Abstract
Hepatocellular cancer (HCC) is the fifth most prevalent cancer worldwide and the third leading cause of cancer-related deaths. Non-alcoholic fatty liver disease (NAFLD), a spectrum of hepatic disorders associated with obesity and the metabolic syndrome, is a recognized risk factor for HCC. NAFLD that is advanced to cirrhosis carries the highest risk for HCC, but there is increasing concern that NAFLD-associated HCC may also occur in non-cirrhotic liver. As NAFLD is rapidly becoming the most common liver condition, it has been implicated in the worrisome trend of rising HCC incidence in a number of countries, which may offset successful measures in reducing the effect of virus-related liver cancer. Independently or in synergy with cirrhosis, NAFLD may provide a special oncogenic microenvironment through its pathogenic association with chronic nutrient excess and adipose tissue remodeling, characterized by pro-inflammatory adipokine profiles, lipotoxicity, altered hepatocellular bioenergetics, and insulin resistance. Better understanding of this complex process, and development of reliable biomarkers for HCC will be critical for early recognition and risk prediction. Moreover, correcting deranged lipid metabolism and restoring insulin sensitivity by lifestyle measures and targeted pharmacotherapy holds major promise for effective prevention of NAFLD-associated HCC.
Keywords: Lipotoxicity, Insulin resistance, Cirrhosis, Hepatocarcinogenesis, Chemoprevention
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by abnormal hepatic lipid deposition without a history of excessive alcohol consumption.1 NAFLD is associated with obesity, and has been regarded as the hepatic presentation of metabolic syndrome.2 NAFLD has become extremely common in developed societies, affecting between 20 and 34% of adults3,4 and up to 13% of children,5 while also reaching high prevalence in other parts of the world.6,7
The term NAFLD refers to a spectrum of liver disorders, ranging from isolated fatty liver (IFL) to non-alcoholic steatohepatitis (NASH).1,8 IFL represents about 80% of all cases of NAFLD, and carries minimal risk for advanced liver disease, although NAFLD in general has been associated with an adverse cardiovascular risk profile.9,10 NASH of varying severity accounts for the remaining cases and displays complex pathology beyond steatosis including hepatocellular injury, inflammation, and fibrosis.1,8 NASH progresses to cirrhosis in 10–20% of cases within 10 years and may lead to major complications such as portal hypertension, liver failure, and hepatocellular carcinoma (HCC).1,11
Based on long-term follow-up studies, HCC is a rare complication of NAFLD.12,13 However, HCC is becoming more common in the USA, with an age-adjusted incidence rising from 1.5 to 4.9 per 100,000 individuals over the past 30 years.14 This trend has been attributed to increased incidence of chronic hepatitis C virus (HCV) infection, although the cause of HCC remains unclear in 50% of cases, and NAFLD is increasingly implicated in the rising HCC prevalence.15–17 It is therefore critical to improve our understanding of the link between NAFLD and HCC.
Epidemiology of HCC associated with NAFLD
HCC emerging in NAFLD that has advanced to cirrhosis
Autopsy records and imaging data indicate that 70–80% of individuals with obesity have increased liver fat content, suggesting that every third person in the general adult population of the USA and other industrialized countries has some form of NAFLD.4,18 Recent reports indicate that NASH can be verified by histological evaluation in up to 47% of all NAFLD cases among obese individuals.19,20 Moreover, there are 26 million individuals with manifest diabetes in the USA, and the presence of biopsy-proven NAFLD among people with diabetes has been reported to reach up to 74%, with evidence of NASH in every fourth case.21,22 The pool of individuals at risk of NAFLD advancing to NASH and cirrhosis is therefore a substantial one.
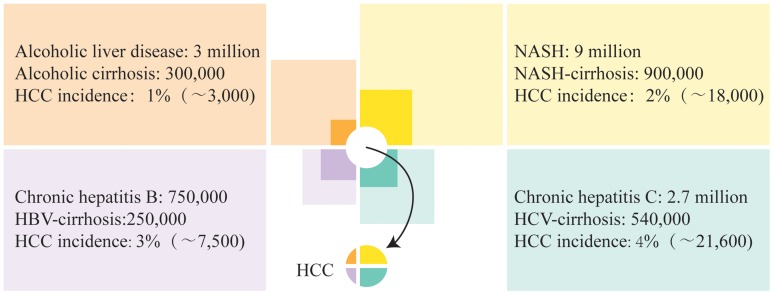
There is good evidence that HCC may complicate NAFLD cirrhosis in the absence of other risk factors.11,23 In a prospective study conducted over 10 years, HCC developed in 10 out of 149 patients with NAFLD cirrhosis in comparison with 25 out of 147 American patients with HCV cirrhosis.24 In another analysis from the USA, the yearly cumulative incidence of NAFLD cirrhosis was 2.6%, whereas that of HCV cirrhosis was 4.0%.25 In a Japanese study, HCC developed in 11.3% of patients with NAFLD cirrhosis compared with 30.5% of patients with HCV cirrhosis over 5 years.26 The overall contribution of NAFLD to the burden of liver cancer appears therefore comparable with that of major established causes of HCC such as chronic hepatitis B virus, chronic HCV, and alcoholic liver disease (Fig. 1).
Fig. 1. Disease burden of HCC by major etiologies in the USA.
The number of individuals with chronic liver disease (prevalence shown by light colored squares) including those with cirrhosis (prevalence shown by dark color squares) who develop HCC in any given year (incidence shown by small pie chart in the center) is illustrated by areas corresponding to low estimates from data published for alcoholic liver disease,111,112 NASH,4,24,25 chronic hepatitis B,113,114 and chronic HCV.24,25,115,116 Accordingly, the highest number of cases of HCC are associated with chronic HCV, with the next highest number being associated with NAFLD. Note that the actual incidence of HCC is less than the sum of these estimates, indicating overlap between groups of individuals with chronic liver disease of various etiologies.
Based on the significant worldwide prevalence of obesity and diabetes, there is an increasing probability that NAFLD coexists with chronic liver disease of other etiologies, increasing the risk of HCC. A study in Taiwan found that body mass index (BMI) ≥ 30 kg/m2 was independently associated with a four-fold increased risk of HCC among HCV-seropositive subjects, while this risk was only two-fold higher among obese subjects who were HCV-seronegative.27 In a French study, the combined presence of increased BMI and clinically manifest diabetes predicted an adjusted hazard ratio of 6.0 for HCC among 431 patients with cirrhosis associated with HCV and alcohol.28
NAFLD is recognized as a major cause of cryptogenic cirrhosis, a condition in which histological features of IFL or NASH may have disappeared, and only thorough medical historytaking can verify this relationship.29,30 Cryptogenic cirrhosis in developed societies is linked to 30–40% of diagnosed HCC cases without other obvious risk factors.17 Cryptogenic cirrhosis may be the cause of HCC diagnosed at more advanced stages, as demonstrated in a single-center study in which HCC was primarily identified by clinical symptoms, rather than via surveillance of patients with cryptogenic cirrhosis.31
HCC emerging in non-cirrhotic NAFLD
An additional concern is that HCC may complicate NAFLD with minimal or no fibrosis.11,32 Analysis of a healthcare claims database accounting each year for 18 million American lives identified 4,406 cases of HCC between 2002 and 2008, with NAFLD being the most common underlying risk factor (59%) followed by diabetes (36%) and chronic HCV (22%).33 Intriguingly, the majority of NAFLD-associated HCC cases (54%) had no International Classification of Diseases code for cirrhosis, in comparison with only 22% of HCV-associated HCC documented in the absence of cirrhosis.33 In a compilation of case reports and case series from various geographical areas, the degree of fibrosis was F2 or lower in 28% of cases with NAFLD-associated HCC.11 Finally, in a recent study of 17,895 patients with HCC identified from the SEER-Medicare database, non-cirrhotic NAFLD was the only etiology in 1,031 cases (5.8%), of which 186 cases (approximately 1% of the total HCC cohort) developed in isolated fatty liver.34
HCC risk remains very low when the analysis includes patients with all stages of NAFLD, not just those with biopsy-proven NASH. In a cohort study conducted on Japanese patients with echogram-based diagnosis of NAFLD, the annual incidence rate of HCC was 0.043%, amounting to a cumulative rate of 0.51% after a period of 12 years.35 A systematic review found that NAFLD cohorts with few or no cases of cirrhosis had a cumulative HCC mortality of 0–3% for study periods of up to 20 years.36 Based on large-scale epidemiological and long-term follow-up studies, HCC affects no more than 1 out of 3,000–4,000 individuals who have NAFLD in the absence of other liver disease.12,13 With the growing prevalence of obesity and diabetes, however, these numbers may increase, and additional studies are warranted to predict and understand the societal impact of NAFLD on HCC.
Pathogenesis of HCC associated with NAFLD
General considerations
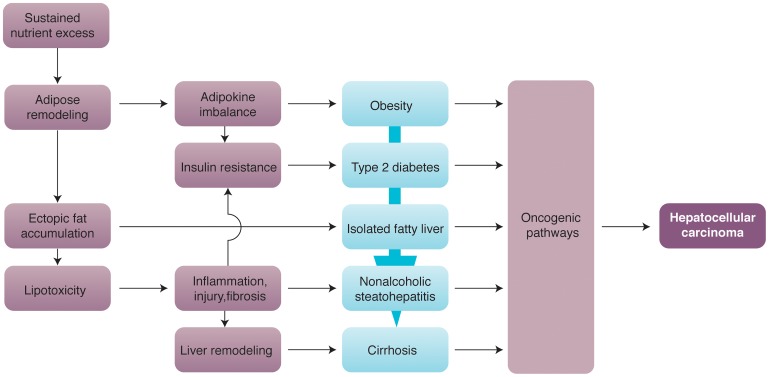
NAFLD has a complex pathogenesis, with cellular and molecular mechanisms providing multiple links to the development of liver cancer.37,38 Most cases of NAFLD-associated HCC occur in cirrhosis, which provides a unique and strong tumorigenic microenvironment.39 However, NAFLD usually develops in the context of obesity and diabetes, which are independently associated with increased risk for cancer in a variety of tissues, including the liver.40,41 Therefore, interaction of oncogenic pathways related to adipose tissue dysfunction and established cirrhosis may provide a particularly conducive setting for the emergence of HCC (Fig. 2).
Fig. 2. Major mechanisms of hepatocarcinogenesis in NAFLD.
Schematic illustration of metabolic derangements resulting from sustained nutrient excess, which involves remodeling of adipose tissue linked to the development and progression of NAFLD culminating in HCC. Key elements include a pro-inflammatory milieu promoted by adipokine imbalance and lipotoxicity caused by ectopic fat accumulation, both contributing to insulin resistance as a hallmark of pathogenesis. Various oncogenic pathways are activated at successive steps of progression, with interactions and cumulative effects accounting for the highest risk of HCC in cirrhosis, but allowing emergence of HCC at earlier disease stages.
Molecular mechanisms of hepatocarcinogenesis in cirrhosis
There is substantial evidence that cirrhosis is one of the most important risk factors of HCC.39,42 Erratic liver remodeling in cirrhosis creates a microenvironment with repeated cycles of hepatocellular destruction and compensatory regeneration to foster the development of HCC.43,44 This complex process involves many different cell types and oncogenic pathways. Triggered by chronic hepatocellular or cholangiocellular injury, resident liver macrophages (Kupffer cells), hepatic stellate cells, sinusoidal endothelial cells, and intrahepatic lymphocytes gradually change their phenotypes and acquire pro-oncogenic properties. Along with the recruitment of additional players, these cells produce a variety of cytokines, chemokines, growth factors, free radicals, and other bioactive substances that drive the initiation and progression of HCC.45 Through stepwise changes that may take many years, emergence of HCC follows a dysplasia–carcinoma sequence seen in other cancers.43,44 Accordingly, identification of molecular signatures that distinguish dysplastic nodules from early HCC in cirrhotic liver may allow enhanced surveillance, reliable prognostication, and timely intervention.46
Essentially, all major mechanisms of oncogenesis have been implicated in the development of HCC, including structural defects of the genome, epigenetic silencing or activation, and overly active or aberrant signal transduction cascades.47,48 This extraordinary heterogeneity of HCC and the need to correlate molecular alterations with clinical characteristics have been the subject of several recent and excellent reviews.49,50 Initiation and progression of HCC involves numerous oncogenic pathways that may offer pharmacological targets for different subsets of patients.51,52 Thus, hepatocyte growth factor acting on c-met is critical for G1 to S transition in the cell cycle, making this receptor a candidate for targeted chemotherapy.53 Oncogenic signaling that depends on activation of Frizzled receptors by Wnt occurs through β-catenin, c-Jun N-terminal kinases (JNKs), and protein kinase C.54 Single nucleotide polymorphisms in the gene encoding epidermal growth factor, another major cell growth stimulator acting through cell surface receptors such as erbB1 and Her2/neu, have been linked to a variable risk of HCC.55 Recent research suggests that defects in the post-transcriptional regulation of gene expression via microRNA (miRNA) also contribute to development of HCC, and that different miRNA signatures may point to the underlying etiology.56,57
Adipose tissue remodeling and molecular mechanisms of hepatocarcinogenesis
So far, no structural or functional aberrations of liver cells have been specifically associated with the pathogenesis of HCC in NAFLD, although there are multiple candidates that may fulfill this role. Emergence of HCC in non-cirrhotic NAFLD suggests sufficient complexity to initiate hepatocarcinogenesis without the tumorigenic microenvironment of cirrhosis. Chronic nutrient excess results in expansion, redistribution, and remodeling of adipose tissue, which is associated with inflammation, lipotoxicity, and insulin resistance.58,59 These events are linked to numerous oncogenic pathways, and have a major role in the progression of NAFLD. Expansion of adipose tissue is characterized by disproportionate growth of visceral and deep subcutaneous fat depots, with an adverse adipokine secretory profile that includes higher levels of leptin and lower levels of adiponectin.60,61 Leptin has pro-inflammatory, pro-fibrogenic, and growth-promoting effects mediated by the Janus kinase/signal transducer and activator of transcription (JAK/STAT), phosphoinositide 3-kinase (PI3K)/Akt, and extracellular signal-regulated kinases (ERKs).62 By contrast, adiponectin, a potent activator of 5′-adenosine monophosphate activated protein kinase (AMPK), has anti-inflammatory, anti-angiogenic, and tumor growth-limiting properties by opposing the actions of leptin and suppressing signal transduction through Akt, STAT3, and mammalian target of rapamycin (mTOR).63 Thus, coinciding leptin surplus and adiponectin deficiency may activate numerous oncogenic pathways.
Adipose tissue remodeling also results in increased secretion of the pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), which promote cell growth and inhibit apoptosis by targeting multiple signaling effectors such as ERKs, JNKs, nuclear factor-κB (NF-κB), signal transducer and activator of transcription 3 (STAT3), and mTOR.37 The role of these cytokines in the development of HCC is supported by observations that obesity-associated promotion of malignant liver lesions in mice treated with diethylnitrosamine requires intact TNF and IL-6 signaling.64
Oncogenic pathways associated with lipotoxicity
When the capacity of expanding adipose tissue is exhausted, lipid molecules are stored in non-adipose tissues (e.g., liver, skeletal muscle, pancreas, and heart) and initiate lipotoxicity, defined as chronic cellular and tissue damage related to ectopic lipid accumulation.65–67 Liver lipotoxicity is advanced by de novo lipogenesis resulting from increased activation of steroid response element binding protein-1c (SREBP-1c) caused by elevated insulin levels.68,69 Removal of excess hepatic lipids in NAFLD may be hampered by insufficient mitochondrial β-oxidation (a common cause of microvesicular steatosis) and impaired export of very low-density lipoproteins.70,71 Moreover, lysosome-mediated degradation of lipid molecules in intracellular autophagosomes (lipophagy) is inhibited by excess fatty acids, representing a self-amplifying mechanism of lipotoxicity in hepatocytes.72
Experimental evidence indicates that lipotoxicity depends on the altered composition rather than the source or amount of ectopically deposited lipids.73 Thus, lipotoxicity has been attributed primarily to the harmful effect of free fatty acids and free cholesterol rather than triglycerides, which are considered relatively innocuous, and possibly actually protective.74,75 The panel of fatty acids implicated in lipotoxicity is broad, and tissue damage may depend on the length (short, medium, long, and very long), saturation (saturated, mono- and polyunsaturated), and isomerism (cis vs. trans) of acyl chains.76 A recent lipidomic analysis by thin-layer chromatography of liver tissue obtained from patients with NAFLD indicated that free cholesterol content and the ratio of n-6 to n-3 polyunsaturated fatty acids were significantly higher in NASH compared with IFL, and diminished levels of phosphatidylcholine and arachidonic acid allowed further distinction of NASH.73 Recent reports indicate that magnetic resonance-based methods may eventually provide similar qualitative information on hepatic lipid content, although this approach is still in the experimental phase.77,78 If fully developed, these non-invasive methods hold the promise of predicting disease severity in NAFLD.
Lipotoxicity results in various adverse conditions such as mitochondrial dysfunction, oxidative injury, endoplasmic reticulum stress, and insulin resistance.65,67 These mechanisms provide multiple links between lipotoxicity and hepatocarcinogenesis. For example, hepatocellular death induced by lipotoxicity (lipoapoptosis) correlates with the severity of NAFLD, and implicates the effects of liver macrophages activated by damaged and dying hepatocytes.79,80 Enhanced lipid peroxidation may activate macrophages by generating ligands (e.g., oxidized LDL) of scavenger receptors.81 Stimulation of the pivotal danger recognition receptor, toll-like receptor 4, by saturated fatty acids is another mechanism of macrophage activation that may contribute to the liver inflammatory response.82 Importantly, macrophage-mediated stimulation of surviving hepatocytes via NF-κB and other cell-proliferation pathways is a major component of hepatocarcinogenesis, which has been established in various experimental models.83,84
As a fundamental consequence of obesity-associated adipokine imbalance, chronic inflammation, and lipotoxicity, insulin resistance results from multiple defects of the insulin-mediated cellular signaling network, and leads to compensatory hyperinsulinemia.85,86 This process contains important self-amplification loops such as increased lipolysis from peripheral adipose depots due to uninhibited hormone-sensitive lipase, and increased hepatic de novo lipogenesis due to retained insulin responsiveness of SREBP-1c.68 Elevated insulin levels stimulate the production of insulin-like growth factor (IGF)-binding protein and increase bioavailability of IGF1 and IGF2, further promoting oncogenic pathways such as PI3K/Akt, mitogen-activated protein kinase, and vascular endothelial growth factor.87
Prevention of HCC associated with NAFLD
HCC reduction by weight management and physical exercise
Measures aimed at preventing NAFLD progression may diminish the risk of HCC associated with this condition. Controlled caloric intake and regular exercise is the mainstay of therapy, although the extent to which these lifestyle changes may reduce the chance of developing HCC in NAFLD remains unclear. As recently reported, development of malignant liver lesions in hepatocyte-specific PTEN-deficient mice fed for 32 weeks on a high-fat diet was significantly less in the group that had 60 minutes of exercise daily on a motorized treadmill compared with ‘sedentary’ controls (71% vs. 100%).88
The cancer-prevention effect of regular exercise is associated with physiological benefits, which include but are not limited to metabolic changes resulting from weight loss, such as decreased oxidative stress and improved adipokine balance.89 Although intentional weight loss in humans is difficult to achieve and keep, obesity can be reduced dramatically by bariatric surgery. Several large-scale studies indicate that cancer-prevention benefits can be expected from a weight loss of 10–30% sustained over 10 years.90,91 However, evidence for the effect of bariatric surgery on lowering the risk of HCC is limited.92
HCC risk reduction by insulin-sensitizing agents
Pharmacological therapy for the metabolic derangements associated with NAFLD such as insulin resistance and hyperlipidemia may provide additional opportunities to prevent hepatocarcinogenesis. There is evidence that insulin-sensitizing agents reduce the risk of HCC in NAFLD associated with manifest diabetes.93–95 Most of these data relate to the effect of metformin, although thiazolidinediones may carry similar benefit.95,96 By contrast, use of insulin and sulfonylureas has been associated with increased risk of HCC.93,95 Metformin has been reported to have tumor inhibitory properties in a variety of cancers, and this effect was confirmed in a xenograft model of human hepatoma cells.97
The anti-proliferative action of metformin is linked to activation of AMPK, a master regulator of cellular energy metabolism, including inhibition of de novo lipogenesis, which has additional molecular targets such as the mTOR pathway and the retinoblastoma protein.98 A recent meta-analysis encompassing 105,495 patients with diabetes found that the risk of primary liver cancer was reduced by 62% (pooled odds ratio 0.38; 95% CI 0.24–0.59; p < 0.001) in those who were regularly taking metformin.99 It remains to be seen whether metformin has a similar chemopreventive effect on the risk of HCC in patients with NAFLD who have insulin resistance but no manifest diabetes.
The use of thiazolidinediones to improve insulin resistance has been associated with a reduction in risk for HCC of 44–70% in patients with diabetes.95,96 There are conflicting data on the molecular mechanisms by which these agents exert tumor prevention. Peroxisome proliferator active receptor γ (PPARγ), a nuclear hormone receptor and the main target of thiazolidinediones, has been shown to inhibit tumor growth and metastasis formation in HCC, although some findings suggest that the effect of thiazolidinediones on HCC is PPARγ-independent.100 Specific molecular mechanisms of tumor inhibition by thiazolidinediones include pro-apoptotic effects via activation of p53 and PTEN, as well as inhibition of Bcl2 and the ERK pathway.101 Because most members of this class of anti-diabetic drugs have been implicated in major adverse events, the role of thiazolidinediones in NAFLD management and HCC prevention remains controversial.
Additional opportunities for chemoprevention of HCC in NAFLD
Statins are inhibitors of endogenous cholesterol synthesis that work by targeting 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase, and are widely used for primary and secondary prevention of cardiovascular diseases. Statins have been reported to decrease the risk of a number of cancer types through anti-proliferative, pro-apoptotic, anti-angiogenic, and immunomodulatory mechanisms.102 Specific molecular targets of statins include G proteins of the Ras/Rho superfamily, the RAF/MEK/ERK pathway, and the Myc oncoprotein.103,104 In a recent meta-analysis of 4,298 cases of HCC, statin users were less likely to develop HCC than non-users, with an adjusted odds ratio of 0.63 (95% CI, 0.52–0.76).102
Farnesoid X receptor (FXR) is a nuclear hormone receptor with high levels of expression in the liver, and has been implicated primarily in bile acid sensing.105 Similar to other nuclear hormone receptors, FXR regulates a large number of genes involved in lipid and glucose metabolism, liver regeneration, inflammation, and cancer.105–107 FXR-deficient mice display sustained activation of the Wnt/β-catenin pathway, and reduced expression of the tumor suppressor N-myc downstream-regulated gene 2 (NDRG2) with spontaneous development of HCC and other malignancies.108,109 FXR inhibits gankyrin, a proteasome factor that promotes degradation of multiple tumor suppressor proteins.110 Human studies indicate that FXR is downregulated in HCC, further supporting a protective role for FXR in tumor development.109 Overexpression of FXR in human hepatoma cells or stimulation by synthetic agonists such as GW4064 inhibited tumor growth in an orthotopic mouse xenograft model.108 These findings provide a basis for novel approaches in the prevention and treatment of HCC.
Conclusions
HCC is one of the most common malignancies in the world and has one of the worst survival rates of all the major cancers. HCC is among the few malignancies with an increasing incidence in the USA, a worrisome trend initially attributed to the aging population with chronic HCV, but increasingly linked to the rapidly spreading epidemic of obesity, diabetes, and NAFLD. Although cirrhosis provides the most conducive microenvironment for hepatocarcinogenesis, NAFLD-associated HCC may emerge in its absence, fittingly described as “a wolf in sheep's clothing.” Because NAFLD could be a game changer for liver cancer, it is imperative to develop reliable risk assessment and prevention tools, explore the pathogenesis in non-cirrhotic HCC, and identify new molecular targets for effective therapy.
Abbreviations
- AMPK
5′-adenosine monophosphate activated protein kinase
- ERK
extracellular signal-regulated kinases
- FXR
farnesoid X receptor
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFL
isolated fatty liver
- IGF
insulin-like growth factor
- IL
interleukin
- LDL
low-density lipoprotein
- MEK
mitogen-activated protein/extracellular signal-regulated kinase kinase
- mTOR
mammalian target of rapamycin
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-κB
nuclear factor-κB
- PI3K
phosphoinositide 3-kinase
- PPAR
peroxisome proliferator active receptor
- SREBP
steroid response element binding protein
- STAT
signal transducer and activator of transcription (STAT)
- PTEN
phosphatase and tensin homolog
- TNF
tumor necrosis factor
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S, Farrell GC, Hashimoto E, Saibara T, Lau GK, Sollano JD. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol. 2007;22:778–787. doi: 10.1111/j.1440-1746.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol. 2012;27:1555–1560. doi: 10.1111/j.1440-1746.2012.07222.x. [DOI] [PubMed] [Google Scholar]
- 8.Torres DM, Williams CD, Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:837–858. doi: 10.1016/j.cgh.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz Y, Kurt R, Yonal O, Polat N, Celikel CA, Gurdal A, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211:182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 11.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in nonalcoholic fatty liver disease: An emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 13.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: Molehill or mountain? Hepatology. 2008;47:1431–1433. doi: 10.1002/hep.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 18.Bellentani S, Tiribelli C. The spectrum of liver disease in the general population: lesson from the Dionysos study. J Hepatol. 2001;35:531–537. doi: 10.1016/s0168-8278(01)00151-9. [DOI] [PubMed] [Google Scholar]
- 19.Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 20.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 21.Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 2009;116:539–564. doi: 10.1042/CS20080253. [DOI] [PubMed] [Google Scholar]
- 22.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1–14. doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 25.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 26.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 28.N'Kontchou G, Paries J, Htar MT, Ganne-Carrie N, Costentin L, Grando-Lemaire V, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062–1068. doi: 10.1016/j.cgh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 30.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 31.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 32.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 34.Rahman RN, Ibdah JA. Nonalcoholic fatty liver disease without cirrhosis is an emergent and independent risk factor of hepatocellular carcinoma: A population based study. Hepatology. 2012;56:241A. [Google Scholar]
- 35.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 36.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 38.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 39.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 41.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 42.Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–1420. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 44.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 45.Wu SD, Ma YS, Fang Y, Liu LL, Fu D, Shen XZ. Role of the microenvironment in hepatocellular carcinoma development and progression. Cancer Treat Rev. 2012;38:218–225. doi: 10.1016/j.ctrv.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Kondo Y, Kanai Y, Sakamoto M, Mizokami M, Ueda R, Hirohashi S. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis–A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on 8 CpG islands in microdissected specimens from patients with hepatocellular carcinoma. Hepatology. 2000;32:970–979. doi: 10.1053/jhep.2000.19797. [DOI] [PubMed] [Google Scholar]
- 48.Breuhahn K, Schirmacher P. Signaling networks in human hepatocarcinogenesis–novel aspects and therapeutic options. Prog Mol Biol Transl Sci. 2010;97:251–277. doi: 10.1016/B978-0-12-385233-5.00009-X. [DOI] [PubMed] [Google Scholar]
- 49.Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 50.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular classification and novel targets in hepatocellular carcinoma: recent advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 52.Zhu AX. Molecularly targeted therapy for advanced hepatocellular carcinoma in 2012: current status and future perspectives. Semin Oncol. 2012;39:493–502. doi: 10.1053/j.seminoncol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 53.Chen X, Ding G, Gao Q, Sun J, Zhang Q, Du L, et al. A human anti-c-Met Fab fragment conjugated with doxorubicin as targeted chemotherapy for hepatocellular carcinoma. PLoS One. 2013;8:e63093. doi: 10.1371/journal.pone.0063093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abu Dayyeh BK, Yang M, Fuchs BC, Karl DL, Yamada S, Sninsky JJ, et al. A functional polymorphism in the epidermal growth factor gene is associated with risk for hepatocellular carcinoma. Gastroenterology. 2011;141:141–149. doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 59.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 60.Van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 61.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 62.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl):28–32. doi: 10.1038/sj.ijo.0801498. [DOI] [PubMed] [Google Scholar]
- 66.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008;36:935–940. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 67.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57–69. doi: 10.1055/s-2001-12929. [DOI] [PubMed] [Google Scholar]
- 71.Fujita K, Nozaki Y, Wada K, Yoneda M, Fujimoto Y, Fujitake M, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 72.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 74.Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogenesis. Part 1: The metabolic movers. J Gastroenterol Hepatol. 2010;25:672–690. doi: 10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- 75.Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- 76.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson NA, Walton DW, Sachinwalla T, Thompson CH, Smith K, Ruell PA, et al. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513–1523. doi: 10.1002/hep.22220. [DOI] [PubMed] [Google Scholar]
- 78.Van Werven JR, Schreuder TC, Nederveen AJ, Lavini C, Jansen PL, Stoker J. Hepatic unsaturated fatty acids in patients with non-alcoholic fatty liver disease assessed by 3.0T MR spectroscopy. Eur J Radiol. 2010;75:e102–e107. doi: 10.1016/j.ejrad.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 79.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 80.Cazanave SC, Gores GJ. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5:71–85. doi: 10.2217/clp.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terpstra V, van Amersfoort ES, van Velzen AG, Kuiper J, van Berkel TJ. Hepatic and extrahepatic scavenger receptors: function in relation to disease. Arterioscler Thromb Vasc Biol. 2000;20:1860–1872. doi: 10.1161/01.atv.20.8.1860. [DOI] [PubMed] [Google Scholar]
- 82.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 86.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 87.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 88.Piguet AC. Amsterdam: International Liver Congress; 2013. Effect of regular training on hepatocellular carcinoma development in hepatocyte-specific PTEN-deficient mice. [Google Scholar]
- 89.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 91.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial: a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 92.Burza MA. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig Liver Dis. 2012;44:1037–1041. doi: 10.1016/j.dld.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 93.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 94.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–2608. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 95.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lai S-W, Chen P-C, Liao K-F, Muo C-H, Lin C-C, Sung F-C. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: A population-based cohort study. Am J Gastroenterol. 2011;107:46–52. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 97.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 98.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544–552. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 100.Wu CW, Farrell GC, Yu J. Functional role of peroxisome-proliferator-activated receptor gamma in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1665–1669. doi: 10.1111/j.1440-1746.2012.07213.x. [DOI] [PubMed] [Google Scholar]
- 101.Okumura T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J Gastroenterol. 2010;45:1097–1102. doi: 10.1007/s00535-010-0310-9. [DOI] [PubMed] [Google Scholar]
- 102.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Cao Z, Fan-Minogue H, Bellovin DI, Yevtodiyenko A, Arzeno J, Yang Q, et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 2011;71:2286–2297. doi: 10.1158/0008-5472.CAN-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 105.Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 106.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 107.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 108.Deuschle U, Schuler J, Schulz A, Schluter T, Kinzel O, Abel U, et al. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS One. 2012;7:e43044. doi: 10.1371/journal.pone.0043044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338:12–21. doi: 10.1124/jpet.111.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jiang Y, Iakova P, Jin J, Sullivan E, Sharin V, Hong IH, et al. Farnesoid X receptor inhibits gankyrin in mouse livers and prevents development of liver cancer. Hepatology. 2013;57:1098–1106. doi: 10.1002/hep.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209–219. [PMC free article] [PubMed] [Google Scholar]
- 112.Jepsen P, Ott P, Andersen PK, Sorensen HT, Vilstrup H. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis: a Danish nationwide cohort study. Ann Intern Med. 2012;156:841–847. doi: 10.7326/0003-4819-156-12-201206190-00004. [DOI] [PubMed] [Google Scholar]
- 113.Fung J, Lai CL, But D, Wong D, Cheung TK, Yuen MF. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103:1421–1426. doi: 10.1111/j.1572-0241.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 114.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43:S173–181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 115.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 116.Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hurter D, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]