Abstract
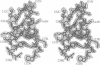
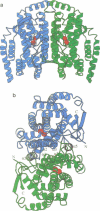
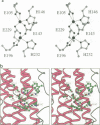
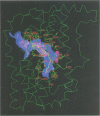
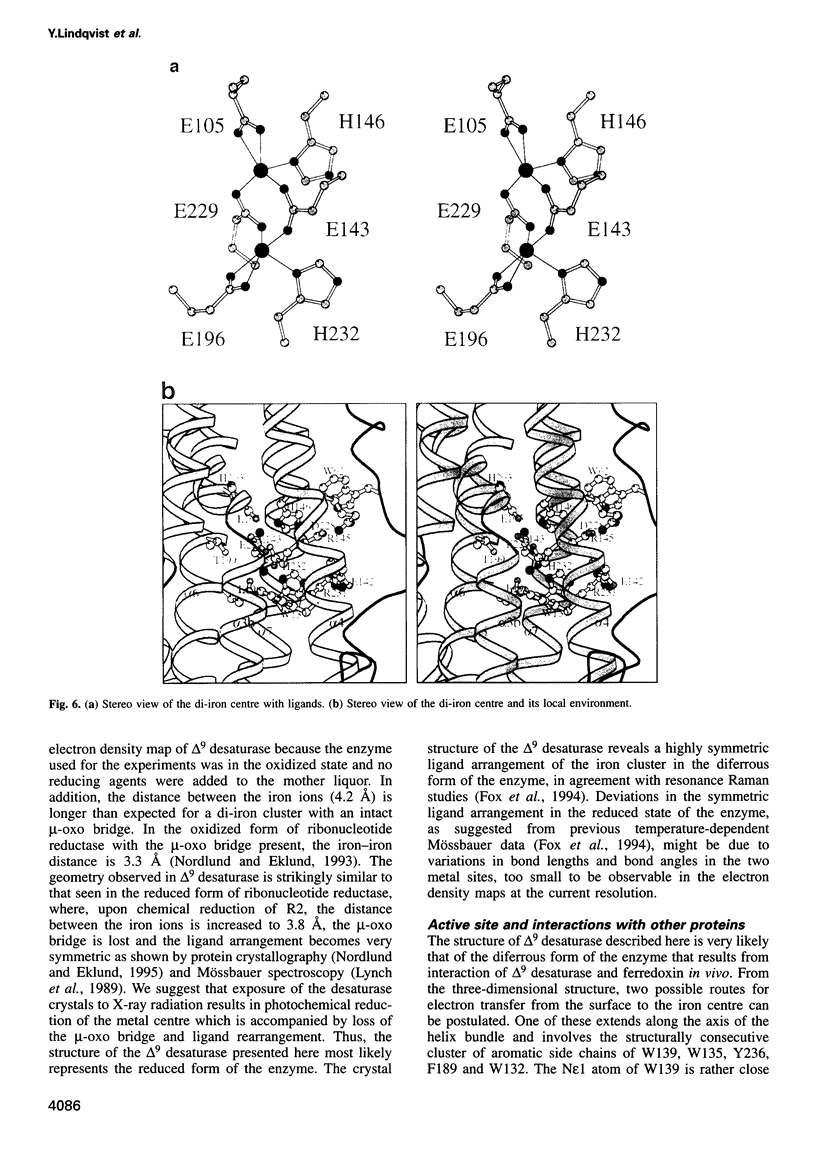
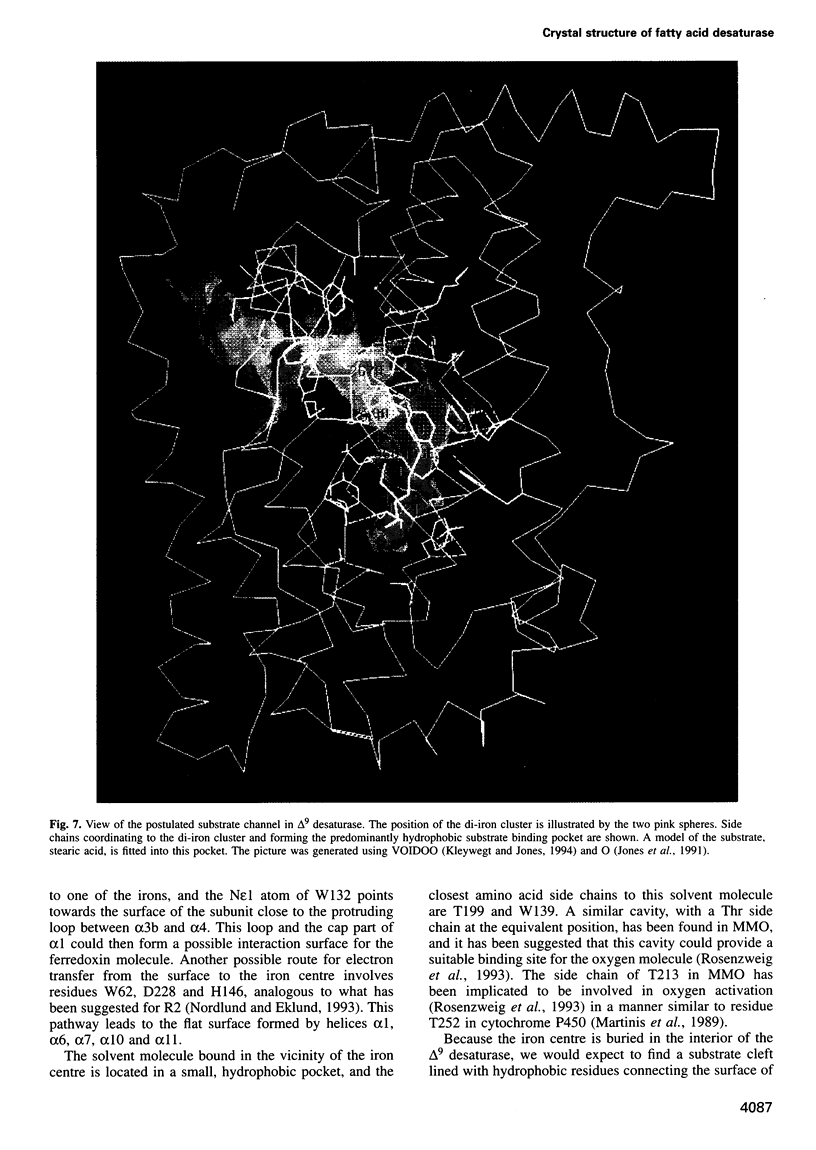
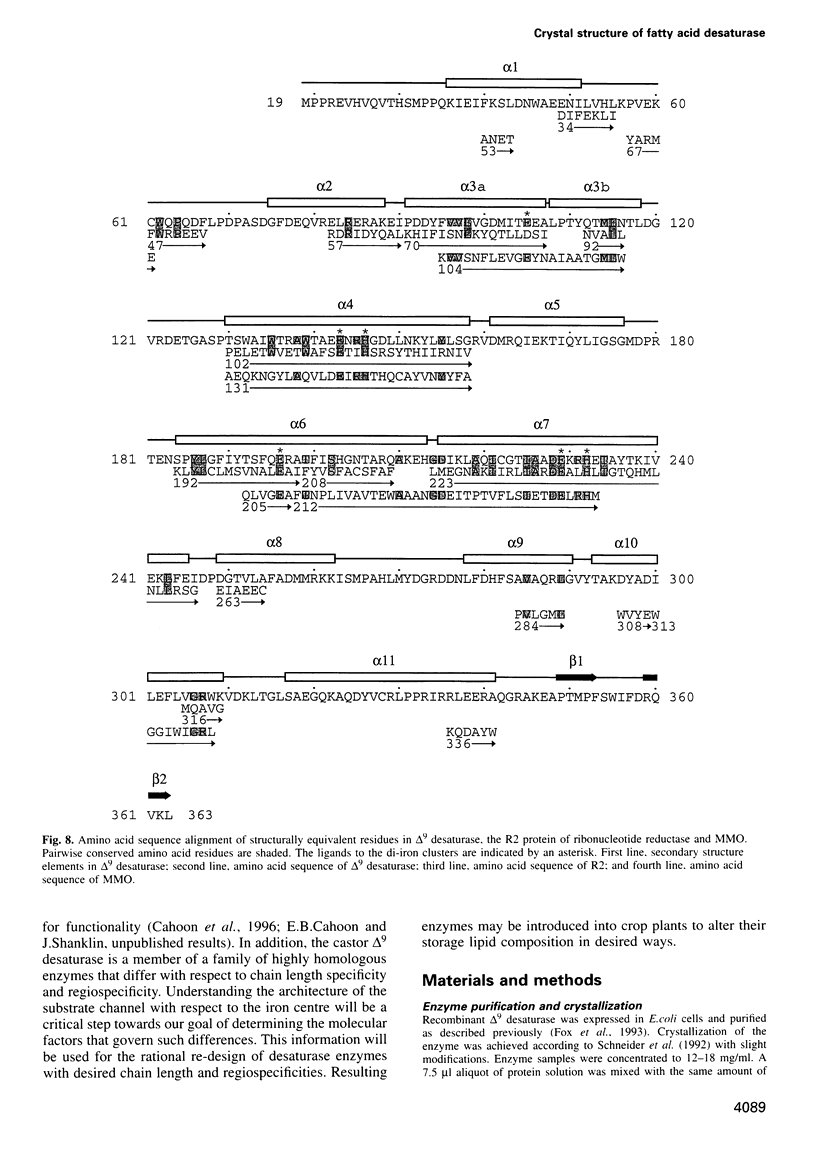
The three-dimensional structure of recombinant homodimeric delta9 stearoyl-acyl carrier protein desaturase, the archetype of the soluble plant fatty acid desaturases that convert saturated to unsaturated fatty acids, has been determined by protein crystallographic methods to a resolution of 2.4 angstroms. The structure was solved by a combination of single isomorphous replacement, anomalous contribution from the iron atoms to the native diffraction data and 6-fold non-crystallographic symmetry averaging. The 363 amino acid monomer consists of a single domain of 11 alpha-helices. Nine of these form an antiparallel helix bundle. The enzyme subunit contains a di-iron centre, with ligands from four of the alpha-helices in the helix bundle. The iron ions are bound in a highly symmetric environment, with one of the irons forming interactions with the side chains of E196 and H232 and the second iron with the side chains of E105 and H146. Two additional glutamic acid side chains, from E143 and E229, are within coordination distance to both iron ions. A water molecule is found within the second coordination sphere from the iron atoms. The lack of electron density corresponding to a mu-oxo bridge, and the long (4.2 angstroms) distance between the iron ions suggests that this probably represents the diferrous form of the enzyme. A deep channel which probably binds the fatty acid extends from the surface into the interior of the enzyme. Modelling of the substrate, stearic acid, into this channel places the delta9 carbon atom in the vicinity of one of the iron ions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. J., Thornton J. M. Ion-pairs in proteins. J Mol Biol. 1983 Aug 25;168(4):867–885. doi: 10.1016/s0022-2836(83)80079-5. [DOI] [PubMed] [Google Scholar]
- Cahoon E. B., Becker C. K., Shanklin J., Ohlrogge J. B. cDNAs for isoforms of the delta 9-stearoyl-acyl carrier protein desaturase from Thunbergia alata endosperm. Plant Physiol. 1994 Oct;106(2):807–808. doi: 10.1104/pp.106.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon E. B., Cranmer A. M., Shanklin J., Ohlrogge J. B. delta 6 Hexadecenoic acid is synthesized by the activity of a soluble delta 6 palmitoyl-acyl carrier protein desaturase in Thunbergia alata endosperm. J Biol Chem. 1994 Nov 4;269(44):27519–27526. [PubMed] [Google Scholar]
- Cahoon E. B., Mills L. A., Shanklin J. Modification of the fatty acid composition of Escherichia coli by coexpression of a plant acyl-acyl carrier protein desaturase and ferredoxin. J Bacteriol. 1996 Feb;178(3):936–939. doi: 10.1128/jb.178.3.936-939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon E. B., Shanklin J., Ohlrogge J. B. Expression of a coriander desaturase results in petroselinic acid production in transgenic tobacco. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11184–11188. doi: 10.1073/pnas.89.23.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox B. G., Shanklin J., Ai J., Loehr T. M., Sanders-Loehr J. Resonance Raman evidence for an Fe-O-Fe center in stearoyl-ACP desaturase. Primary sequence identity with other diiron-oxo proteins. Biochemistry. 1994 Nov 1;33(43):12776–12786. doi: 10.1021/bi00209a008. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Shanklin J., Somerville C., Münck E. Stearoyl-acyl carrier protein delta 9 desaturase from Ricinus communis is a diiron-oxo protein. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2486–2490. doi: 10.1073/pnas.90.6.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K. J. Palmitoleate formation by soybean stearoyl-acyl carrier protein desaturase. Biochim Biophys Acta. 1993 Sep 8;1169(3):231–235. doi: 10.1016/0005-2760(93)90245-5. [DOI] [PubMed] [Google Scholar]
- Hamlin R. Multiwire area X-ray diffractometers. Methods Enzymol. 1985;114:416–452. doi: 10.1016/0076-6879(85)14029-2. [DOI] [PubMed] [Google Scholar]
- Holmes M. A., Le Trong I., Turley S., Sieker L. C., Stenkamp R. E. Structures of deoxy and oxy hemerythrin at 2.0 A resolution. J Mol Biol. 1991 Apr 5;218(3):583–593. doi: 10.1016/0022-2836(91)90703-9. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kleywegt G. J., Jones T. A. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr. 1994 Mar 1;50(Pt 2):178–185. doi: 10.1107/S0907444993011333. [DOI] [PubMed] [Google Scholar]
- Lynch J. B., Juarez-Garcia C., Münck E., Que L., Jr Mössbauer and EPR studies of the binuclear iron center in ribonucleotide reductase from Escherichia coli. A new iron-to-protein stoichiometry. J Biol Chem. 1989 May 15;264(14):8091–8096. [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1966 Apr 25;241(8):1925–1927. [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1968 Sep 10;243(17):4626–4633. [PubMed] [Google Scholar]
- Nordlund P., Dalton H., Eklund H. The active site structure of methane monooxygenase is closely related to the binuclear iron center of ribonucleotide reductase. FEBS Lett. 1992 Aug 3;307(3):257–262. doi: 10.1016/0014-5793(92)80690-i. [DOI] [PubMed] [Google Scholar]
- Nordlund P., Eklund H. Di-iron-carboxylate proteins. Curr Opin Struct Biol. 1995 Dec;5(6):758–766. doi: 10.1016/0959-440x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Nordlund P., Eklund H. Structure and function of the Escherichia coli ribonucleotide reductase protein R2. J Mol Biol. 1993 Jul 5;232(1):123–164. doi: 10.1006/jmbi.1993.1374. [DOI] [PubMed] [Google Scholar]
- Nordlund P., Sjöberg B. M., Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature. 1990 Jun 14;345(6276):593–598. doi: 10.1038/345593a0. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B. Design of New Plant Products: Engineering of Fatty Acid Metabolism. Plant Physiol. 1994 Mar;104(3):821–826. doi: 10.1104/pp.104.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J., Browse J. Lipid biosynthesis. Plant Cell. 1995 Jul;7(7):957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi N., Prickril B. C., Kurtz D. M., Jr, Huynh B. H. Spectroscopic characterization of 57Fe-reconstituted rubrerythrin, a non-heme iron protein with structural analogies to ribonucleotide reductase. Biochemistry. 1993 Aug 24;32(33):8487–8491. doi: 10.1021/bi00084a013. [DOI] [PubMed] [Google Scholar]
- Rosenzweig A. C., Frederick C. A., Lippard S. J., Nordlund P. Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane. Nature. 1993 Dec 9;366(6455):537–543. doi: 10.1038/366537a0. [DOI] [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Shanklin J., Somerville C. Preliminary crystallographic data for stearoyl-acyl carrier protein desaturase from castor seed. J Mol Biol. 1992 May 20;225(2):561–564. doi: 10.1016/0022-2836(92)90941-c. [DOI] [PubMed] [Google Scholar]
- Shanklin J., Somerville C. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2510–2514. doi: 10.1073/pnas.88.6.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin J., Whittle E., Fox B. G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry. 1994 Nov 1;33(43):12787–12794. doi: 10.1021/bi00209a009. [DOI] [PubMed] [Google Scholar]
- Sheriff S., Hendrickson W. A., Smith J. L. Structure of myohemerythrin in the azidomet state at 1.7/1.3 A resolution. J Mol Biol. 1987 Sep 20;197(2):273–296. doi: 10.1016/0022-2836(87)90124-0. [DOI] [PubMed] [Google Scholar]
- Somerville C., Browse J. Plant lipids: metabolism, mutants, and membranes. Science. 1991 Apr 5;252(5002):80–87. doi: 10.1126/science.252.5002.80. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Spatz L., Corcoran D., Rogers M. J., Setlow B., Redline R. Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4565–4569. doi: 10.1073/pnas.71.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sträter N., Klabunde T., Tucker P., Witzel H., Krebs B. Crystal structure of a purple acid phosphatase containing a dinuclear Fe(III)-Zn(II) active site. Science. 1995 Jun 9;268(5216):1489–1492. doi: 10.1126/science.7770774. [DOI] [PubMed] [Google Scholar]
- Stukey J. E., McDonough V. M., Martin C. E. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J Biol Chem. 1990 Nov 25;265(33):20144–20149. [PubMed] [Google Scholar]
- Thiede M. A., Ozols J., Strittmatter P. Construction and sequence of cDNA for rat liver stearyl coenzyme A desaturase. J Biol Chem. 1986 Oct 5;261(28):13230–13235. [PubMed] [Google Scholar]
- Thompson G. A., Scherer D. E., Foxall-Van Aken S., Kenny J. W., Young H. L., Shintani D. K., Kridl J. C., Knauf V. C. Primary structures of the precursor and mature forms of stearoyl-acyl carrier protein desaturase from safflower embryos and requirement of ferredoxin for enzyme activity. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2578–2582. doi: 10.1073/pnas.88.6.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]