Abstract
Autoimmune hepatitis (AIH) is an important disorder that predominantly results in inflammatory liver disease in genetically predisposed women. The clinicopathological picture is characterized by symptoms associated with both systemic inflammation and hepatic dysfunction, and with increased serum aminotransferases, elevated IgG, autoantibodies, and interface hepatitis on liver biopsy. AIH usually results in liver injury as a consequence of chronic hepatitis and cirrhosis. However, rarely, patients may present with fulminant liver failure. Early diagnosis is important in all instances because the disease can be highly responsive to immunosuppressive therapeutic options. Left untreated, the disease is associated with high morbidity and mortality. Here we provide an overview of the current state of knowledge on AIH and summarize the treatment options for this serious condition in adults. We also discuss the pathogenesis of the disease as a possible consequence of autoimmunity and the breakdown of hepatic tolerance. We focus on regulatory T cell impairments as a consequence of changes in CD39 ectonucleotidase expression and altered purinergic signaling. Further understanding of hepatic tolerance may aid in the development of specific and well-tolerated therapies for AIH.
Keywords: Autoimmune hepatitis, Pathogenesis, Immunology, Purinergic, CD39, Therapeutic overview
Introduction
In 1950, Jan Waldenstrom described the first case of autoimmune hepatitis (AIH) in a woman with hepatic dysfunction and hypergammaglobulinemia.1,2 Since then, AIH has become a well-established clinical entity, albeit uncommon, with an estimated annual incidence of 1.9 per 100,000, and a prevalence of 10–20/100,000.3 Early diagnosis is important, as AIH usually responds to immunosuppressive treatment. However, if left untreated, AIH can progress to liver failure, cirrhosis, and death.
In this updated review, we explore the pathogenesis of AIH, consider the immunological basis for the pathogenesis of liver-directed immune injury, and present new concepts in the understanding of immune tolerance that seem to be perturbed in AIH. We also comment on various developments in innovative treatment modalities.
Clinical presentation
AIH may present with a variety of clinical manifestations, ranging from asymptomatic disease to fulminant liver failure. Although up to 25% of patients may be asymptomatic at diagnosis,4 the condition most commonly presents in an insidious manner with non-specific complaints in young or middle-aged women.5 Approximately 30% of patients may have evidence of advanced liver disease and cirrhosis at the time of diagnosis.6 Extrahepatic manifestations of AIH may include inflammatory bowel disease, thyroiditis, type-1 diabetes mellitus, and celiac disease.7,8
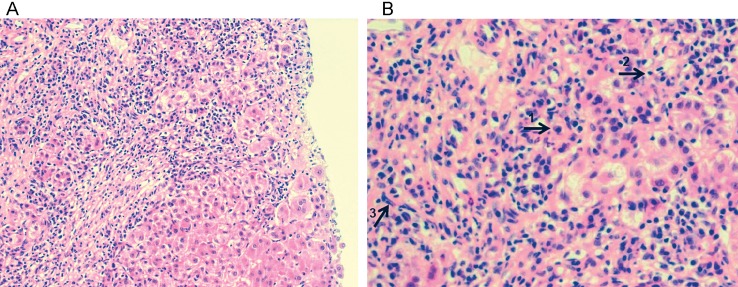
There are no pathognomonic features of AIH. Therefore, the diagnosis depends on a set of clinicopathological, histological, biochemical, and immunological criteria. Interface hepatitis is the histological hallmark of AIH (see Fig. 1) and is present in 84–98% of cases.8 Biopsy findings of cirrhosis and/or bridging necrosis carry a poorer prognosis than those lacking these features.5,8,9
Fig. 1. Histological picture of AIH. (A) Portal and peri-portal inflammatory infiltrate, characteristic of autoimmune hepatitis, consists of (B) plasma cells, lymphocytes and monocytes/macrophages, as phenotypically indicated by arrows labeled 1, 2, and 3, respectively. There is evidence of interface hepatitis and piecemeal necrosis of hepatocytes. Hematoxylin and eosin staining, magnification ×200 (A) and x400 (B). (Credit: Dr Alberto Quaglia).
Multiple biochemical derangements can be found in AIH. Most commonly, elevated aminotransferases with or without elevated bilirubin and alkaline phosphatase are frequently seen. Serum immunoglobulins, notably IgG, are elevated in approximately 85% of cases.8
Several scoring systems are available to aid in making an objective diagnosis and prognostication of AIH. In 1992, the International Autoimmune Hepatitis Group (IAIHG) published the first scoring system for AIH, with a revision released in 1999.10 A simplified scoring system was published in 2008, which was subsequently vetted and shown to have high specificity.11,12
Serologic markers of disease
Autoantibodies are important in making the diagnosis of AIH, and aside from confirming the diagnosis, serologic markers may assist in subtyping the disease and determining the prognosis.11
Diagnostic markers
Conventional markers for AIH include anti-smooth muscle antibody (SMA) and anti-nuclear antibody (ANA), both of which characterize the classic type 1 AIH. SMA positivity appears to be more specific for AIH than ANA, which is associated with multiple sero-reactants to centromere, histones, double-stranded DNA, chromatin, and ribonucleoprotein complex components, and thus has yet to yield a specific antigenic target.13–17 The specific antigen of SMA is also not clear, although multiple studies suggest that these autoantibodies react with actin components.18,19
It has also been reported that anti-nuclear and other autoantibodies are frequently noted in patients with non-alcoholic steatohepatitis. These observations may represent either nonspecific antibody responses associated with liver injury, or an autoimmune diathesis that may be linked pathogenetically to chronic inflammation, as in steatohepatitis.20–22
Antibodies to liver-kidney microsome type 1 (anti-LKM1) and liver cytosol type 1 (anti-LC1) characterize type 2 AIH. This subtype was described in the 1980s after the discovery of antibodies to liver-kidney microsomes.23–25 Typically, patients are young women with more severe disease. The antigenic target of anti-LKM1 was identified as the cytochrome P450 liver enzyme CYP2D6.26,27
Adjunctive markers
Anti-soluble liver antigen (SLA) and liver pancreas (LP) reactivity are specific to and positive in 58% of adult patients with type 1 AIH.1,28,29 The antigens for SLA/LP include ribonucleoprotein complex and O-phosphoseryl-tRNA:selenocysteinyl-tRNA synthase (SepSecS).30 Anti-SLA is often associated with severe disease.31
Liver cytosol antibody (LC1) was described in the 1980s,32 and is useful as an adjunct in the diagnosis of AIH type 2. The LC1 antigen is the liver-expressed enzyme formiminotransferase cyclodeaminase (FTCD), and appears to be associated with early onset of disease, concurrent autoimmunity, and rapid progression to cirrhosis.31 LC-1 titers fluctuate with disease activity.33,34 Rarely, anti-neutrophil cytoplasmic antibodies (ANCA) are associated with primary sclerosing cholangitis (PSC) overlap syndrome, and high rates of cirrhosis.35–38
Pathogenesis
The following pathogenetic model has been proposed: in a genetically predisposed host, defined environmental agent(s) catalyze(s) and trigger a series of T cell-mediated immune events directed at hepatic cellular antigens, resulting in unfettered inflammation, which ultimately culminates in fibrotic transformation of the liver, aberrant regeneration, and cirrhosis.5,29,39
Genetic predisposition
The genetic predisposition to AIH has been attributed, at least in part, to specific allelic variations in the major histocompatibility complex (MHC), located on chromosome 6 in the human leukocyte antigen (HLA) region.31,40 Among Caucasian populations, associations between AIH type 1 and DRB1 alleles (DRB1*0301, DRB1*0401), as well as between AIH type 2 and allele DRB1*0701 have been described. Patients with DRB1*0301 tend to be younger, more likely to require liver transplant, and experience higher rates of acute liver failure and steroid treatment failure.34,41 HLA allelic associations vary globally.37
Genetic risk factors outside of the MHC include polymorphisms of the gene for cytotoxic T lymphocyte antigen 4 (CTLA-4) in white North American and European populations. The CTLA-4 molecule interaction with antigen-presenting cells has been shown to mitigate T cell activation.
Further, a polymorphism of the gene encoding for tumor necrosis factor alpha (TNFα2), which is involved in the up-regulation of type 1 cytokines, is associated with more severe AIH in young white patients whose disease may be steroid-resistant.42,43
Molecular mimicry
Discovery and understanding of the target antigens for the autoantibodies in AIH may be important for developing specific treatments and understanding the mechanisms of the disease. Molecular mimicry describes natural genetic homologies between autoantigens and common viral genomes (hepatitis C virus, herpes simplex virus 1, cytomegalovirus (CMV)) that spawn autoantibodies.33,44 This genetic interplay is feasible because of incomplete specificity at CD4+ T-cell antigen receptors.33
Cellular immunoregulation
Immune system homeostasis is accomplished through regulation of effector CD8+ and CD4+ T cells by CD4+CD25+CD39+FOXP3+ regulatory T cells (Tregs).45,46 A major contributor to AIH pathogenesis is the failure of immunoregulation as a result of diminished function and sheer number of Tregs, with consequent massive recruitment of inflammatory effector cells, which inflict hepatic injury.47,48
Tregs express unique markers including the interleukin-2Rα (IL-2Rα) chain (CD25), the glucocorticoid induced tumor necrosis factor receptor (GITR), CD62L, CTLA-4, and forkhead/winged helix transcription factor (FOXP3) as well as CD39, an ectonucleotidase responsible for extracellular nucleotide phosphohydrolysis, culminating in the production of immunosuppressive adenosine and regulated purinergic signaling.39,47,49–53
There is fairly recent evidence indicating that adenosine modulates effector cells by up-regulating inhibitory molecules (i.e. CTLA-4 and programmed-cell-death-protein-1 (PD1)), by decreasing IL-2 production and proliferation, and by inhibiting differentiation of effector cells into T helper 1 (Th1) and Th17 cell lineages.54 The immunomodulating effects of adenosine are mediated by the binding of the nucleoside to A2A adenosine receptors on effector T cells. There are multiple putative Treg defects in common diseases that might contribute to a model of impaired immunoregulation in AIH and other immunological illnesses.55 In very recent work, we and our colleagues showed that the CD39-expressing Tregs are decreased and lack the functional capacity to efficiently limit production of IL17, a pro-inflammatory cytokine elevated in the serum of patients with AIH.56,57 The mechanism by which CD39+ Tregs limit IL17 production is unclear, although it has been previously suggested that CD39 can decrease IL17 levels by removal of ATP.58
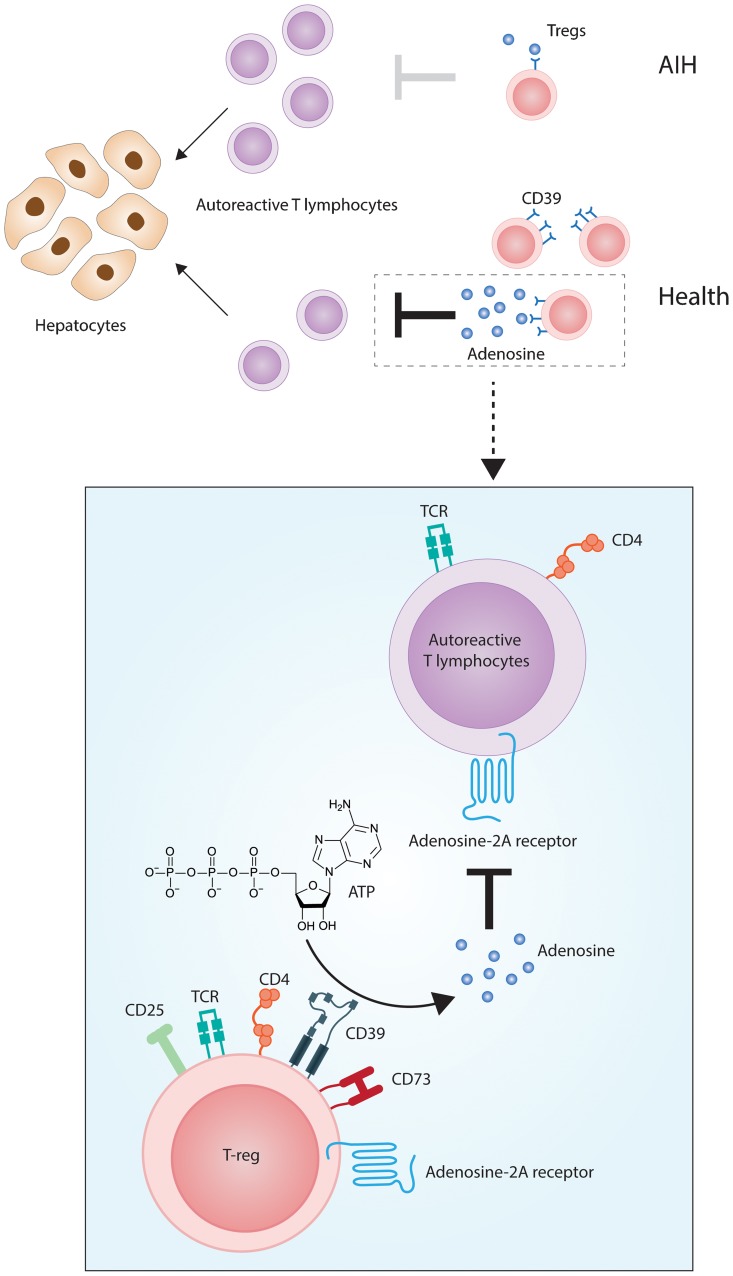
Tregs in AIH exhibit slow rates of phosphohydrolysis of pro-inflammatory nucleotides compared to matched control cells. It has been proposed that defective immunoregulation in AIH is associated not only with decreased Treg number and functions, but also increased conversion of Tregs into effectors as a result of predominance of pro-inflammatory cytokines in the environment. The reasons for CD39 down-regulation or loss from Tregs in AIH are unknown, although reduced levels of transforming growth factor-β (TGF-β), an inhibitory cytokine that promotes CD39 upregulation on human leukocytes,59 may account for this observation. Such a model of Treg CD39 dysregulation leading to autoimmune attack is depicted in Fig. 2. Such pathways may also be implicated in other autoimmune disorders of the gastrointestinal tract, such as inflammatory bowel disease.60
Fig. 2. Pathogenesis of liver attack in AIH: the role of CD39.
In health, immunotolerance to liver autoantigens is maintained by effective control of CD4+CD25+FOXP3+Tregs over CD4+ and CD8+ autoreactive T lymphocytes. The machinery enabling Tregs to modulate effector immune responses relies on the expression of CD39, an ectonucleotidase ultimately leading to the generation of immunomodulatory adenosine. In AIH, Tregs are numerically defective and express low levels of CD39. This results in poor generation of adenosine and ineffective control over autoreactive lymphocytes, with consequent perpetuation of hepatocyte damage. Details of Treg adenosinergic suppression are depicted in the box. Adenosine is generated from ATP through the action of CD39 and CD73 ectonucleotidases in tandem, expressed by Tregs. Adenosine mediates immunomodulation by binding to A2A adenosine receptors on autoreactive T lymphocytes.
Treatment-induced remission of AIH is associated with restoration of Treg function.61 Some groups have looked at expansion of Treg populations or diminishing effects of cytokine IL-17 as possible therapeutic interventions.56,57,62
Natural killer T (NKT) cells may also be involved in the pathogenesis of AIH. NKT cells are found in the vascular sinusoids, potentially providing an immunological bridge between innate and adaptive immune responses in immune liver reactions.
The purinergic receptor P2X7 recognizes extracellular ATP, and is crucial in regulating the function of NKT cells.63 It has been proposed that the P2X7 receptor constitutes a sensor that can modulate NKT cell functions, which would also be affected by the ectonucleotidase CD39 expressed by these cells.64 Curiously, genetic deletion in CD39, which limits Treg functions exacerbating adaptive immune responses in transplant rejection models,65 also results in increased rates of stimulated NKT cell apoptosis in mouse models of AIH.66 Hence, concanavalin-A hepatitis induction in Cd39 null mice results in enhanced levels of NKT cell loss and paradoxical protection from liver injury. This unexpected experimental finding illustrates the complexity of purinergic signaling in influencing diverse immune cell types (Treg vs. NKT cells) and in dictating opposing outcomes in the immune liver injury.
Treatment
Prednisone/azathioprine
The standard treatment for AIH comprises corticosteroid, and prednisone at an initial dose of 40–60 mg daily followed by combinations of prednisone in tapering doses to the lowest levels required to maintain remission with the anti-metabolite immune suppressant azathioprine (AZA) added to the therapeutic regimen. IN the USA, the daily dose of AZA is generally 50 mg, whereas in Europe, a higher dose of 1–2 mg/kg is usually preferred.65 Several clinical trials in the 1970's demonstrated the safety and efficacy of this regimen, with remission rates of 65–80%.5,67 However, many of the American Association for the Study of Liver Diseases (AASLD) and British Society of Gastroenterology consensus guidelines for the standardized management of AIH are based on suboptimal studies, and there remain clear uncertainties as to the management of refractory or resistant cases.68–71
Prolonged therapy may potentially result in a variety of adverse events that may lead to non-compliance and early cessation of therapy in a minority of patients.
Approximately 13% of patients discontinue conventional therapy because of intolerable prednisone-related side effects, with nearly half of these patients discontinuing therapy because of intolerable cosmetic issues. Similarly, long-term AZA use is associated with a constellation of potential side effects. Approximately 5% of patients treated with AZA cannot tolerate the side effects, and require early discontinuation of therapy. The most notable side effect is pancytopenia. Risk factors for this complication include malnutrition, cirrhosis, and absent (1:300) or low-level expression (10%) of thiopurine methyltransferase (TMPT).72,73 It is increasingly accepted that TPMT testing should be performed prior to starting thiopurine drugs.
Other potential side effects of AZA include pancreatitis, cholestatic liver injury, vascular sinusoidal injury with nodular regenerative hyperplasia, and development of opportunistic infection.74
In 2010, the updated guidelines for the management of AIH redefined remission as sustained normalization of liver enzymes,9,69,75–77 including a goal of normalized IgG/gammaglobulins.9 Histological remission lags behind biochemical remission by 3–8 months. Although remission is achieved in the majority of patients with conventional management, 50% of patients relapse within 6 months of cessation of immunosuppressive therapy, and nearly 70% within 3 years.71 Achieving histological remission reduces the frequency of relapse to approximately 28%, while evidence of even mild portal hepatitis or inflammation increases the frequency to greater than 50%.78
Budesonide
Budesonide is a synthetic, orally administered corticosteroid with a rate of hepatic first-pass metabolism of 80–90%.79 The drug is metabolized in the liver to by-products that have negligible glucocorticoid activity, but a marked affinity for glucocorticoid receptors. In 1994, Danielsson and Prytz studied the use of budesonide with or without AZA80 (see Table 1). Since then multiple small studies81–83 have preceded the first prospective, clinical trial studying the use of budesonide in AIH by Manns et al. in 2010.84
Table 1. Review of major literature on the use of budesonide in AIH.
| Author | Year | No. of Patients | Budesonide Dose | Study End Points | Results |
| Danielsson | 1994 | 13 | 6–8 mg daily | Decrease in ALT, AST, IgG | Complete response: 100% |
| Zandieh | 2008 | 9 | 3 mg every other day to 9 mg daily | Complete response: Normal ALT, AST | Complete response: 78% No response: 22% |
| Wiegand | 2005 | 12 | Day 1: 6 mg daily Day 2: 9 mg daily Upon remission: 6 mg daily | Complete remission: AST and ALT drop ≤ two times the upper limit of normal Partial response: ALT or AST ≤ two times the upper limit of normal or AST/ALT improvement > 80% from baseline | Complete response: 58% Partial response: 25% |
| Csepregi | 2006 | 11 | 9 mg daily | Remission: Absence of symptoms Normal ALT, ALP, IgG | Treatment-naïve: 57% Treatment-experienced: 100% |
| Manns | 2010 | 203 (100 received Budesonide and 103 received prednisone) | 6 mg daily or 9 mg daily | Complete response: Normal ALT Normal AST Absence of steroid-related side effects | Budesonide group: 47% Prednisone group: 18.4% |
| Czaja | 2000 | 10 | 9 mg daily | Remission: Asymptomatic Normal or near normal AST Normal bilirubin Normal γ-globulin Failure: Clinical/biochemical deterioration | Remission: 30%Treatment failure: 40% |
There is increasing evidence supporting budesonide as an alternative to prednisone. As demonstrated in published accounts, budesonide appears to be similar in efficacy to but more tolerable than prednisone. Although the Mayo Clinic series did not find any benefit with this alternative use of budesonide, other studies have demonstrated its efficacy and favorable side effect profile.85
Because the drug is metabolized almost exclusively in the liver, it may be intolerable to patients with cirrhosis. Additionally, patients exposed to prednisone may experience significant corticoid-related side effects when transitioning to budesonide.85 Further follow-up regarding sustained remission with budesonide is required.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is the prodrug of mycophenolic acid, which is a potent, irreversible inhibitor of inosine monophosphate dehydrogenase, and has prominent cytostatic effects on lymphocytes. Richardson and colleagues published the first case series investigating usage of MMF in patients resistant to or intolerant of AZA.86 Multiple subsequent case series and retrospective reviews noted achievement of biochemical remission in the majority of refractory cases of AIH, with a significant steroid-sparing effect.87–91 However, Czaja and Carpenter studied the use of MMF in eight patients, and found that none of the patients who previously failed conventional therapy responded to MMF as salvage therapy.92
In 2010, another group studied the role of MMF in treatment-naïve patients. At the end of the study there were no non-responders, while 59.3% of patients achieved a complete response, and 28.8% of patients had a complete response initially, followed by a relapse.93
MMF appears to be an effective agent in treatment-naïve disease. However, there is no clear consensus on the use of MMF as a second line agent in those patients who fail conventional management. It appears that MMF has a role to play in patients who were previously intolerant to conventional therapy. The role of MMF as a definitive second line salvage agent requires further studies in the form of prospective controlled trials. The most common adverse reactions with MMF are gastrointestinal side effects, and significant thrombocytopenia, leukopenia, and rarely CMV infection. Considering that the treatment duration is often measured in years, the adverse effects associated with prolonged use of MMF in this population are yet to be determined. Additionally, judicious use of MMF must be employed given its greater cost compared to conventional treatment.89
Cyclosporin
Cyclosporin is a calcineurin inhibitor that acts by binding to cyclophilin, thus creating a complex.94 Mistilis and colleagues first reported the use of cyclosporin in the management of AIH in 1985.95 Since then, several isolated case reports and case series have described the successful use of cyclosporin in adults intolerant or non-responsive to conventional management.96–100 Malekzadeh and colleagues in 2001 published the largest case series reporting the use of cyclosporin as an alternative to steroid-based treatment in AIH.101 Although cyclosporin appears to have a promising role in the treatment of AIH, the potential long-term adverse effects and nephrotoxicity of the drug have yet to be studied in this particular patient population.
Tacrolimus
Tacrolimus is a macrolide with a similar mechanism of action to cyclosporin but with greater immunosuppressive potency. Tacrolimus binds to the FK506 binding protein, thus inhibiting phosphatase activity, which is required for cytokine gene transcription and T-cell activation. The net result of tacrolimus activity is inhibition of both T and B cells.102
Treatment of AIH with tacrolimus was first proposed in 1995.103 In an open label study, 15 of 21 patients demonstrated biochemical improvement. Multiple follow-up single-center studies on patients who were either steroid-refractory or steroid-intolerant demonstrated similar results.104,105 A review of the literature suggests that tacrolimus has a role to play in the management of patients intolerant and/or refractory to conventional therapy, and may be effective as a second line therapy in AIH. Adverse effects include nephrotoxicity, hypertension, bone marrow toxicity, diabetes, neurotoxicity, and opportunistic infections.106,107
Alternative therapies
Several isolated case reports have investigated other agents as potential alternatives to conventional management, including ursodeoxycholic acid, infliximab, etanercept, methotrexate, rapamycin and rituximab; however, rigorous supportive data is lacking.
Transplantation
Orthotopic liver transplantation (OLT) is reserved for those patients who have failed medical therapy or who present with acute fulminant hepatitis that is too advanced for medical management.
AIH is the indication for OLT in approximately 4–6% of adult transplants occurring in the USA and Europe.108 Outcomes are excellent in patients who undergo OLT for treatment of AIH, with 5-year and 10-year survival rates close to 75%. Recent studies indicate that the rate of recurrence of AIH post-OLT is approximately 23%.109 In these patients, modification of the immunosuppressive regimen and close follow-up is mandatory.9
Conclusions
AIH remains clinically challenging despite decades of awareness of this complex disease. Although standard therapy with prednisone and AZA frequently results in an excellent treatment response, the need for novel and steroid-sparing treatments remains. The goal is to optimize care for the wide spectrum of patients afflicted with this condition. Further exploration of the underlying immunologic processes in AIH, particularly those which, at least in part, involve purinergic signaling, should be undertaken. This will lead to a deeper understanding of how the usual mechanisms of hepatic tolerance are rendered incompetent in this dangerous and yet fascinating liver disease.
Acknowledgments
We thank Dr Alberto Quaglia for kindly providing the histological image used in this publication.
Abbreviations
- AIH
autoimmune hepatitis
- ANA
anti-nuclear antibody
- ANCA
anti-neutrophil cytoplasmic antibodies
- anti-LC1
antibodies to liver cytosol type 1
- anti-LKM1
antibodies to liver-kidney microsome type 1
- AZA
azathioprine
- FTCD
formiminotransferase cyclodeaminase
- HLA
human leukocyte antigen
- LC1
liver cytosol antibody
- LP
liver pancreas
- MHC
major Histocompatibility Complex
- MMF
mycophenolate mofetil
- NKT
natural killer T
- OLT
orthotopic liver transplantation
- SepSecS
O-phosphoseryl-tRNA: selenocysteinyl-tRNA synthase
- SLA
soluble liver antigen
- SMA
smooth muscle antibody
- TMPT
thiopurine methyltransferase
- Treg
regulatory T cell
References
- 1.Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet. 2013 doi: 10.1016/S0140-6736(12)62163-1. [DOI] [PubMed] [Google Scholar]
- 2.WJ L. Blutproteine und Nahrungseiweib. Dtsch Z Verdau Stoffwechselkr. 1950;15:113–119.el. [Google Scholar]
- 3.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 4.Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53–62. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 5.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 6.Werner M, Prytz H, Ohlsson B, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol. 2008;43:1232–1240. doi: 10.1080/00365520802130183. [DOI] [PubMed] [Google Scholar]
- 7.Kaukinen K, Halme L, Collin P, et al. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881–888. doi: 10.1053/gast.2002.32416. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 9.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 11.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 12.Miyake Y, Iwasaki Y, Kobashi H, et al. Clinical features of autoimmune hepatitis diagnosed based on simplified criteria of the International Autoimmune Hepatitis Group. Dig Liver Dis. 2010;42:210–215. doi: 10.1016/j.dld.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Hargraves MM, Richmond H, Morton R. Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proc Staff Meet Mayo Clin. 1948;23:25–28. [PubMed] [Google Scholar]
- 14.Mackay IR, Weiden S, Hasker J. Autoimmune hepatitis. Ann N Y Acad Sci. 1965;124:767–780. doi: 10.1111/j.1749-6632.1965.tb19000.x. [DOI] [PubMed] [Google Scholar]
- 15.Cowling DC, Mackay IR, Taft LI. Lupoid hepatitis. Lancet. 1956;271:1323–1326. doi: 10.1016/s0140-6736(56)91483-0. [DOI] [PubMed] [Google Scholar]
- 16.Parveen S, Morshed SA, Arima K, et al. Antibodies to Ro/La, Cenp-B, and snRNPs antigens in autoimmune hepatitis of North America versus Asia: patterns of immunofluorescence, ELISA reactivities, and HLA association. Dig Dis Sci. 1998;43:1322–1331. doi: 10.1023/a:1018880429469. [DOI] [PubMed] [Google Scholar]
- 17.Bottazzo GF, Florin-Christensen A, Fairfax A, Swana G, Doniach D, Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976;29:403–410. doi: 10.1136/jcp.29.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabbiani G, Ryan GB, Lamelin JP, et al. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to “nonmuscular” cells. Am J Pathol. 1973;72:473–488. [PMC free article] [PubMed] [Google Scholar]
- 19.Villalta D, Bizzaro N, Da Re M, Tozzoli R, Komorowski L, Tonutti E. Diagnostic accuracy of four different immunological methods for the detection of anti-F-actin autoantibodies in type 1 autoimmune hepatitis and other liver-related disorders. Autoimmunity. 2008;41:105–110. doi: 10.1080/08916930701619896. [DOI] [PubMed] [Google Scholar]
- 20.Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. J Clin Gastroenterol. 2004;38:801–804. doi: 10.1097/01.mcg.0000139072.38580.a0. [DOI] [PubMed] [Google Scholar]
- 21.Bleibel W, Thukral C, Robson SC. Overlap between systemic lupus erythematosus and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40:561–562. doi: 10.1097/00004836-200607000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Tsuneyama K, Baba H, Kikuchi K, et al. Autoimmune features in metabolic liver disease: a single-center experience and review of the literature. Clin Rev Allergy Immunol. 2013;45:143–148. doi: 10.1007/s12016-013-8383-x. [DOI] [PubMed] [Google Scholar]
- 23.Homberg JC. Autoimmunity induced by drugs. Immunological characteristics and etiopathogenic hypotheses. Presse Med. 1984;13:2755–2760. [PubMed] [Google Scholar]
- 24.Homberg JC, Andre C, Abuaf N. A new anti-liver-kidney microsome antibody (anti-LKM2) in tienilic acid-induced hepatitis. Clin Exp Immunol. 1984;55:561–570. [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzetto M, Doniach D. Types of ‘reticulin’ antibodies detected in human sera by immunofluorescence. J Clin Pathol. 1973;26:841–851. doi: 10.1136/jcp.26.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueguen M, Meunier-Rotival M, Bernard O, Alvarez F. Anti-liver kidney microsome antibody recognizes a cytochrome P450 from the IID subfamily. J Exp Med. 1988;168:801–806. doi: 10.1084/jem.168.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest. 1989;83:1066–1072. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mix H, Weiler-Normann C, Thimme R, et al. Identification of CD4 T-cell epitopes in soluble liver antigen/liver pancreas autoantigen in autoimmune hepatitis. Gastroenterology. 2008;135:2107–2118. doi: 10.1053/j.gastro.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Okamoto M, Thomas MG, et al. Antibodies to conformational epitopes of soluble liver antigen define a severe form of autoimmune liver disease. Hepatology. 2002;35:658–664. doi: 10.1053/jhep.2002.32092. [DOI] [PubMed] [Google Scholar]
- 30.Palioura S, Herkel J, Simonovic M, Lohse AW, Soll D. Human SepSecS or SLA/LP: selenocysteine formation and autoimmune hepatitis. Biol Chem. 2010;391:771–776. doi: 10.1515/BC.2010.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fallatah HI, Akbar HO. Autoimmune hepatitis as a unique form of an autoimmune liver disease: immunological aspects and clinical overview. Autoimmune diseases. 2012;2012:312817. doi: 10.1155/2012/312817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martini E, Abuaf N, Cavalli F, Durand V, Johanet C, Homberg JC. Antibody to liver cytosol (anti-LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology. 1988;8:1662–1666. doi: 10.1002/hep.1840080632. [DOI] [PubMed] [Google Scholar]
- 33.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72 e54. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 34.Bogdanos DP, Mieli-Vergani G, Vergani D. Autoantibodies and their antigens in autoimmune hepatitis. Semin Liver Dis. 2009;29:241–253. doi: 10.1055/s-0029-1233533. [DOI] [PubMed] [Google Scholar]
- 35.Terjung B, Bogsch F, Klein R, et al. Diagnostic accuracy of atypical p-ANCA in autoimmune hepatitis using ROC- and multivariate regression analysis. Eur J Med Res. 2004;9:439–448. [PubMed] [Google Scholar]
- 36.Terjung B, Spengler U, Sauerbruch T, Worman HJ. “Atypical p-ANCA” in IBD and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310–322. doi: 10.1053/gast.2000.9366. [DOI] [PubMed] [Google Scholar]
- 37.Yokosawa S, Yoshizawa K, Ota M, et al. A genomewide DNA microsatellite association study of Japanese patients with autoimmune hepatitis type 1. Hepatology. 2007;45:384–390. doi: 10.1002/hep.21518. [DOI] [PubMed] [Google Scholar]
- 38.Roozendaal C, de Jong MA, van den Berg AP, van Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J Hepatol. 2000;32:734–741. doi: 10.1016/s0168-8278(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 39.Longhi MS, Robson SC, Bernstein SH, Serra S, Deaglio S. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med. 2013;91:165–172. doi: 10.1007/s00109-012-0991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vergani D, Mieli-Vergani G. Aetiopathogenesis of autoimmune hepatitis. World J Gastroenterol. 2008;14:3306–3312. doi: 10.3748/wjg.14.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Genetic predispositions for the immunological features of chronic active hepatitis. Hepatology. 1993;18:816–822. doi: 10.1002/hep.1840180411. [DOI] [PubMed] [Google Scholar]
- 42.Czaja AJ, Cookson S, Constantini PK, Clare M, Underhill JA, Donaldson PT. Cytokine polymorphisms associated with clinical features and treatment outcome in type 1 autoimmune hepatitis. Gastroenterology. 1999;117:645–652. doi: 10.1016/s0016-5085(99)70458-0. [DOI] [PubMed] [Google Scholar]
- 43.Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology. 2002;35:126–131. doi: 10.1053/jhep.2002.30084. [DOI] [PubMed] [Google Scholar]
- 44.Manns MP, Griffin KJ, Sullivan KF, Johnson EF. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J Clin Invest. 1991;88:1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011;23:282–292. doi: 10.1016/j.smim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 48.Longhi MS, Hussain MJ, Bogdanos DP, et al. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 49.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaughn BP, Robson SC, Burnstock G. Pathological roles of purinergic signaling in the liver. J Hepatol. 2012;57:916–920. doi: 10.1016/j.jhep.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2013;368:1260. doi: 10.1056/NEJMc1300259. [DOI] [PubMed] [Google Scholar]
- 53.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liberal R, Longhi MS, Mieli-Vergani G, Vergani D. Pathogenesis of autoimmune hepatitis. Best Pract Res Clin Gastroenterol. 2011;25:653–664. doi: 10.1016/j.bpg.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Grant CR, Liberal R, Holder BS, et al. Dysfunctional CD39 regulatory T cells and aberrant control of T helper type 17 cells in autoimmune hepatitis. Hepatology. 2013 doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao L, Tang Y, You Z, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PloS One. 2011;6:e18909. doi: 10.1371/journal.pone.0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 59.Regateiro FS, Howie D, Nolan KF, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. 2011;41:2955–2965. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- 60.Friedman DJ, Kunzli BM, YI AR, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longhi MS, Ma Y, Mitry RR, et al. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Longhi MS, Liberal R, Holder B, et al. Inhibition of interleukin-17 promotes differentiation of CD25(-) cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology. 2012;142:1526–1535. doi: 10.1053/j.gastro.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 63.Swain MG. Hepatic NKT cells: friend or foe? Clin Sci. 2008;114:457–466. doi: 10.1042/CS20070328. [DOI] [PubMed] [Google Scholar]
- 64.Dennert G, Aswad F. The role of NKT cells in animal models of autoimmune hepatitis. Crit Rev Immunol. 2006;26:453–473. doi: 10.1615/critrevimmunol.v26.i5.50. [DOI] [PubMed] [Google Scholar]
- 65.Beldi G, Wu Y, Banz Y, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Summerskill WH, Korman MG, Ammon HV, Baggenstoss AH. Prednisone for chronic active liver disease: dose titration, standard dose, and combination with azathioprine compared. Gut. 1975;16:876–883. doi: 10.1136/gut.16.11.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 69.Soloway RD, Summerskill WH, Baggenstoss AH, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 70.Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 71.Czaja AJ. Review article: the management of autoimmune hepatitis beyond consensus guidelines. Aliment Pharmacol Ther. 2013;38:343–364. doi: 10.1111/apt.12381. [DOI] [PubMed] [Google Scholar]
- 72.Stellon AJ, Hegarty JE, Portmann B, Williams R. Randomised controlled trial of azathioprine withdrawal in autoimmune chronic active hepatitis. Lancet. 1985;1:668–670. doi: 10.1016/s0140-6736(85)91329-7. [DOI] [PubMed] [Google Scholar]
- 73.van Gerven NM, Verwer BJ, Witte BI, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2013 doi: 10.1111/bcp.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Saf. 2008;7:319–333. doi: 10.1517/14740338.7.3.319. [DOI] [PubMed] [Google Scholar]
- 76.Bajaj JS, Saeian K, Varma RR, et al. Increased rates of early adverse reaction to azathioprine in patients with Crohn's disease compared to autoimmune hepatitis: a tertiary referral center experience. Am J Gastroenterol. 2005;100:1121–1125. doi: 10.1111/j.1572-0241.2005.41598.x. [DOI] [PubMed] [Google Scholar]
- 77.Heneghan MA, Allan ML, Bornstein JD, Muir AJ, Tendler DA. Utility of thiopurine methyltransferase genotyping and phenotyping, and measurement of azathioprine metabolites in the management of patients with autoimmune hepatitis. J hepatol. 2006;45:584–591. doi: 10.1016/j.jhep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol. 2007;102:1005–1012. doi: 10.1111/j.1572-0241.2007.01153.x. [DOI] [PubMed] [Google Scholar]
- 79.Czaja AJ. Nonstandard drugs and feasible new interventions for autoimmune hepatitis: part I. Inflamm Allergy Drug Targets. 2012;11:337–50. doi: 10.2174/187152812803251006. [DOI] [PubMed] [Google Scholar]
- 80.Danielsson A, Prytz H. Oral budesonide for treatment of autoimmune chronic active hepatitis. Aliment Pharmacol Ther. 1994;8:585–590. doi: 10.1111/j.1365-2036.1994.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 81.Zandieh I, Krygier D, Wong V, et al. The use of budesonide in the treatment of autoimmune hepatitis in Canada. Can J Gastroenterol. 2008;22:388–392. doi: 10.1155/2008/509459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiegand J, Schuler A, Kanzler S, et al. Budesonide in previously untreated autoimmune hepatitis. Liver Int. 2005;25:927–934. doi: 10.1111/j.1478-3231.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- 83.Csepregi A, Rocken C, Treiber G, Malfertheiner P. Budesonide induces complete remission in autoimmune hepatitis. World J Gastroenterol. 2006;12:1362–1366. doi: 10.3748/wjg.v12.i9.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manns MP, Woynarowski M, Kreisel W, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198–1206. doi: 10.1053/j.gastro.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 85.Czaja AJ, Lindor KD. Failure of budesonide in a pilot study of treatment-dependent autoimmune hepatitis. Gastroenterology. 2000;119:1312–1316. doi: 10.1053/gast.2000.0010000001. [DOI] [PubMed] [Google Scholar]
- 86.Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371–375. doi: 10.1016/s0168-8278(00)80271-8. [DOI] [PubMed] [Google Scholar]
- 87.Baven-Pronk AM, Coenraad MJ, van Buuren HR, et al. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34:335–343. doi: 10.1111/j.1365-2036.2011.04727.x. [DOI] [PubMed] [Google Scholar]
- 88.Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24:588–592. doi: 10.1155/2010/891252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inductivo-Yu I, Adams A, Gish RG, et al. Mycophenolate mofetil in autoimmune hepatitis patients not responsive or intolerant to standard immunosuppressive therapy. Clin Gastroenterol Hepatol. 2007;5:799–802. doi: 10.1016/j.cgh.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 90.Devlin SM, Swain MG, Urbanski SJ, Burak KW. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory to standard therapy. Can J Gastroenterol. 2004;18:321–326. doi: 10.1155/2004/504591. [DOI] [PubMed] [Google Scholar]
- 91.Hlivko JT, Shiffman ML, Stravitz RT, et al. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol. 2008;6:1036–1040. doi: 10.1016/j.cgh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: comparison with conventional treatment for refractory disease. J Clin Gastroenterol. 2005;39:819–825. doi: 10.1097/01.mcg.0000177260.72692.e8. [DOI] [PubMed] [Google Scholar]
- 93.Zachou K, Gatselis N, Papadamou G, Rigopoulou EI, Dalekos GN. Mycophenolate for the treatment of autoimmune hepatitis: prospective assessment of its efficacy and safety for induction and maintenance of remission in a large cohort of treatment-naive patients. J Hepatol. 2011;55:636–646. doi: 10.1016/j.jhep.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 94.Heneghan MA, Al-Chalabi T, McFarlane IG. Cost-effectiveness of pharmacotherapy for autoimmune hepatitis. Expert Opin Pharmacother. 2006;7:145–156. doi: 10.1517/14656566.7.2.145. [DOI] [PubMed] [Google Scholar]
- 95.Matsuda S, Koyasu S. Mechanisms of action of cyclosporin. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 96.Jackson LD, Song E. Cyclosporin in the treatment of corticosteroid resistant autoimmune chronic active hepatitis. Gut. 1995;36:459–461. doi: 10.1136/gut.36.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hyams JS, Ballow M, Leichtner AM. Cyclosporin treatment of autoimmune chronic active hepatitis. Gastroenterology. 1987;93:890–893. doi: 10.1016/0016-5085(87)90454-9. [DOI] [PubMed] [Google Scholar]
- 98.Person JL, McHutchison JG, Fong TL, Redeker AG. A case of cyclosporin-sensitive, steroid-resistant, autoimmune chronic active hepatitis. J Clin Gastroenterol. 1993;17:317–320. doi: 10.1097/00004836-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Sherman KE, Narkewicz M, Pinto PC. Cyclosporin in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol. 1994;21:1040–1047. doi: 10.1016/s0168-8278(05)80615-4. [DOI] [PubMed] [Google Scholar]
- 100.Fernandes NF, Redeker AG, Vierling JM, Villamil FG, Fong TL. Cyclosporin therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol. 1999;94:241–248. doi: 10.1111/j.1572-0241.1999.00807.x. [DOI] [PubMed] [Google Scholar]
- 101.Malekzadeh R, Nasseri-Moghaddam S, Kaviani MJ, Taheri H, Kamalian N, Sotoudeh M. Cyclosporin A is a promising alternative to corticosteroids in autoimmune hepatitis. Dig Dis Sci. 2001;46:1321–1327. doi: 10.1023/a:1010683817344. [DOI] [PubMed] [Google Scholar]
- 102.Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805–809. doi: 10.1097/01.mcg.0000139050.67178.be. [DOI] [PubMed] [Google Scholar]
- 103.Van Thiel DH, Wright H, Carroll P, et al. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:771–776. [PMC free article] [PubMed] [Google Scholar]
- 104.Larsen FS, Vainer B, Eefsen M, Bjerring PN, Adel Hansen B. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol. 2007;13:3232–3236. doi: 10.3748/wjg.v13.i23.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tannous MM, Cheng J, Muniyappa K, et al. Use of tacrolimus in the treatment of autoimmune hepatitis: a single centre experience. Aliment Pharmacol Ther. 2011;34:405–407. doi: 10.1111/j.1365-2036.2011.04749.x. [DOI] [PubMed] [Google Scholar]
- 106.Czaja AJ. Advances in the current treatment of autoimmune hepatitis. Dig Dis Sci. 2012;57:1996–2010. doi: 10.1007/s10620-012-2151-2. [DOI] [PubMed] [Google Scholar]
- 107.Jothimani D, Cramp ME, Mitchell JD, Cross TJ. Treatment of autoimmune hepatitis: a review of current and evolving therapies. J Gastroenterol Hepatol. 2011;26:619–627. doi: 10.1111/j.1440-1746.2010.06579.x. [DOI] [PubMed] [Google Scholar]
- 108.Ilyas JA, O'Mahony CA, Vierling JM. Liver transplantation in autoimmune liver diseases. Best Pract Res Clin Gastroenterol. 2011;25:765–782. doi: 10.1016/j.bpg.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813–1824. doi: 10.1002/lt.20910. [DOI] [PubMed] [Google Scholar]