for the classification of primary SS, not because it mimics primary SS, but because it seems to be directly responsible for the development of SS in a specific subset of HCV patients.17 HCV-related SS patients should be considered as a separate subset to those with primary SS. It would be more appropriate to classify these patients as having an HCV-related SS. The term “SS secondary to HCV” might be used in those cases in which infection of salivary gland epithelium by HCV is directly demonstrated.
Some recent studies have investigated etiopathogenic factors involved in HCV-related SS. Genetic factors may play a role in the development of HCV-related SS. Smyth et al28 have reported that HCV patients carrying the HLA DQB1*02 allele had a higher frequency of sicca syndrome. In addition, human La protein has been recently implicated in facilitating the internal initiation of translation as well as replication of HCV RNA.29 It could be hypothesized that patients carrying anti-La antibodies may be protected against chronic HCV infection. We analyzed the possible association between anti-La antibodies and chronic HCV infection in a large series of patients with SS, and found that the main differential aspect between primary and HCV-related SS was the immunological pattern, with a predominance of cryoglobulinemia-related markers [mixed cryoglobulins, rheumatoid factor (RF), hypocomplementemia, monoclonal band] over SS-related specific markers (anti-Ro/SS-A and anti-La/SS-B autoantibodies) in HCV-related SS. We confirmed that cryoglobulinemia was the key immunological marker of SS associated with HCV, while anti-La antibodies were less frequently detected in HCV patients, and do not seem to protect against chronic HCV infection in SS patients.30
In Mediterranean countries, chronic HCV infection is the main cause of liver involvement in patients with SS, with a prevalence of 13% – nearly three-fold greater than that observed for autoimmune liver involvement.16 A recent study by Nawito et al31 found a prevalence of sicca syndrome of 55% in 120 Egyptian patients with chronic HCV infection. This underlies the importance of chronic HCV infection as a cause of liver disease in SS patients from specific geographical regions that have high prevalences of HCV infection in the general population.
Hepatitis B virus infection
Chronic hepatitis B virus (HBV) infection is associated with various extrahepatic manifestations, including skin rash, arthritis and glomerular disease.32 In addition, a close association between HBV and polyarteritis nodosa was reported by Guillevin et al in 1981,33 while other studies have suggested a possible association between HBV and other systemic autoimmune diseases, such as RA, rheumatic polymyalgia, antiphospholipid syndrome (APS) and SLE.34 However, there are no data suggestive of a causal role of HBV in these diseases,35 and one study has even suggested a lower frequency of HBV infection in patients with autoimmune diseases.36 It has also been suggested that previous exposure to HBV might protect against the development of autoimmune diseases due to mechanisms such as antigen competition or down-regulation of allergic and autoimmune responses.36–38 Although the reasons for the specific predilection of HCV for exocrine tissue are unknown, differences either in the viral structure (HBV is a DNA virus, while HCV is an RNA virus) or in the autoimmune responses they trigger might explain the variation in sialotropism.
The association between SS and HBV depends on the geographical area in which the association is investigated. In European studies, this association is very infrequent. Only one case of chronic HBV infection was found in 475 Spanish SS patients, in comparison with 63 patients with chronic HCV infection.39 Three additional cases40–42 of HBV-related SS have been reported (one associated with HBV vaccination). In a recent study,43 we found a prevalence of chronic HBV infection of 0.83% in SS, a very similar prevalence to that found in the general population in Spain (0.7%).44 In spite of the few reported cases of HBV-related SS, a comparison between primary SS and HBV-related SS reveals some differences. The clinical expression of HBV-related SS is similar to that of primary SS with respect to the prevalence of sicca features, except for a higher percentage of patients with joint involvement. With respect to immunological expression, HBV-related SS patients had a higher frequency of RF, but a lower frequency of some immunological features typically described in HCV-related SS patients, such as hypocomplementemia and cryoglobulinemia. In contrast to the close association between SS and HCV, chronic HBV infection is not associated with SS in our geographical area (Barcelona, Spain), with a ratio of HBV-related SS:HCV-related SS cases of 1:10.
However, recent studies have suggested a different role of HBV in SS patients from Asian countries. Chen et al45 have recently reported a 10% prevalence of HBV infection in patients with primary SS from Taiwan. Approximately three million people in Taiwan are infected with HBV, and a large-scale survey found a prevalence rate of HBsAg (hepatitis B surface antigen)-positive patients of 17%.46 Although the prevalence of HBV infection in Taiwanese patients with SS was 10-fold higher than that found in European SS patients, the prevalence was lower than that found in the general population of Taiwan (10% vs 17%, p<0.001). In contrast, Kang et al47 have reported that Taiwanese patients with SS had a 2.3-fold higher risk of having associated HBV infection in comparison with the general Taiwanese population. The differing results obtained by these two studies suggest that further research is needed to evaluate whether the prevalence of HBV infection in Asian patients with primary SS is higher or lower with respect to the prevalence of HBV infection found in the general population of the same geographical area.
Other chronic hepatitis viral infections
No cases of association between other hepatitis viral infections (hepatitis A, D, E) and SS have been reported. In 1998, we reviewed the prevalence and clinical significance of hepatitis G virus (HGV) infection in 100 Spanish patients with primary SS. Four patients (4%) and six volunteer blood donors (3%) were found to have HGV-RNA sequences in serum. HGV infection was associated with biochemical signs of liver involvement in only two (50%) of the patients with primary SS. When compared with patients without HGV infection, no significant differences were found in terms of clinical or immunological features. HCV co-infection occurred in one (25%) of the four SS patients with HGV infection. We concluded that HGV infection alone was not a significant cause of chronic liver involvement in patients with primary SS.48
Organ-specific autoimmune liver diseases
Primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune liver disease, characterized by the chronic progressive loss of interlobular bile ducts, that primarily affects middle-aged women of all races. An immune-mediated destruction of the bile duct epithelium is thought to mediate its pathogenesis, histologically characterized by portal inflammation comprising aggregates of lymphoid cells and/or granulomas, which invade and destroy biliary epithelial cells.49 Although there are no standardized diagnostic criteria, the majority of studies consider a diagnosis of PBC in the presence of at least two of the following features: (1) biochemical data of cholestatic liver disease, (2) positive AMAs, and (3) histological features of PBC in liver biopsy (Table 1).
Prevalence of AMA in SS
Serologically, the diagnostic hallmark of PBC is the presence of significant titers of AMA, which is possibly the most specific autoantibody in clinical immunology. Elevated AMA is detected in nearly 100% of PBC patients when diagnostic tests based on recombinant antigens are used.49 AMA is directed against the 2-oxo-acid dehydrogenase complex (2-OADC),36 most frequently against the E2- and E3-binding protein components of the pyruvate dehydrogenase complex, and against the E2 components of the 2-oxo- glutarate dehydrogenase and branched-chain 2-oxo-acid dehydrogenase complexes.49
Several studies have analyzed the prevalence of AMA in patients with primary SS using the currently available methods for its detection: indirect immunofluorescence (IIF), enzyme-linked immunosorbent assays (ELISA) or Western blot tests. Studies using IIF found a prevalence ranging from 1.6% to 13%,11,12,16,50 while studies using ELISA/Western blot found higher prevalences (22–27%).12,51–53 The discrepancy in prevalences may be explained by the low sensitivity of IIF. Although IIF is widely used for its technical simplicity and cost effectiveness, it lacks sensitivity, and in up to 10% of patients diagnosed with PBC, AMA cannot be detected by this technique.54 Therefore, in patients who are strongly suspected of having PBC, but test negative for AMA with IIF, more sensitive techniques are recommended, such as ELISA. However, there are patients who test negative for AMA by any of the methods described above despite clinical and biochemical findings supporting the diagnosis of PBC. In such cases, a diagnosis of PBC should be confirmed by liver biopsy.
Prevalence of PBC in SS
The prevalence of PBC in patients with primary SS ranges from 4% to 9% according to the five studies found in the literature (Table 2),11,16,53,55,56 with two studies including more than 400 primary SS patients.16,53
Table 2. Studies on the prevalence of PBC and AIH in primary SS patients.
| Author (year) | Country | Primary SS (n) | PBC n (%) | AIH n (%) |
| Lindgren et al (1994) 11 | Sweden | 45 | 4 (9) | 2 (4) |
| Ramos-Casals et al (2006) 16 | Spain | 475 | 16 (4) | 8 (2) |
| Montaño-Loza et al (2007) 55 | Mexico | 95 | 5 (5) | 2 (2) |
| Hatzis et al (2008) 53 | Greece | 410 | 27 (6.6)† | NA |
| Karp et al (2010) 56 | USA | 194‡ | NA | 2 (1) |
AIH, autoimmune hepatitis; NA, not available; PBC, primary biliary cirrhosis; SS, Sjögren syndrome
Including patients with either definite, probable or AMA-negative PBC
Patients with Sjögren syndrome and/or sicca symptoms
Prevalence of SS in PBC
Three studies have analyzed the prevalence of systemic autoimmune diseases in PBC (Table 3).57–59 Two of these studies found SS to be the most prevalent systemic autoimmune disease in patients with PBC.58,59 Wang et al59 found that 36% of 322 patients with PBC had SS, followed by SLE with a prevalence of 4%. Gershwin et al58 found a lower prevalence of SS (10%) in 1032 patients with PBC. The same prevalence (10%) was found for RA. Finally, an Italian study including 170 PBC patients57 found that SSc was the most frequently associated systemic autoimmune disease (12%), followed by SS (3.5%).
Table 3. Studies on the prevalence of systemic autoimmune diseases in PBC patients.
| Author (year) | Country | PBC (n) | SS n (%) | RA n (%) | SLE n (%) | SSc n (%) | PM n (%) | UCTD n (%) |
| Marasini et al (2001) 57 | Italy | 170 | 6 (3.5%) | 3 (2) | 3 (2) | 21 (12) | 1 (1) | 12 (7) |
| Gershwin et al (2005) 58 | USA | 1032 | 102 (10) | 103 (10) | 27 (3) | 24 (2) | 6 (0.6) | - |
| Wang et al (2013) 59 | China | 322 | 121 (36) | 9 (2.8) | 12 (4%) | 9 (3) | 10 (3) | - |
| TOTAL | 1524 | 229 (15) | 112 (7.5) | 42 (3) | 53 (3.5) | 17 (1) | 12 (0.8) |
PBC, primary biliary cirrhosis; PM, polymyositis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjögren syndrome; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease
Clinical expression of PBC in SS
PBC and SS share several clinical, histological and serological features. According to several studies, characteristic symptoms of SS such as dry mouth or dry eyes are also commonly found (47–73%) in PBC. In addition, objective findings of dry eyes or dry mouth (such as abnormal Schirmer test, or diminished salivary flow rate) are also found in 30–50% of patients with PBC.60,61 Furthermore, PBC patients frequently (26–93%) manifest histological changes in salivary gland biopsies that are compatible with a diagnosis of SS,10,62,63 especially at early disease stages of PBC when a CD4+ lymphocyte infiltration predominates.61
Regarding immunological profiles, serum anti-nuclear antibodies (ANA) are frequently observed in both conditions, but with a higher prevalence in SS compared with PBC. Also, patients with SS have significantly higher frequencies of anti-Ro and anti-La autoantibodies, while patients with PBC have significantly higher frequencies of autoantibodies to AMA – Sm, Jo-1, collagen and MPO.64 The frequencies of HLA-B8, -DR3 and -DRW52 are also lower in PBC patients compared with those in primary SS patients.
We found a broad spectrum of abnormalities in the liver laboratory profile of SS patients with AMA-M2, including three patients with no clinical or analytical data suggestive of liver disease,16 as has been reported in five previous cases.11–13 Previous studies in non-SS patients have shown that AMA-M2 patients with any clinical or analytical signs of liver involvement have a high risk of developing symptomatic PBC,65 underlining the key role of AMA-M2 as an early immunological marker of PBC,15 and suggesting the existence of an incipient or incomplete PBC in some patients with primary SS.16 Hatzis et al53 found a diverse clinical scenario: the majority of AMA-positive patients had acholestatic liver biochemistry, and were diagnosed as having definite (n=10) or probable (n=11) PBC according to the histological confirmation, while six additional patients had a biopsy-proven PBC, but negative AMA, and were diagnosed as having AMA-negative PBC.
The high prevalence of PBC in primary SS and vice versa may suggest that both diseases share common etiopathogenic mechanisms. In both conditions, environmental triggers (putatively infectious agents and xenobiotics) may cause salivary or biliary epithelial cell apoptosis, and may contribute to tolerance breakdown to self-antigens exposed on the apoptotic blebs (SSA and SSB) and not protected by post-translational modification (PDC-E2). Salivary and biliary epithelial cells contribute to the autoimmune process by expressing cytokines, HLA class II antigens and adhesion molecules.61,66,67
Outcome and management of PBC-associated SS
Few studies have evaluated the outcome of PBC in patients with primary SS.16,53 Hatzis et al53 reported that PBC in primary SS appears to progress slowly. The authors evaluated clinical, biochemical and histological data during a mean follow-up of 66 months after diagnosis of PBC, and found that only 1 patient with probable PBC showed clinical deterioration, while 8 patients (3 with definite PBC, 4 with probable PBC, and 1 with AMA-negative PBC) showed biochemical deterioration. A second liver biopsy was carried out in five patients, and no progression was found between the first and the second biopsies after a mean follow-up of nearly 46 months.
After eliminating viral hepatitis, PBC should be considered as the main cause of liver disease in patients with primary SS. Although historically these patients have been considered as having a “secondary” SS, it seems more rational to use the term “PBC-associated SS”, owing to the clinical-based evidence that SS is associated with (and not secondary to) other autoimmune diseases. The inclusion of AMA in the routine immunological follow-up of SS patients should be recommended, independently of whether the serum liver profile is altered or not, because of the strong association between AMA and the development of PBC, and because a significant percentage of patients with primary SS may have an asymptomatic underlying PBC. Although there are no therapeutic guidelines for such asymptomatic patients, early use of ursodeoxycholic acid (UDCA) may be considered, since some studies on non-SS patients with mild serum test abnormalities have suggested that treatment with UDCA might prevent a possible evolution to liver cirrhosis.68
Autoimmune hepatitis
Autoimmune hepatitis (AIH) is a chronic autoimmune liver disease characterized histologically by interface hepatitis, biochemically by elevated transaminase levels, and serologically by the presence of autoantibodies and hypergammaglobulinemia49 (Table 1). Anti-smooth muscle and/or anti-nuclear antibodies defines type-1 AIH, while positivity for liver kidney microsomal type-1 antibodies defines type-2 AIH, which is more common in children. 49
Type-1 AIH in SS
Type-1 AIH is the second most frequently found autoimmune liver disease associated with SS. The frequency of AIH in primary SS ranges from 1% to 4% according to four studies (Table 2).11,16,55,56 Up to 2009, 56 cases of type-1 AIH had been reported in patients with primary SS.60 A specific characteristic of AIH associated with primary SS is that two-thirds of cases have been reported from Asian countries. In addition, nearly 10% of AIH patients had positive AMA (AIH-PBC overlap).These patients may have histological features compatible with both AIH and PBC, and have a cholestatic biochemical pattern.60 A recent study showed that SS was the systemic autoimmune disease most frequently reported to be associated with AIH-PBC overlap syndrome (6 out of 71 patients, 8%).69
A recent study55 evaluated the prognostic implications of antibodies to Ro/SSA in patients with type-1 AIH, and reported that anti-Ro52 antibodies (alone or in combination with antibodies to soluble liver antigen) were independently associated with a poor prognosis.
Type-2 AIH in SS
There are no reported cases of type-2 AIH in patients with primary SS, a fact that is consistent with the lack of positive anti-LKM-1 antibodies in SS. The prevalence of anti-LKM-1 antibodies has been evaluated in a large series of patients with primary SS, and none of the 335 patients tested had these autoantibodies.50
Sclerosing cholangitis
Sclerosing cholangitis (SC) is a chronic cholestatic liver disease characterized by the presence of intrahepatic and/or extrahepatic biliary duct concentric and obliterative fibrosis.49 This organ-specific autoimmune hepatobiliary disease is frequently associated with positive autoantibodies including ANAs, anti-cardiolipin antibodies and perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) (Table 1).
Thirteen cases of SC have been described in patients with primary SS.16 Certain characteristics of patients with SS and associated SC should be highlighted: a specific pattern of clinical features at presentation of SC (abdominal pain, jaundice and diarrhea), an overwhelming association with chronic pancreatitis in all but one case (with pancreatic masses demonstrated by CT abdominal scan), and an association with other autoimmune processes such as retroperitoneal fibrosis. These specific features may help to reach an early diagnosis of this rare disease in patients with primary SS. However, investigation of a possible IgG4-related disease should be mandatory in all patients with SS diagnosed with SC, especially when autoimmune pancreatitis or retroperitoneal fibrosis is also present.
Other autoimmune liver diseases
Other autoimmune liver diseases in patients with primary SS have been described rarely, including seven cases of autoimmune cholangitis, one case of nodular regenerative hyperplasia of the liver16 and one case of granulomatous hepatitis.70
Conclusions
The most recent studies have shown that the two main causes of liver disease in patients with primary SS are associated processes such as chronic viral infections and autoimmune liver diseases (Table 4).
Table 4. Diagnosis of liver involvement in Sjögren syndrome: Clinical pearls.
|
|
|
|
|
|
|
|
|
|
|
|
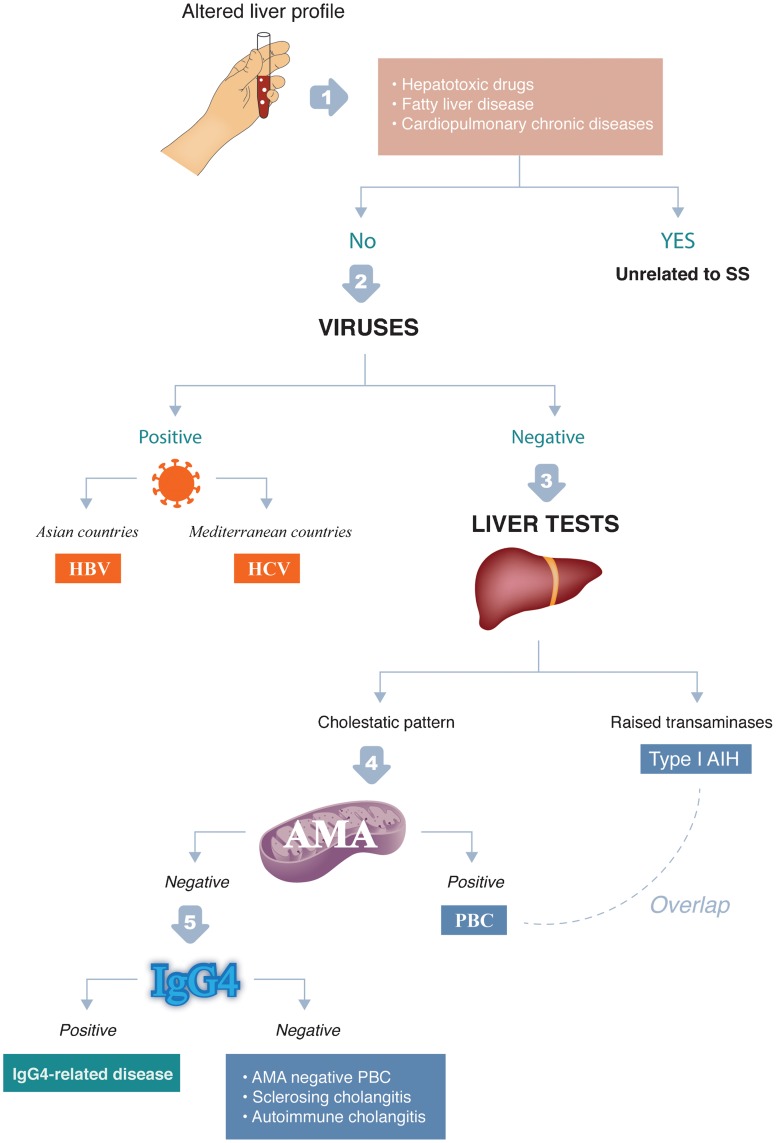
Detection of an altered liver profile in a patient with SS requires a sequential diagnosis (Fig. 2):
Fig. 2. Sequential diagnosis for patients with Sjögren syndrome presenting with altered liver profile.
The first step is to eliminate processes not associated with SS, mainly the chronic use of potential hepatotoxic drugs, fatty liver and congestive heart failure.
The second step is to evaluate epidemiological features. With respect to viral infections, HCV infection will be more frequently found in SS patients from the Mediterranean area, while HBV infection should be principally investigated in Asian patients. With respect to gender and age, the diagnosis of HCV will be more frequent in older and male SS patients, while younger and female SS patients will be more likely to have an associated autoimmune liver disease.
The third step is the evaluation of liver tests: raised aminotransferases suggest viral or autoimmune hepatitis, while a cholestatic pattern suggests PBC or SC. Liver profiles are not useful in differentiating between viral- and autoimmune-related liver diseases in all cases.
The fourth step is the evaluation of the immunological profile, which plays a key role in differentiating between the main etiologies: patients with chronic HCV infection will have a higher frequency of cryoglobulins and hypocomplementemia, while those with autoimmune liver disease will have, predominantly, hypergammaglobulinemia and positive autoantibodies (AMA, ANA, SMA, Ro and La). In SS patients with a suspected autoimmune liver disease, the existence of AMA with a specific M2 pattern clearly indicates PBC, while high titers of ANA and anti-SMA suggest type-1 AIH.
The fifth step is to eliminate IgG4-related disease in SS patients presenting with autoimmune or SC, especially in cases with associated autoimmune pancreatitis, retroperitoneal fibrosis or inflammatory bowel disease, or presenting with atypical autoantibodies such as antiphospholipid antibodies or p-ANCA.
Acknowledgments
Supported by Grants La Marató de TV3 (071810), Fondo de Investigaciones Sanitarias (080103/1201009), and “Ajut per a la Recerca Josep Font” from Hospital Clinic-Barcelona (PBZ, 2012)
Abbreviations
- 2-OADC
2-oxo-acid dehydrogenase complex
- AIH
autoimmune hepatitis
- ANA
anti-nuclear antibodies
- ANCA
anti-neutrophil cytoplasmic antibodies
- AMA
anti-mitochondrial antibodies
- APS
antiphospholipid syndrome
- ELISA
enzyme-linked immunosorbent assays
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HGV
hepatitis G virus
- IIF
immunofluorescence
- LC-1
liver cytosol type 1
- LKM-1
liver kidney microsome type 1
- p-ANCA
perinuclear anti-neutrophil cytoplasmic antibodies
- PBC
primary biliary cirrhosis
- PM
polymyositis
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SC
Sclerosing cholangitis
- SLA/LPA
soluble liver antigen/liver-pancreas antigen
- SLE
systemic lupus erythematosus
- SMA
smooth muscle antibodies
- SS
Sjögren syndrome
- SSc
systemic sclerosis
- UCTD
undifferentiated connective tissue disease
- UDCA
ursodeoxycholic acid
References
- 1.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X. Primary Sjögren syndrome. Br Med J. 2012;344:e3821. doi: 10.1136/bmj.e3821. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M, Font J. Primary Sjögren's syndrome. In: Imboden J, Hellmann D, Stone J, editors. Current Diagnosis and Treatment in Rheumatology. USA: McGraw-Hill; 2007. pp. 237–245. [Google Scholar]
- 4.García-Carrasco M, Ramos-Casals M, Rosas J, Pallares L, Calvo-Alén J, Cervera R, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine. 2002;81:270–280. doi: 10.1097/00005792-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren's syndrome. Semin Arthritis Rheum. 2000;29:296–304. doi: 10.1016/s0049-0172(00)80016-5. [DOI] [PubMed] [Google Scholar]
- 6.Haga HJ, Gjesdal CG, Koksvik HS, Skomsvoll JF, Irgens LM, Ostensen M. Pregnancy outcome in patients with primary Sjögren's syndrome. A case-control study. J Rheumatol. 2005;32:1734–1736. [PubMed] [Google Scholar]
- 7.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjögren's syndrome. A clinical, pathological, and serological study of sixty-two cases. Medicine. 1965;44:187–231. [PubMed] [Google Scholar]
- 8.Golding PL, Bown R, Mason AM, Taylor E. “Sicca complex” in liver disease. Br Med J. 1970;4:340–342. doi: 10.1136/bmj.4.5731.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alarcón-Segovia D, Díaz-Jouanen E, Fishbein E. Features of Sjögren's syndrome in primary biliary cirrhosis. Ann Intern Med. 1973;79:31–36. doi: 10.7326/0003-4819-79-1-31. [DOI] [PubMed] [Google Scholar]
- 10.Tsianos EV, Hoofnagle JH, Fox PC, Alspaugh M, Jones EA, Schafer DF, et al. Sjögren's syndrome in patients with primary biliary cirrhosis. Hepatology. 1990;11:730–734. doi: 10.1002/hep.1840110504. [DOI] [PubMed] [Google Scholar]
- 11.Lindgren S, Manthorpe R, Eriksson S. Autoimmune liver disease in patients with primary Sjögren's syndrome. J Hepatol. 1994;20:354–358. doi: 10.1016/s0168-8278(94)80007-3. [DOI] [PubMed] [Google Scholar]
- 12.Skopouli FN, Barbatis C, Moutsopoulos HM. Liver involvement in primary Sjögren's syndrome. Br J Rheumatol. 1994;33:745–748. doi: 10.1093/rheumatology/33.8.745. [DOI] [PubMed] [Google Scholar]
- 13.Csepregi A, Szodoray P, Zeher M. Do autoantibodies predict autoimmune liver disease in primary Sjögren's syndrome? Data of 180 patients upon a 5 year follow-up. Scand J Immunol. 2002;56:623–629. doi: 10.1046/j.1365-3083.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramos-Casals M, Loustaud-Ratti V, De Vita S, Zeher M, Bosch JA, Toussirot E, et al. SS-HCV Study Group Sjögren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine. 2005;84:81–89. doi: 10.1097/01.md.0000157397.30055.c9. [DOI] [PubMed] [Google Scholar]
- 15.Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann Rheum Dis. 2004;63:123–129. doi: 10.1136/ard.2002.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Sanchez-Tapias JM, Pares A, Forns X, Brito-Zerón P, Nardi N, et al. Characterization and differentiation of autoimmune versus viral liver involvement in patients with Sjögren's syndrome. J Rheumatol. 2006;33:1593–1599. [PubMed] [Google Scholar]
- 17.Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126–139. doi: 10.1016/j.jaut.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez Tapias JM, FornsBernhardt X. Hepatitis vírica crónica. In: Farreras P, Rozman C, editors. Medicina Interna. XVII edition. Vol. 1. Barcelona: Elsevier; 2012. pp. 300–305. [Google Scholar]
- 20.De Vita S, Sansonno D, Dolcetti R, Ferraccioli G, Carbone A, Cornacchiulo V, et al. Hepatitis C virus infection within a malignant lymphoma lesion in the course of type II mixed cryoglobulinemia. Blood. 1995;86:1887–1892. [PubMed] [Google Scholar]
- 21.Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, et al. Sialadenitis histologically resembling Sjögren syndrome in mice transgenic for hepatitis C virus envelope genes. Proc Natl Acad Sci U S A. 1997;94:233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arrieta JJ, Rodríguez-Inigo E, Ortiz-Movilla N, Bartolome J, Pardo M, Manzarbeitia F, et al. In situ detection of hepatitis C virus RNA in salivary glands. Am J Pathol. 2001;158:259–264. doi: 10.1016/S0002-9440(10)63964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toussirot E, Le Huede G, Mougin C, Balblanc JC, Bettinger D, Wendling D. Presence of hepatitis C virus RNA in the salivary glands of patients with Sjögren's syndrome and hepatitis C virus infection. J Rheumatol. 2002;29:2382–2385. [PubMed] [Google Scholar]
- 24.De Vita S, Damato R, De Marchi G, Sacco S, Ferraccioli G. True primary Sjögren's syndrome in a subset of patients with hepatitis C infection: a model linking chronic infection to chronic sialadenitis. Isr Med Assoc J. 2002;4:1101–1105. [PubMed] [Google Scholar]
- 25.Jorgensen C, Legouffe MC, Perney P, Coste J, Tissot B, Segarra C, et al. Sicca syndrome associated with hepatitis C virus infection. Arthritis Rheum. 1996;39:1166–1171. doi: 10.1002/art.1780390714. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Casals M, García-Carrasco M, Cervera R, Rosas J, Trejo O, de la Red G, et al. Hepatitis C virus infection mimicking primary Sjögren syndrome. A clinical and immunologic description of 35 cases. Medicine. 2001;80:1–8. doi: 10.1097/00005792-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379:348–360. doi: 10.1016/S0140-6736(11)60242-0. [DOI] [PubMed] [Google Scholar]
- 28.Smyth CM, McKiernan SM, Hagan R, Pilkington R, O'Regan M, Lawlor E, et al. Chronic hepatitis C infection and sicca syndrome: a clear association with HLA DQB1*02. Eur J Gastroenterol Hepatol. 2007;19:493–498. doi: 10.1097/MEG.0b013e328010687d. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Ray U, Das S. Human La protein interaction with GCAC near the initiator AUG enhances hepatitis C Virus RNA replication by promoting linkage between 5' and 3' untranslated regions. J Virol. 2013;87:6713–6726. doi: 10.1128/JVI.00525-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brito-Zerón P, Kaveri SV, Bové A, Retamozo S, Akasbi M, Gandía M, et al. Anti-La antibodies as a potential protection for chronic hepatitis C virus infection in patients with Sjögren syndrome: analysis in 663 patients. Rev Clin Esp. 2013 (in press) [Google Scholar]
- 31.Nawito Z, Amin A, El-Fadl SA, Abu El Einen K. Sicca complex among Egyptian patients with chronic hepatitis C virus infection. Clin Rheumatol. 2011;30:1299–1304. doi: 10.1007/s10067-011-1746-x. [DOI] [PubMed] [Google Scholar]
- 32.Han SH. Extrahepatic manifestations of chronic hepatitis. B. Clin Liver Dis. 2004;8:403–418. doi: 10.1016/j.cld.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Godeau P, Guillevin L, Bletry O, Wechsler B. Periarteritis nodosa associated with hepatitis B virus. 42 cases. Nouv Presse Med. 1981;10:1289–1292. [PubMed] [Google Scholar]
- 34.Maya R, Gershwin ME, Shoenfeld Y. Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol. 2008;34:85–102. doi: 10.1007/s12016-007-8013-6. [DOI] [PubMed] [Google Scholar]
- 35.Permin H, Aldershvile J, Nielsen JO. Hepatitis B virus infection in patients with rheumatic diseases. Ann Rheum Dis. 1982;41:479–482. doi: 10.1136/ard.41.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ram M, Anaya JM, Barzilai O, Izhaky D, Porat Katz BS, Blank M, et al. The putative protective role of hepatitis B virus (HBV) infection against autoimmune disorders. Autoimmun Rev. 2008;7:621–625. doi: 10.1016/j.autrev.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol. 2005;12:217–224. doi: 10.1080/17402520500285247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bach JF. Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun. 2001;16:347–353. doi: 10.1006/jaut.2000.0478. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Casals M, Muñoz S, Zerón PB. Hepatitis C virus and Sjögren's syndrome: trigger or mimic? Rheum Dis Clin North Am. 2008;34:869–884. doi: 10.1016/j.rdc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Aprosin ZG, Serov VV, Lopatkina TN. The hepatitis B virus as a probable etiological factor in Sjögren's disease. Ter Arkh. 1993;65:73–78. [PubMed] [Google Scholar]
- 41.Iakimtchouk K, Myrmel H, Jonsson R. Serological screening for hepatitis B and C and human herpes virus 6 in Norwegian patients with primary Sjögren's syndrome. J Rheumatol. 1999;26:2065–2066. [PubMed] [Google Scholar]
- 42.Toussirot E, Lohse A, Wendling D, Mougin C. Sjögren's syndrome occurring after hepatitis B vaccination. Arthritis Rheum. 2000;43:2139–2140. doi: 10.1002/1529-0131(200009)43:9<2139::AID-ANR27>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Marcos M, Alvarez F, Brito-Zerón P, Bové A, Perez-De-Lis M, Diaz-Lagares C, et al. Chronic hepatitis B virus infection in Sjögren's syndrome. Prevalence and clinical significance in 603 patients. Autoimmun Rev. 2009;8:616–620. doi: 10.1016/j.autrev.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Salleras L, Dominguez A, Bruguera M, Plans P, Costa J, Cardenosa N, et al. Declining prevalence of hepatitis B virus infection in Catalonia (Spain) 12 years after the introduction of universal vaccination. Vaccine. 2007;25:8726–8731. doi: 10.1016/j.vaccine.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Chen MH, Hsiao LT, Chen MH, Tsai CY, Huang YH, Chou CT. Clinical significance of chronic hepatitis B virus infection in patients with primary Sjögren's syndrome. Clin Rheumatol. 2012;31:309–315. doi: 10.1007/s10067-011-1814-2. [DOI] [PubMed] [Google Scholar]
- 46.Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatic screening participants. J Formos Med Assoc. 2007;106:148–155. doi: 10.1016/S0929-6646(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 47.Kang JH, Lin HC. Comorbidities in patients with primary Sjögren's syndrome: a registry-based case-control study. J Rheumatol. 2010;37:1188–1194. doi: 10.3899/jrheum.090942. [DOI] [PubMed] [Google Scholar]
- 48.Font J, Tàssies D, García-Carrasco M, Ramos-Casals M, Cervera R, Reverter JC, et al. Hepatitis G virus infection in primary Sjögren's syndrome: analysis in a series of 100 patients. Ann Rheum Dis. 1998;57:42–44. doi: 10.1136/ard.57.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czaja AJ. Immunopathogenesis of autoimmune liver damage. In: Font J, Ramos-Casals M, Rodés J, editors. Digestive Involvement in Systemic Autoimmune Diseases. Amsterdam: Elsevier; 2008. pp. 121–140. [Google Scholar]
- 50.Nardi N, Brito-Zerón P, Ramos-Casals M, Aguilo S, Cervera R, Ingelmo M, et al. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren's syndrome: prevalence and clinical significance in 335 patients. Clin Rheumatol. 2006;25:341–346. doi: 10.1007/s10067-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 51.Zurgil N, Bakimer R, Moutsopoulos HM, Tzioufas AG, Youinou P, Isenberg DA, et al. Antimitochondrial (pyruvate dehydrogenase) autoantibodies in autoimmune rheumatic diseases. J Clin Immunol. 1992;12:201–209. doi: 10.1007/BF00918090. [DOI] [PubMed] [Google Scholar]
- 52.Fujikura S, Davis PA, Prindiville T, Leung P, Fox RI, Gershwin ME. Sjögren's syndrome and primary biliary cirrhosis: presence of autoantibodies to purified mitochondrial 2-oxoacid dehydrogenases. J Rheumatol. 1990;17:1453–1457. [PubMed] [Google Scholar]
- 53.Hatzis GS, Fragoulis GE, Karatzaferis A, Delladetsima I, Barbatis C, Moutsopoulos HM. Prevalence and longterm course of primary biliary cirrhosis in primary Sjögren's syndrome. J Rheumatol. 2008;35:2012–2016. [PubMed] [Google Scholar]
- 54.Tanaka A, Miyakawa H, Luketic VA, Kaplan M, Storch WB, Gershwin ME. The diagnostic value of anti-mitochondrial antibodies, especially in primary biliary cirrhosis. Cell Mol Biol (Noisy-le-grand) 2002;48:295–299. [PubMed] [Google Scholar]
- 55.Montano-Loza AJ, Crispin-Acuña JC, Remes-Troche JM, Uribe M. Abnormal hepatic biochemistries and clinical liver disease in patients with primary Sjögren syndrome. Ann Hepatol. 2007;6:150–155. [PubMed] [Google Scholar]
- 56.Karp JK, Akpek EK, Anders RA. Autoimmune hepatitis in patients with primary Sjögren's syndrome: a series of two-hundred and two patients. Int J Clin Exp Pathol. 2010;3:582–586. [PMC free article] [PubMed] [Google Scholar]
- 57.Marasini B, Gagetta M, Rossi V, Ferrari P. Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis. 2001;60:1046–1049. doi: 10.1136/ard.60.11.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, et al. USA PBC Epidemiology Group. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Zhang FC, Chen H, Zhang X, Xu D, Li YZ, et al. Connective tissue diseases in primary biliary cirrhosis: a population-based cohort study. World J Gastroenterol. 2013;19:5131–5137. doi: 10.3748/wjg.v19.i31.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fragoulis GE, Skopouli FN, Selmi C, Gershwin ME. Liver involvement in Primary Sjögren Syndrome. In: Ramos-Casals M, Stone J, Moutsopoulos H, editors. Sjögren syndrome. Diagnosis and Therapeutics. London: Springer-Verlag; 2012. pp. 237–246. [Google Scholar]
- 61.Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren's syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34–42. doi: 10.1016/j.jaut.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uddenfeldt P, Danielsson A, Forssell A, Holm M, Ostberg Y. Features of Sjogren's syndrome in patients with primary biliary cirrhosis. J Intern Med. 1991;230:443–448. doi: 10.1111/j.1365-2796.1991.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 63.Kaplan MJ, Ike RW. The liver is a common non-exocrine target in primary Sjogren's syndrome: a retrospective review. BMC Gastroenterol. 2002;2:21. doi: 10.1186/1471-230X-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tishler M, Alosachie I, Barka N, Lin HC, Gershwin ME, Peter JB, et al. Primary Sjogren's syndrome and primary biliary cirrhosis: differences and similarities in the autoantibody profile. Clin Exp Rheumatol. 1994;13:497–500. [PubMed] [Google Scholar]
- 65.Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865–870. doi: 10.1136/gut.2003.023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Selmi C, Mackay IR, Gershwin ME. The autoimmunity of primary biliary cirrhosis and the clonal selection theory. Immunol Cell Biol. 2011;89:70–80. doi: 10.1038/icb.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009;33:190–196. doi: 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beswick DR, Klatskin G, Boyer JL. Asymptomatic primary biliary cirrhosis. A progress report on long-term follow-up and natural history. Gastroenterology. 1985;89:267–271. [PubMed] [Google Scholar]
- 69.Efe C, Wahlin S, Ozaslan E, Berlot AH, Purnak T, Muratori L, et al. Autoimmune hepatitis/primary biliary cirrhosis overlap syndrome and associated extrahepatic autoimmune diseases. Eur J Gastroenterol Hepatol. 2012;24:531–534. doi: 10.1097/MEG.0b013e328350f95b. [DOI] [PubMed] [Google Scholar]
- 70.Miller EB, Shichmanter R, Friedman JA, Sokolowski N. Granulomatous hepatitis and Sjögren's syndrome: an association. Semin Arthritis Rheum. 2006;36:153–158. doi: 10.1016/j.semarthrit.2006.07.002. [DOI] [PubMed] [Google Scholar]