Abstract
Background:
Cisplatin (CP) is a chemotherapy drug and nephrotoxicity is a major concern for CP therapy. CP-induced nephrotoxicity is gender-dependent, and the effect of aerobic exercise in females has not been reported yet while it has a beneficial effect in males. Hence, this study was designed to determine the protective role of aerobic exercise against CP-induced nephrotoxicity in female rats.
Methods:
Twenty-eight adult female rats were divided into four groups. Groups I and II had aerobic exercise on a treadmill for 8 weeks. Then, the exercise protocol was continued for another week in group I and stopped in group II. All animals in these groups received CP (2.5 mg/kg/day; i.p.) for 1-week. Groups III and IV were treated with CP and vehicle, respectively, without exercise. Finally, the animals were sacrificed for biochemical measurements and tissue histopathology investigations.
Results:
CP alone without exercise increased serum levels of blood urea nitrogen (BUN) and creatinine (Cr), kidney weight, and kidney tissue damage score (KTDS); while exercise could not attenuate these parameters in female rats. Exercise in females increased the serum levels of BUN and Cr and KTDS and weight loss (P < 0.05). Kidney nitrite levels reduce significantly in group I in compared to positive and negative control groups. Exercise also did not have beneficial effects on malondialdehyde levels in plasma and kidney.
Conclusions:
Aerobic exercise cannot reduce CP-induced nephrotoxicity in female rats. Increasing the damage in female rats may be related to female sex hormone estrogen or gender differences in renal hemodynamic and renin-angiotensin system activity in the presence of exercise. In general, it is recommended that the females under CP chemotherapy avoid exercising during treatment.
Keywords: Aerobic exercise, cisplatin, female rats, nephrotoxicity
INTRODUCTION
Cancer is a major cause of morbidity and mortality throughout the world. Chemotherapy is recommended to inhibit tumor cells growth and platinum compounds are categorized as chemotherapeutic agents.[1,2] Cisplatin (cis-diamminedichloroplatinum) (CP) is a platinum compound that is used to treat various types of cancers.[1,3] Despite the antineoplastic effect of CP, its use is limited due to severe side effects such as neurotoxicity,[4] nausea and vomiting cardiac toxicity, ototoxicity, myelotoxicity, and hemolytic anemia.[5,6,7] Nephrotoxicity is a major concern for CP therapy.[8] CP is the inhibitor of deoxyribonucleic acid synthesis,[9] and induce tubular damage by various mechanisms including oxidative stress.[8,10] It is reported that CP-induced nephrotoxicity is ameliorated by free radical scavenging agents such as Vitamin E,[11] Vitamin C, losartan,[12,13] gamma-amino butyric acid,[14] and melatonin.[15] CP-induced nephrotoxicity is also gender-related.[16,17] Several studies indicated exercise activity changes antioxidant content.[18,19,20] Prolonged exercise activity reduces erythrocyte glutathione peroxidase (GSH-PX) level.[18] Furthermore, moderate regular exercise for 9 months decreased malondialdehyde (MDA) levels.[21] It is reported that long-term aerobic exercise increases GSH erythrocyte PX enzyme (GSH) activity and superoxide dismutase activity.[19] Our previous study indicated that aerobic exercise reduce CP-induced nephrotoxicity with a favorable effect on renal function by reducing lipid peroxidation.[22] Exercise has been also shown to have a protective effect against CP-induced cell death in the kidney,[23] and it is suggested that physical exercise before and during chemotherapy could attenuate the side-effects of medications and improve the quality of life for patients.[23] As mentioned before, gender plays a role in CP-induced nephrotoxicity, and previously we reported that aerobic exercise could attenuate the CP-induced kidney damage in male rats.[22] However, the effect has not been reported in females. Furthermore, inhibition of tumor cell proliferation by CP chemotherapy is different in male and female patients.[24] Therefore, this study was designed to investigate the protective effect of aerobic exercise against CP-induced nephrotoxicity in female rats.
METHODS
Experimental protocol
Twenty-eight adult female Wistar rats (weighing 211 ± 7 g) (Animal Centre, Isfahan University of Medical Sciences, Isfahan, Iran) were used. The animals were housed at the room temperature of 23–25°C and 12 h light/12 h dark cycle and free access to water and rat chow. The experimental procedures were in advance approved by the Isfahan University of Medical Sciences Ethics Committee.
The animals were categorized into four groups as described previously.[22] Groups I (called EX + CP + EX) (n = 7) and II (called EX + CP) (n = 8) were subjected to aerobic exercise on a treadmill 1 h/day and 5 days/week for 8 weeks. Then, exercise was continued in group 1 in the 9th week. The animals in groups III (positive control) (n = 5) and IV (negative control) (n = 8) did not have exercise during the experiment. In addition, all animals in groups I, II, and III received CP (2.5 mg/kg/day; i.p.) and animals in group IV received saline in the 9th week. The animals in the positive and negative control groups were placed in the apparatus for the same duration of time without running.
Treadmill exercise
The rats were exposed to treadmill exercise as a moderate regular exercise on a motorized rodent treadmill with an inclination of 0% during the study. The animals in groups I and II underwent training adaptation for 7 days (each day 15 min) at 16 m/min. Then, groups I and II received a progressive exercise. During the first 2 weeks, the speed was increased to 20 m/min for 60 min/day for 5 days/week. Then, the speed was raised to 23, 25, and 28 m/min in the second (weeks 3 and 4), third (weeks 5 and 6), and fourth (weeks 7 and 8) 2 weeks, respectively. After endurance training for 8 weeks, group I with reduced aerobic exercise to 23 m/min and group II with stopped aerobic exercise were treated with CP for 1-week. The animals in this study were exposed to moderate exercise. The oxygen consumption in this position was about 65% for the rats.[25,26]
Measurements and histopathological procedures
At the end of the experiment, the rats were anesthetized with chloral hydrate injection (450 mg/kg; i.p.) to obtain blood samples via heart puncture. Moreover, right and left kidneys and uterus were removed and weighed immediately. After hematoxylin and eosin staining, the left kidney was fixed in 10% formalin solution. The damage was evaluated and scored by a pathologist who was blind to the study protocol. The right kidney was homogenized to obtain supernatant for tissue nitrite measurements. The levels of serum creatinine (Cr) and blood urea nitrogen (BUN) were determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum and tissue levels of nitrite (stable nitric oxide metabolite) were measured using a colorimetric assay kit (Promega Corporation, USA) that involves Griess reaction. The serum level of MDA was measured by preparing a mixture of trichloroacetic acid (15%) and thiobarbituric acid (0.375%) (Merck). Then, 1 ml of the sample was added to 2 ml prepared the mixture and incubated in the boiling water for 60 min. After cooling, the samples were centrifuged for 10 min and finally the supernatant light absorbance was read at 535 nm.
Statistical analyses
Data were expressed as mean ± standard error of the mean one-way ANOVA followed by the Tukey posttest was applied to compare the groups in terms of BUN, Cr, NO, and MDA levels, kidney weight (KW), and bodyweight (BW). To compare the pathology damage score, the Kruskal–Wallis and Mann–Whitney U-test were applied. P < 0.05 was considered statistically significant.
RESULTS
Effect of cisplatin and exercise on serum levels of blood urea nitrogen and creatinine, body weight change, kidney weight, and kidney tissue damage score
The serum levels of BUN and Cr significantly increased in groups I to III when compared with the negative control group (sham) (P < 0.05). However, these parameters increased non-significantly in the EX + CP + EX group more than that in the EX + CP and CP groups [Figure 1]. This was also observed for kidney tissue damage score (KTDS); as KTDS was significantly higher than that in the positive control group (P < 0.05) [Figures 1 and 2]. On the contrary, BW decreased significantly in groups I to III in comparison with the sham group (P < 0.05). In addition, the BW changes in group II was less than group I (P < 0.05) [Figure 1]. KW increased significantly in all the CP-treated groups when compared with the sham group (P < 0.05) [Figure 1].
Figure 1.
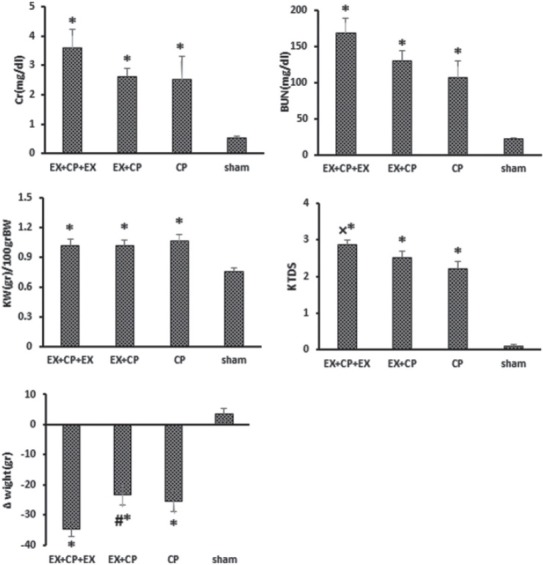
Serum levels of blood urea nitrogen and creatinine, kidney weight, body weight change (ΔBW), kidney tissue damage score *indicates significant difference from the negative control (sham) group and #indicates significant difference from the EX + cisplatin (CP) + EX group. ×indicates significant difference from the positive control (CP) group (P < 0.05)
Figure 2.
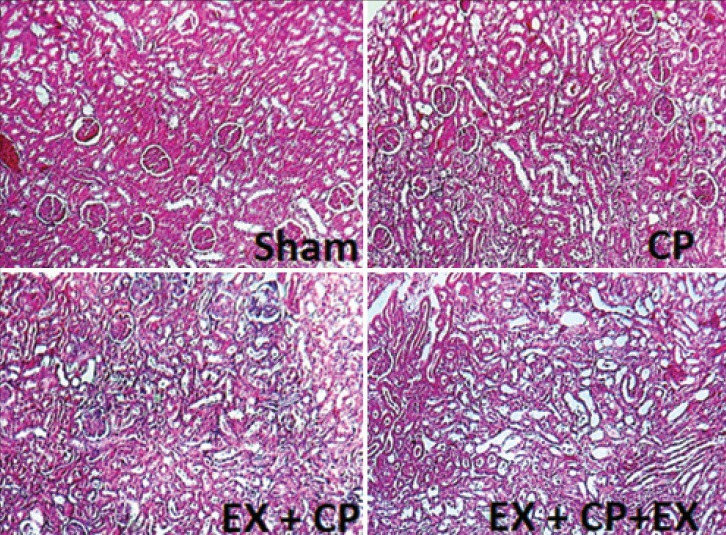
The images of kidney tissue with H and E staining to examine tissue damage in four experimental groups. The images were presented at ×100
Effect of cisplatin and exercise on serum and kidney nitrite
No significant difference was observed among the groups with regard to the serum level of nitrite. The kidney level of nitrite reduced significantly in EX + CP + EX group when compared with the positive and negative control groups (P < 0.05) [Figure 3]. The serum levels of MDA significantly increased in the EX + CP + EX and EX + CP groups when compared with the negative and positive control groups (P < 0.05).
Figure 3.
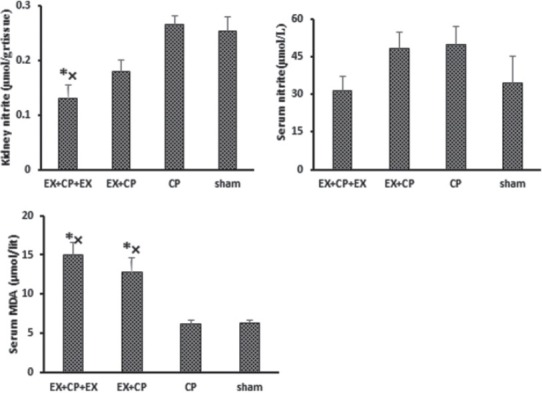
Serum levels of nitrite and malondialdehyde, and kidney tissue level of nitrite. *indicates significant difference from the negative control (sham) group, ×indicates significant difference from the positive control (cisplatin) group (P < 0.05)
DISCUSSION
Previously, we reported the beneficial effect of aerobic exercise against CP-induced nephrotoxicity in male rats.[22] In the current study, female rats were subjected to treadmill exercise and CP therapy simultaneously to find the role of exercise in females. Our results were in agreement with previous studies in CP alone treated groups.[27,28,29,30,31,32] Histopathological examination has shown 50–75% of tubular degeneration due to CP administration[33] by the mechanisms such as oxidative stress and apoptosis.[10,34,35] Our study indicated that exercise did not protect the kidney against CP-induced nephrotoxicity. This finding is in contrast to the findings of our previous study on male rats.[22] Moderate regular exercise before or simultaneously with CP therapy has a protective effect against CP-induced nephrotoxicity in the male rats.[22] In our study, exercise increased the serum MDA level in groups I and II. A study has indicated the high intensity of exercise increased MDA in skeletal muscle[36] as well as, exhaustive maximal exercise-induced free radical generation while short periods of submaximal exercise may inhibit it and lipid peroxidation.[37] CP is known as a drug that causes endothelial dysfunction and decreases endothelial NO synthase (eNOS).[38] On the other hand, it enhances NO production by the inducible isoform of NO synthase.[39] In our study, CP alone treated group did not show a significant difference in nitrite levels in compared to the negative control group. Alteration of NO production due to CP influences male rat more than female rat.[40] A study indicated mild, regular aerobic endurance exercise increased NO production in elderly woman[41] but the result of this study indicated reduction of nitrite in EX + CP + EX group. Maybe reduction of nitrite in female rat under the combination of CP and exercise was related to increase endothelial dysfunction in the female rat and reduced NO production by eNOS. Our data indicated BW decreased in groups I, II, and III when compared with the negative control group. This reduction was higher in EX + CP + EX group than other CP treated groups. CP reduced BW by induced gastrointestinal disturbance.[42] Furthermore, physical activity decreased BW.[43,44,45] It was reported that administration of CP increased KW, which is directly related to kidney injury.[46] This is while several studies indicated that increase in the levels of biochemistry markers such as BUN or Cr after CP therapy is higher in males.[16,17] Nevertheless, when CP is co-administrated with another compound, contradictory results are achieved in males and females. For example L-arginine, losartan, and vitamin E have protective effects against CP-induced nephrotoxicity in male rats, but these agents did not have ameliorative effects on CP-induced nephrotoxicity in female rats.[11,47,48] Inhibition of tumor cell proliferation is different in male and female patients under CP chemotherapy.[24] Males have higher respiratory exchange ratios (mean 0.94 vs. 0.87), higher muscle glycogen utilization (by 25%), and higher urea nitrogen excretion (by 30%) than the females.[49] Furthermore, there is a high basal level of vasodilator prostaglandins in renal medulla of female rats that is affected by blood flow autoregulation in this area, and medullary flow in female rats is partially influenced by endogenous angiotensin (AT).[50] Moreover, physical activity affects the sensitivity of the renin-angiotensin system (RAS).[51,52] There is a gender difference in RAS activity.[53,54,55] Losartan, as an AT II receptor 1 blocker, has higher protective effects against CP-induced nephrotoxicity in males.[47] A study indicated despite a parallel fitness in males and females, development of metabolic responses are different in incremental exercise. These gender differences may be related to body mass because of a positive correlation between body mass and enhancement of systemic oxygen consumption in males and females.[56] This may be also related to male and female sex hormones. Several studies indicated estrogen abolish the protective effect of Vitamins E and C, losartan, and erythropoietin against CP-induced nephrotoxicity in female rats.[31,57,58] This is while testosterone administration reduces CP-induced nephrotoxicity in male rats.[59]
CONCLUSIONS
Aerobic exercise cannot reduce CP-induced nephrotoxicity in female rats. Increasing the damage in female rats may be related to the female sex hormone, estrogen or gender differences in renal hemodynamic and RAS activity in the presence of exercise. In general, it is recommended that the females under CP chemotherapy avoid exercise during the treatment.
ACKNOWLEDGMENTS
This research was supported by Isfahan University of Medical Sciences.
Footnotes
Source of Support: Isfahan University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Lind M. Principles of cytotoxic chemotherapy. Medicine. 2008;36:19–23. [Google Scholar]
- 2.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–79. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 3.Mascharak PK, Sugiura Y, Kuwahara J, Suzuki T, Lippard SJ. Alteration and activation of sequence-specific cleavage of DNA by bleomycin in the presence of the antitumor drug cis-diamminedichloroplatinum(II) Proc Natl Acad Sci U S A. 1983;80:6795–8. doi: 10.1073/pnas.80.22.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milosavljevic N, Duranton C, Djerbi N, Puech PH, Gounon P, Lagadic-Gossmann D, et al. Nongenomic effects of cisplatin: Acute inhibition of mechanosensitive transporters and channels without actin remodeling. Cancer Res. 2010;70:7514–22. doi: 10.1158/0008-5472.CAN-10-1253. [DOI] [PubMed] [Google Scholar]
- 5.Levi JA, Aroney RS, Dalley DN. Haemolytic anaemia after cisplatin treatment. Br Med J (Clin Res Ed) 1981;282:2003–4. doi: 10.1136/bmj.282.6281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kris MG, Gralla RJ, Clark RA, Tyson LB, O’Connell JP, Wertheim MS, et al. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3:1379–84. doi: 10.1200/JCO.1985.3.10.1379. [DOI] [PubMed] [Google Scholar]
- 7.Windsor RE, Strauss SJ, Kallis C, Wood NE, Whelan JS. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: A pilot study. Cancer. 2012;118:1856–67. doi: 10.1002/cncr.26472. [DOI] [PubMed] [Google Scholar]
- 8.Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 1984;100:704–13. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 9.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Jilanchi S, Nematbakhsh M, Bahadorani M, Talebi A, Eshraghi-Jazi F, Mansouri A, et al. Vitamin E is a nephroprotectant agent in male but not in female in a model of Cisplatin-induced nephrotoxicity. ISRN Nephrol. 2013;2013:280395. doi: 10.5402/2013/280395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashrafi F, Nematbakhsh M, Safari T, Talebi A, Nasri H, Khazaei M, et al. A combination of vitamin C and losartan for cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis. 2012;6:361–5. [PubMed] [Google Scholar]
- 13.Nematbakhsh M, Ashrafi F, Safari T, Talebi A, Nasri H, Mortazavi M, et al. Administration of vitamin E and losartan as prophylaxes in cisplatin-induced nephrotoxicity model in rats. J Nephrol. 2012;25:410–7. doi: 10.5301/jn.5000018. [DOI] [PubMed] [Google Scholar]
- 14.Ali BH, Al-Salam S, Al Za’abi M, Al Balushi KA, AlMahruqi AS, Beegam S, et al. Renoprotective effects of gamma-aminobutyric acid on cisplatin-induced acute renal injury in rats. Basic Clin Pharmacol Toxicol. 2015;116:62–8. doi: 10.1111/bcpt.12291. [DOI] [PubMed] [Google Scholar]
- 15.Nasri H, Tavakoli M, Ahmadi A, Baradaran A, Nematbakhsh M, Rafieian-Kopaei M. Ameliorative effect of melatonin against contrast media induced renal tubular cell injury. Pak J Med Sci. 2014;30:261–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Nematbakhsh M, Ebrahimian S, Tooyserkani M, Eshraghi-Jazi F, Talebi A, Ashrafi F. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: Greater intensity of damage in male than female. Nephrourol Mon. 2013;5:818–21. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nematbakhsh M, Talebi A, Nasri H, Safari T, Dolatkhah S, Ashrafi F, et al. Some evidence for sex-based differences in cisplatin-induced nephrotoxicity in rats. Med Sci Technol. 2012;53:RA29–32. [Google Scholar]
- 18.Duthie GG, Robertson JD, Maughan RJ, Morrice PC. Blood antioxidant status and erythrocyte lipid peroxidation following distance running. Arch Biochem Biophys. 1990;282:78–83. doi: 10.1016/0003-9861(90)90089-h. [DOI] [PubMed] [Google Scholar]
- 19.Shin YA, Lee JH, Song W, Jun TW. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev. 2008;129:254–60. doi: 10.1016/j.mad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Berzosa C, Cebrián I, Fuentes-Broto L, Gómez-Trullén E, Piedrafita E, Martínez-Ballarín E, et al. Acute exercise increases plasma total antioxidant status and antioxidant enzyme activities in untrained men. J Biomed Biotechnol 2011. 2011 doi: 10.1155/2011/540458. 540458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naziroglu M, Simsek M, Kutlu M. Moderate exercise with a dietary vitamin C and E combination protects against streptozotocin-induced oxidative damage to the blood and improves fetal outcomes in pregnant rats. Clin Chem Lab Med. 2004;42:511–7. doi: 10.1515/CCLM.2004.087. [DOI] [PubMed] [Google Scholar]
- 22.Zeynali F, Nematbakhsh M, Mojtahedi H, Porshahnazari A, Talebi A, Pezeshki Z, et al. Protective role of aerobic exercise against cisplatin-induced nephrotoxicity in rats. Asian J Sports Med. 2015 doi: 10.5812/asjsm.24901. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyagi MY, Seelaender M, Castoldi A, de Almeida DC, Bacurau AV, Andrade-Oliveira V, et al. Long-term aerobic exercise protects against cisplatin-induced nephrotoxicity by modulating the expression of IL-6 and HO-1. PLoS One. 2014;9:e108543. doi: 10.1371/journal.pone.0108543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta V, Singh SM. Sex dimorphism in antitumor response of chemotherapeutic drug cisplatin in a murine host-bearing a T-cell lymphoma. Anticancer Drugs. 2008;19:583–92. doi: 10.1097/CAD.0b013e3282fb97bf. [DOI] [PubMed] [Google Scholar]
- 25.Baranowski M, Zabielski P, Błachnio-Zabielska A, Harasiuk D, Górski J. LXR activation prevents exhaustive exercise-induced hypoglycaemia and spares muscle glycogen but does not enhance running endurance in untrained rats. Acta Physiol. 2011;201:373–9. doi: 10.1111/j.1748-1716.2010.02199.x. [DOI] [PubMed] [Google Scholar]
- 26.Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, et al. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993;265:H2094–8. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- 27.Deegan PM, Nolan C, Ryan MP, Basinger MA, Jones MM, Hande KR. The role of the renin-angiotensin system in cisplatin nephrotoxicity. Ren Fail. 1995;17:665–74. doi: 10.3109/08860229509037634. [DOI] [PubMed] [Google Scholar]
- 28.Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective effects of the angiotensin II receptor blocker losartan on cisplatin-induced kidney injury. Chemotherapy. 2009;55:399–406. doi: 10.1159/000262453. [DOI] [PubMed] [Google Scholar]
- 29.Stakisaitis D, Dudeniene G, Jankunas RJ, Grazeliene G, Didziapetriene J, Pundziene B. Cisplatin increases urinary sodium excretion in rats: Gender-related differences. Medicina (Kaunas) 2010;46:45–50. [PubMed] [Google Scholar]
- 30.Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, et al. Effects of fennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol Int. 2013;20:138–45. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, et al. Estrogen abolishes protective effect of erythropoietin against cisplatin-induced nephrotoxicity in ovariectomized rats. ISRN Oncol 2012. 2012 doi: 10.5402/2012/890310. 890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezeshki Z, Nematbakhsh M, Nasri H, Talebi A, Pilehvarian AA, Safari T, et al. Evidence against protective role of sex hormone estrogen in Cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol Int. 2013;20:43–7. doi: 10.4103/0971-6580.111568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleisa AM, Al-Majed AA, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, Al-Shabanah OA, et al. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34:1252–9. doi: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- 34.Okuda M, Masaki K, Fukatsu S, Hashimoto Y, Inui K. Role of apoptosis in cisplatin-induced toxicity in the renal epithelial cell line LLC-PK1. Implication of the functions of apical membranes. Biochem Pharmacol. 2000;59:195–201. doi: 10.1016/s0006-2952(99)00303-2. [DOI] [PubMed] [Google Scholar]
- 35.Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: Apoptosis vs. necrosis. Am J Physiol. 1996;270:F700–8. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- 36.Alessio HM, Goldfarb AH, Cutler RG. MDA content increases in fast-and slow-twitch skeletal muscle with intensity of exercise in a rat. Am J Physiol. 1988;255:C874–7. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- 37.Lovlin R, Cottle W, Pyke I, Kavanagh M, Belcastro AN. Are indices of free radical damage related to exercise intensity. Eur J Appl Physiol Occup Physiol. 1987;56:313–6. doi: 10.1007/BF00690898. [DOI] [PubMed] [Google Scholar]
- 38.Saleh S, El-Demerdash E. Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: Role of nitric oxide. Basic Clin Pharmacol Toxicol. 2005;97:91–7. doi: 10.1111/j.1742-7843.2005.pto_114.x. [DOI] [PubMed] [Google Scholar]
- 39.Chirino YI, Trujillo J, Sánchez-González DJ, Martínez-Martínez CM, Cruz C, Bobadilla NA, et al. Selective iNOS inhibition reduces renal damage induced by cisplatin. Toxicol Lett. 2008;176:48–57. doi: 10.1016/j.toxlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Nematbakhsh M, Pezeshki Z. Sex-Related Difference in Nitric Oxide Metabolites Levels after Nephroprotectant Supplementation Administration against Cisplatin-Induced Nephrotoxicity in Wistar Rat Model: The Role of Vitamin E, Erythropoietin, or N-Acetylcysteine. ISRN Nephrol 2013. 2013 doi: 10.5402/2013/612675. 612675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, et al. Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res. 2004;27:947–53. doi: 10.1291/hypres.27.947. [DOI] [PubMed] [Google Scholar]
- 42.Arivarasu NA, Priyamvada S, Mahmood R. Oral administration of caffeic acid ameliorates the effect of cisplatin on brush border membrane enzymes and antioxidant system in rat intestine. Exp Toxicol Pathol. 2013;65:21–5. doi: 10.1016/j.etp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE – A randomized controlled study. Arch Intern Med. 2004;164:31–9. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 44.Gao Y, Wang C, Pan T, Luo L. Impact of metformin treatment and swimming exercise on visfatin levels in high-fat-induced obesity rats. Arq Bras Endocrinol Metabol. 2014;58:42–7. doi: 10.1590/0004-2730000002840. [DOI] [PubMed] [Google Scholar]
- 45.Wilmore JH, Costill DL. Cham Paign, Illinois, USA: Human Kinetics Publishers; 1994. Physiology of Sport and Exercise. [Google Scholar]
- 46.Nematbakhsh M, Ashrafi F, Nasri H, Talebi A, Pezeshki Z, Eshraghi F, et al. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J Res Med Sci. 2013;18:370–3. [PMC free article] [PubMed] [Google Scholar]
- 47.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K, et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: Gender-related differences. Ren Fail. 2012;34:1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 48.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z, et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16:1389–96. [PMC free article] [PubMed] [Google Scholar]
- 49.Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol (1985) 1990;68:302–8. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- 50.Parekh N, Zou AP, Jüngling I, Endlich K, Sadowski J, Steinhausen M. Sex differences in control of renal outer medullary circulation in rats: Role of prostaglandins. Am J Physiol. 1993;264:F629–36. doi: 10.1152/ajprenal.1993.264.4.F629. [DOI] [PubMed] [Google Scholar]
- 51.Pereira MG, Ferreira JC, Bueno CR, Jr, Mattos KC, Rosa KT, Irigoyen MC, et al. Exercise training reduces cardiac angiotensin II levels and prevents cardiac dysfunction in a genetic model of sympathetic hyperactivity-induced heart failure in mice. Eur J Appl Physiol. 2009;105:843–50. doi: 10.1007/s00421-008-0967-4. [DOI] [PubMed] [Google Scholar]
- 52.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–62. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 53.Sandberg K, Ji H. Sex and the renin angiotensin system: Implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther. 2003;10:15–23. doi: 10.1053/jarr.2003.50006. [DOI] [PubMed] [Google Scholar]
- 54.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the Mas receptor. Acta Physiol (Oxf) 2012;206:150–6. doi: 10.1111/j.1748-1716.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 55.Hilliard LM, Nematbakhsh M, Kett MM, Teichman E, Sampson AK, Widdop RE, et al. Gender differences in pressure-natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension. 2011;57:275–82. doi: 10.1161/HYPERTENSIONAHA.110.166827. [DOI] [PubMed] [Google Scholar]
- 56.Kang J, Hoffman JR, Chaloupka EC, Ratamess NA, Weiser PC. Gender differences in the progression of metabolic responses during incremental exercise. J Sports Med Phys Fitness. 2006;46:71–8. [PubMed] [Google Scholar]
- 57.Nematbakhsh M, Pezeshki Z, Eshraghi-Jazi F, Ashrafi F, Nasri H, Talebi A, et al. Vitamin E, vitamin C, or losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol 2012. 2012:1–10. doi: 10.1155/2012/284896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carraro-Eduardo JC, Oliveira AV, Carrapatoso ME, Ornellas JF. Effect of sex hormones on gentamicin-induced nephrotoxicity in rats. Braz J Med Biol Res. 1993;26:653–62. [PubMed] [Google Scholar]
- 59.Rostami B, Nematbakhsh M, Pezeshki Z, Talebi A, Sharifi MR, Moslemi F, et al. Effect of testosterone on Cisplatin-induced nephrotoxicity in surgically castrated rats. Nephrourol Mon. 2014;6:e21546. doi: 10.5812/numonthly.21546. [DOI] [PMC free article] [PubMed] [Google Scholar]


