Abstract
Background:
The objective of this study was to determine the favorable effects of multi mineral-Vitamin D supplementation on pregnancy outcomes among women at risk for pre-eclampsia.
Methods:
This randomized double-blind controlled clinical trial was conducted among 46 women at risk for pre-eclampsia at 27 weeks’ gestation with positive roll-over test. Pregnant women were randomly assigned to receive either the multi mineral-Vitamin D supplements (n = 23) or the placebo (n = 23) for 9-week. Multi mineral-Vitamin D supplements were containing 800 mg calcium, 200 mg magnesium, 8 mg zinc, and 400 IU Vitamin D3. Fasting blood samples were taken at baseline and after 9-week intervention to measure related factors. Newborn's outcomes were determined.
Results:
Although no significant difference was seen in newborn's weight and head circumference between the two groups, mean newborns’ length (51.3 ± 1.7 vs. 50.3 ± 1.2 cm, P = 0.03) was significantly higher in multi mineral-Vitamin D group than that in the placebo group. Compared to the placebo, consumption of multi mineral-Vitamin D supplements resulted in increased levels of serum calcium (+0.19 vs. −0.08 mg/dL, P = 0.03), magnesium (+0.15 vs. −0.08 mg/dL, P = 0.03), zinc (+8.25 vs. −21.38 mg/dL, P = 0.001) and Vitamin D (+3.79 vs. −1.37 ng/ml, P = 0.01). In addition, taking multi mineral-Vitamin D supplements favorably influenced systolic blood pressure (SBP) (−1.08 vs. 6.08 mmHg, P = 0.001) and diastolic blood pressure (DBP) (−0.44 vs. 3.05 mmHg, P = 0.02).
Conclusions:
Multi mineral-Vitamin D supplementation for 9-week in pregnant women at risk for pre-eclampsia resulted in increased newborn's length, increased circulating levels of maternal serum calcium, magnesium, zinc and Vitamin D, and led to decreased maternal SBP and DBP.
Keywords: Multi mineral-Vitamin D supplementation, pre-eclampsia, pregnancy outcomes
INTRODUCTION
Due to the changes in the women's physiology and the requirements of the growing fetus, pregnancy is associated with additional requirements to several micronutrient including zinc, iron and Vitamin D.[1,2] It is estimated that 57% of pregnant women in the world suffer from calcium deficiency[3] and 31–47.3% from zinc deficiency.[4] Furthermore, the prevalence of Vitamin D deficiency during pregnancy ranges from 18% to 84% in different countries.[5] The prevalence among Kashani pregnant women in Iran women is almost 95.8%.[6] Micronutrient deficiencies during pregnancy might lead to spontaneous abortion, fetal malformation, growth retardation,[7] placental abruption,[8] increased maternal morbidity,[9] low birth weight (LBW) babies,[10] neonatal hypocalcaemia, and also increased incidence of autoimmune diseases.[5]
To reduce maternal and fetal complications in pregnant women at risk for pre-eclampsia, various strategies have been suggested including, but not limited to, diet therapy and physical activity,[11] maternal micronutrient supplementation,[12] increased intake of marine foods[13] and oxidative stress-lowering factors including trace elements (zinc, copper, selenium, and magnesium).[14] Recently, few clinical trials have shown that multi mineral and/or Vitamin D supplementation might improve pregnancy outcomes.[15,16] However, these studies were limited and the findings conflicting.
Calcium and magnesium intake might improve pregnancy outcomes through their effects on reduction of parathyroid calcium release and regulating intracellular calcium concentrations[17] as well as increasing the sensitivity of vascular smooth muscle to nitric oxide (NO).[18] Furthermore, zinc and Vitamin D supplementation might also influence pregnancy outcomes through the influences on regulation of insulin-like growth factor I and its receptor,[19] regulation of gene expression associated with normal implantation and angiogenesis,[20] improvement of insulin sensitivity.[21] Although several studies have examined the effect of single Vitamin or mineral supplementation on pregnancy outcomes, limited data have assessed the combined effect of multi minerals and Vitamin D on these outcomes. Therefore, the current study was conducted to investigate the effects of multi mineral-Vitamin D supplementation on pregnancy outcomes in pregnant women at risk for pre-eclampsia.
METHODS
Participants
This randomized double-blind placebo-controlled clinical trial was performed in Kashan, Iran, during November 2013 to May 2014. For estimating sample size, we used a randomized clinical study sample size formula where type one (α) and type two errors (β) were 0.05 and 0.20 (power = 80%), respectively. Based on a previous study[22] and considering newborns’ weight at birth as a key variable, we considered 0.4 as standard deviation and 0.3 kg as the difference in the mean (d). According to this, we needed 22 subjects in each group to have 80% study power. Pregnant women at risk for pre-eclampsia with positive roll-over test, primigravida, aged 18–40 years old who were carrying singleton pregnancy at their third trimester were recruited in this study. Gestational age was assessed from the date of last menstrual period and concurrent clinical assessment.[23] Individuals with the above-mentioned inclusion criteria were called for participation in the study from among those that attended maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Iran. A total of 70 women that attended maternity clinics affiliated to Kashan University of Medical Sciences, Kashan, Iran, were screened for risk of pre-elampsia, of whom 52 met the inclusion criteria. We did not include those with maternal severe pre-eclampsia, intra-uterine fetal death, premature preterm rupture of membrane (PPROM), completed bed rest (CBR), placenta abruption, preterm delivery, and gestational diabetes mellitus (GDM). A total of 52 pregnant women were recruited in the study and were randomly assigned to receive either the placebo (n = 26) or multi mineral-Vitamin D supplements (n = 26) for 9-week. The study was performed according to the guidelines laid down in the Declaration of Helsinki. The Ethical Committee of Kashan University of Medical Sciences approved the study and informed written consent was obtained from all participants.
Study design
Participants considered as high risk for pre-eclampsia when they had positive roll-over test.[24] At study baseline (25 weeks of gestation), subjects were randomly assigned to receive either the placebo or multi mineral-Vitamin D supplements for 9-week. Random assignment was done by the use of computer-generated random numbers. A trained midwife at maternity clinic enrolled participants. Participants were asked not to alter their routine physical activity or usual diets and not to consume any supplements other than the one provided to them by the investigators. The placebo was provided by Share Darou Pharma Co., Tehran, Iran. The multi mineral-Vitamin D supplements (Calcicare) were containing 800 mg calcium, 200 mg magnesium, 8 mg zinc and 400 IU Vitamin D3 and provided by Vitane Pharma Co., Wolfratshausen, Germany. All subjects were also consuming 400 μg/d folic acid from the beginning of pregnancy and 50 mg ferrous sulfate from the second trimester. We kept all supplements in a cool temperature before using. Compliance with the consumption of supplements was monitored once a week through phone interviews. The compliance, as well as dietary intakes of study participants, were also double-checked by the use of 3-day dietary records completed throughout the study. To obtain nutrient intakes of participants based on these 3-day food diaries, we used Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods.
Assessment of variables
Anthropometric measurements of pregnant women were assessed at baseline (25 weeks of gestation) and after 9-week of intervention (34 weeks of gestation). Body weight was measured in an overnight fasting state, without shoes and in a minimal clothing state using a digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was measured using a nonstretched tape measure (Seca, Hamburg, Germany) to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight in kg divided by height in meters squared. Newborn's length and weight were measured using standard methods (Seca 155 Scale, Hamburg, Germany) during the first 24 h after birth and were recorded to the nearest 1 mm and 10 g, respectively. Newborn's head circumference was measured to the nearest 1 mm with a Seca girth measuring tape. Fasting blood samples (10 ml) from pregnant women were taken at baseline and after 9-week intervention at Kashan reference laboratory in an early morning after an overnight fast. Serum samples were analyzed for serum calcium, magnesium, zinc, iron and 25-hydroxy Vitamin D levels. Serum calcium, magnesium and iron concentrations were assayed using commercial kits (Pars Azmun Inc., Tehran, Iran). Serum zinc concentrations were examined using the appropriate kit (Elitech, France). Serum 25-hydroxy Vitamin D levels were quantified by ELISA using available kits (IDS, Boldon, UK). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) was determined via a sphygmomanometer (ALPK2, Zhejiang, China). Blood pressure values were reported in millimeters of mercury.
Statistical analysis
To ensure the normal distribution of variables, Histogram and Kolmogorov–Smirnov test was applied. We used paired-samples t-test to identify within-group differences. Independent samples Student's t-test was used to detect differences between groups. Pearson Chi-square test was used to detect an association between categorical variables. P <0.05 was considered as statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Among individuals in the placebo group, three women (PPROM [n = 1], GDM [n = 1] and severe pre-eclampsia [n = 1]) were excluded. The exclusions in the multi mineral-Vitamin D group was three persons (CBR [n = 1], severe pre-eclampsia [n = 1] and placenta abruption [n = 1]). Finally, 46 participants (placebo [n = 23] and multi mineral-Vitamin D supplements [n = 23]) completed the trial [Figure 1].
Figure 1.
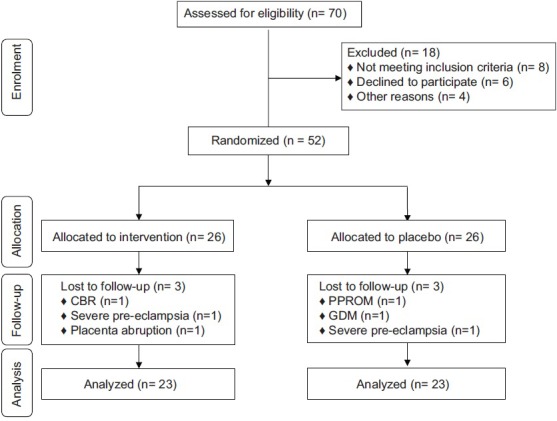
Summary of patient flow
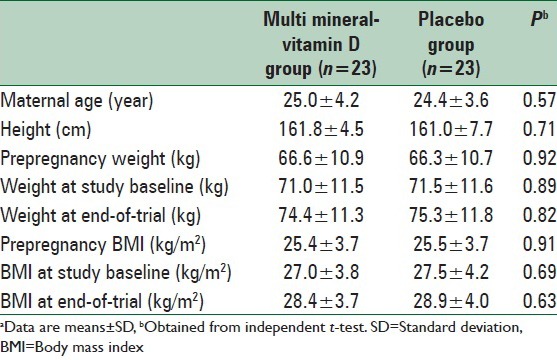
The mean age of study participants was not statistically different between multi mineral-Vitamin D and placebo groups. Baseline prepregnancy weight and BMI, as well as their means before and after intervention, were not significantly different between the two groups [Table 1].
Table 1.
General characteristics of the study participantsa
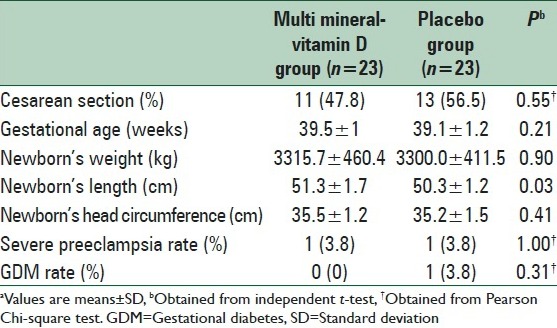
Although no significant difference was seen in newborn's weight and head circumference between the two groups, mean newborns’ length (51.3 ± 1.7 vs. 50.3 ± 1.2 cm, P = 0.03) was significantly higher in multi mineral-Vitamin D group than that in the placebo group [Table 2]. The supplementation did not significantly influence mode of delivery.
Table 2.
The effect of multi mineral-Vitamin D supplementation on pregnancy outcomesa
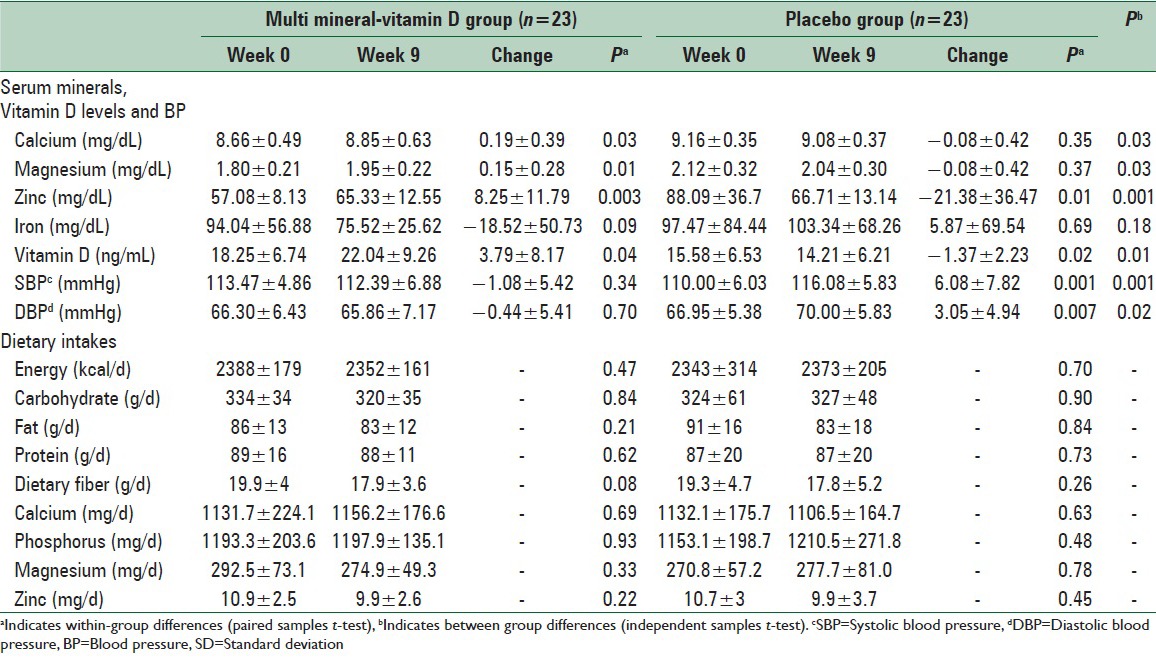
As compared to the placebo, consumption of multi mineral-Vitamin D supplements resulted in increased levels of serum calcium (+0.19 vs. −0.08 mg/dL, P = 0.03), magnesium (+0.15 vs. −0.08 mg/dL, P = 0.03), zinc (+8.25 vs. −21.38 mg/dL, P = 0.001) and Vitamin D (+3.79 vs. −1.37 ng/ml, P = 0.01) [Table 3]. Additionally, taking multi mineral-Vitamin D supplements favorably influenced SBP (−1.08 vs. 6.08 mmHg, P = 0.001) and DBP (−0.44 vs. 3.05 mmHg, P = 0.02). We did not find a significant difference in mean changes of maternal serum iron levels comparing the two groups. Within-group comparisons in the multi mineral-Vitamin D group revealed a significant increase in serum calcium (+0.19 mg/dL, P = 0.03), magnesium (+0.15 mg/dL, P = 0.01), zinc (+8.25 mg/dL, P = 0.003) and Vitamin D levels (+3.79 ng/ml, P = 0.04). In addition, within-group comparisons in placebo group revealed a significant reduction in serum zinc (−21.38 mg/dL, P = 0.01), Vitamin D levels (−1.37 ng/ml, P = 0.02), and a significant increase in SBP and DBP (+6.08 mmHg; P = 0.001, +3.05 mmHg; P = 0.007, respectively). We found no statistically significant difference between the two groups in terms of dietary intakes of energy, carbohydrate, fat, protein, dietary fiber, calcium, phosphorus, magnesium, and zinc throughout the study.
Table 3.
Means (±SD) of serum minerals, vitamin D levels, BP and dietary intakes at baseline and after the intervention
DISCUSSION
This study showed that consumption of multi mineral-Vitamin D supplements for 9-week among pregnant women at risk for pre-eclampsia resulted in a significant increase in newborn's length, maternal serum calcium, magnesium, zinc and Vitamin D levels, and also a significant reduction in maternal SBP and DBP. We failed to find any significant effect of multi mineral-Vitamin D supplementation on gestational age, mode of delivery, newborns’ weight, and head circumference as compared to the placebo.
Pregnant women are susceptible to micronutrients deficiency especially calcium, zinc and Vitamin D due to the growing fetus and the development of the fetal skeleton in the third trimester. Micronutrients deficiency during pregnancy would result in several complications in maternal and fetal life.[9,10] Our data showed that the use of multi mineral-Vitamin D supplements for 9-week among pregnant women at risk for pre-eclampsia led to increased newborns’ length, but did not affect newborns’ weight and head circumference. This finding was in line with the reports of Sabour et al.[25] where higher newborns’ length was seen in mothers who had adequate calcium and Vitamin D intake than those with the inadequate intake. However, oral magnesium supplementation (365 mg/d) among normotensive primigravid mothers who were between 13 and 24 weeks of gestation did not result in a significant difference in SBP, DBP, incidence of pre-eclampsia, fetal growth retardation, preterm labor, birth weight and gestational age at delivery compared with placebo.[26] Maternal supplementation with folic acid + iron + zinc has led to an increase in mean height among children aged 6–8 years.[27] Mild to moderate maternal zinc deficiency has also resulted in LBW, intra-uterine growth retardation, and preterm delivery, whereas severe zinc deficiency led to abortion and congenital malformations.[28] Furthermore, Kalra et al.[29] has reported that the intake of either one oral dose of 1500 μg Vitamin D3 or two doses of 3000 μg Vitamin D3 in the second and third trimesters has been resulted in increased birth weight, length, and head circumference. The particular mechanisms through which prenatal multi mineral-Vitamin D supplementation may have influenced postnatal growth patterns are unknown. Zinc plays an important role in the regulation of insulin-like growth factor I and its receptor,[19] thereby may be act as an activator of insulin-like growth factor I activity within osteoblasts and promoting bone growth.[30] Furthermore, Vitamin D might be associated with a taller knee-heel length, suggesting influences on long bone growth.[31]
The current study revealed that consumption of multi mineral-Vitamin D supplements for 9-week among pregnant women at risk for pre-eclampsia resulted in a significant increase in maternal serum calcium, magnesium, zinc and Vitamin D levels. Inconsistent with our study, Firouzabadi et al.[32] observed increased levels of serum calcium and Vitamin D with 1000 mg/day plus Vitamin D 100,000 IU/month supplementation for 6 months in infertile women with polycystic ovary syndrome. Supplementation with 30 mg elemental zinc during the last 2 trimesters of pregnancy has also been linked to higher serum zinc concentrations in the zinc-supplemented group than in the placebo group.[33] Similar increases in serum Vitamin D and calcium levels were also documented with use of Vitamin D[34] and calcium[16] supplements during pregnancy.
We demonstrated that multi mineral-Vitamin D supplementation for 9-week among pregnant women at risk for pre-eclampsia led to a significant reduction of maternal SBP and DBP. Pfeifer et al.[35] has shown that receiving 1200 mg calcium plus 800 IU Vitamin D3 compared with 1200 mg calcium/day for 8 weeks in elderly women resulted in increased serum Vitamin D and decreased SBP, but did not influence DBP. Oral magnesium supplementation (600 mg of pidolate magnesium daily) for a 12-week period has also been resulted in significant reductions in mean 24-h systolic and in patients with mild hypertension.[36] In addition, the use of 200 mg magnesium, 30 mg zinc, 200 mg Vitamin C and 150 mg Vitamin E significantly reduced SBP, DBP and mean blood pressure in type 2 diabetic patients after 3 months.[37] The same findings have also been reported with Vitamin D supplementation in hypertensive patients after 3 months.[38] Several mechanisms can explain the beneficial effects of multi mineral-Vitamin D supplementation on blood pressure. Firstly, calcium may act as a regulatory factor of the renin-angiotensin system and thus might result in blood pressure regulation via altering cellular concentrations of sodium and calcium ions.[38] Secondly, magnesium might have antihypertensive effects due to increased sensitivity of vascular smooth muscle to NO or decreased production of vasoconstrictor prostanoids.[18] Furthermore, Vitamin D has a critical role in the regulation of the renin-angiotensin system.[38,39]
Several limitations must be considered in the interpretation of our findings. Small sample size was a main limitation of our study. Further trials with a large sample size would be needed to confirm our findings. Moreover, due to budget limitations, we were unable to assess the effect of supplementation in the later life of born babies. In the current study, we could not examine the effects of multi mineral-Vitamin D supplementation on the biochemical indicators of newborn infants. Furthermore, other indicators of pregnancy outcome, including hospitalization in Intensive Care Unit could not be assessed.
In conclusion, multi mineral-Vitamin D supplementation for 9-week in pregnant women at risk for pre-eclampsia resulted in increased newborn's length, increased circulating levels of maternal serum calcium, magnesium, zinc and Vitamin D, and led to decreased maternal SBP and DBP, but did not influence gestational age at delivery, mode of delivery, newborns’ weight and head circumference.
ACKNOWLEDGEMENTS
The present study was supported by a grant from the Vice-chancellor for Research, KUMS, and Iran. The authors would like to thank the staff of Naghavi and Shaheed Beheshti Clinics (Kashan, Iran) for their assistance in this project.
Footnotes
Source of Support: The study was supported by a grant (no. 9304) from Kashan University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.King JC. Determinants of maternal zinc status during pregnancy. Am J Clin Nutr. 2000;71:1334S–43. doi: 10.1093/ajcn/71.5.1334s. [DOI] [PubMed] [Google Scholar]
- 2.Hess SY, King JC. Effects of maternal zinc supplementation on pregnancy and lactation outcomes. Food Nutr Bull. 2009;30:S60–78. doi: 10.1177/15648265090301S105. [DOI] [PubMed] [Google Scholar]
- 3.Darwish AM, Mohamad SN, Gamal Al-Din HR, Elsayed YA, Ahmad SI. Prevalence and predictors of deficient dietary calcium intake during the third trimester of pregnancy: The experience of a developing country. J Obstet Gynaecol Res. 2009;35:106–12. doi: 10.1111/j.1447-0756.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 4.Gebremedhin S, Enquselassie F, Umeta M. Prevalence of prenatal zinc deficiency and its association with socio-demographic, dietary and health care related factors in rural Sidama, Southern Ethiopia: A cross-sectional study. BMC Public Health. 2011;11:898. doi: 10.1186/1471-2458-11-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of Vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(429):e1–9. doi: 10.1016/j.ajog.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asemi Z, Taghizadeh M, Sarahroodi S, Jazayeri S, Tabasi Z, Seyyedi F. Assessment of the relationship of Vitamin D with serum antioxidant Vitamins E and A and their deficiencies in Iranian pregnant women. Saudi Med J. 2010;31:1119–23. [PubMed] [Google Scholar]
- 7.Ashworth CJ, Antipatis C. Micronutrient programming of development throughout gestation. Reproduction. 2001;122:527–35. [PubMed] [Google Scholar]
- 8.Pathak P, Kapil U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J Pediatr. 2004;71:1003–5. doi: 10.1007/BF02828116. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri S. Prevalence of micronutrient deficiency particularly of iron, zinc and folic acid in pregnant women in South East Asia. Br J Nutr. 2001;85(Suppl 2):S87–92. [PubMed] [Google Scholar]
- 10.Pathak P, Kapil U, Kapoor SK, Saxena R, Kumar A, Gupta N, et al. Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana. Indian J Pediatr. 2004;71:1007–14. doi: 10.1007/BF02828117. [DOI] [PubMed] [Google Scholar]
- 11.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with Vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204(503):e1–12. doi: 10.1016/j.ajog.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imhoff-Kunsch B, Briggs V, Goldenberg T, Ramakrishnan U. Effect of n-3 long-chain polyunsaturated fatty acid intake during pregnancy on maternal, infant, and child health outcomes: A systematic review. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):91–107. doi: 10.1111/j.1365-3016.2012.01292.x. [DOI] [PubMed] [Google Scholar]
- 14.Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD. Trace elements and antioxidant enzymes associated with oxidative stress in the pre-eclamptic/eclamptic mothers during fetal circulation. Clin Nutr. 2012;31:946–50. doi: 10.1016/j.clnu.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: A cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202. doi: 10.1093/ajcn/78.6.1194. [DOI] [PubMed] [Google Scholar]
- 16.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and calcium: A systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;183:1–420. [PMC free article] [PubMed] [Google Scholar]
- 17.Imdad A, Jabeen A, Bhutta ZA. Role of calcium supplementation during pregnancy in reducing risk of developing gestational hypertensive disorders: A meta-analysis of studies from developing countries. BMC Public Health. 2011;11(Suppl 3):S18. doi: 10.1186/1471-2458-11-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbagallo M, Dominguez LJ, Resnick LM. Magnesium metabolism in hypertension and type 2 diabetes mellitus. Am J Ther. 2007;14:375–85. doi: 10.1097/01.mjt.0000209676.91582.46. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000;130:1500S–8. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 20.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–55. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Sandler L, Muñoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: A prospective study. J Clin Endocrinol Metab. 2002;87:1563–8. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 22.Roth DE, Perumal N, Al Mahmud A, Baqui AH. Maternal Vitamin D3 supplementation during the third trimester of pregnancy: Effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr. 2013;163:1605–11.e3. doi: 10.1016/j.jpeds.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Jehan I, Zaidi S, Rizvi S, Mobeen N, McClure EM, Munoz B, et al. Dating gestational age by last menstrual period, symphysis-fundal height, and ultrasound in urban Pakistan. Int J Gynaecol Obstet. 2010;110:231–4. doi: 10.1016/j.ijgo.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziaei S, Hantoshzadeh S, Rezasoltani P, Lamyian M. The effect of garlic tablet on plasma lipids and platelet aggregation in nulliparous pregnants at high risk of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2001;99:201–6. doi: 10.1016/s0301-2115(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 25.Sabour H, Hossein-Nezhad A, Maghbooli Z, Madani F, Mir E, Larijani B. Relationship between pregnancy outcomes and maternal Vitamin D and calcium intake: A cross-sectional study. Gynecol Endocrinol. 2006;22:585–9. doi: 10.1080/09513590601005409. [DOI] [PubMed] [Google Scholar]
- 26.Sibai BM, Villar MA, Bray E. Magnesium supplementation during pregnancy: A double-blind randomized controlled clinical trial. Am J Obstet Gynecol. 1989;161:115–9. doi: 10.1016/0002-9378(89)90246-9. [DOI] [PubMed] [Google Scholar]
- 27.Stewart CP, Christian P, LeClerq SC, West KP, Jr, Khatry SK. Antenatal supplementation with folic acid+iron+zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90:132–40. doi: 10.3945/ajcn.2008.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah D, Sachdev HP. Effect of gestational zinc deficiency on pregnancy outcomes: Summary of observation studies and zinc supplementation trials. Br J Nutr. 2001;85(Suppl 2):S101–8. doi: 10.1079/bjn2000301. [DOI] [PubMed] [Google Scholar]
- 29.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of Vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–8. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 30.Matsui T, Yamaguchi M. Zinc modulation of insulin-like growth factor's effect in osteoblastic MC3T3-E1 cells. Peptides. 1995;16:1063–8. doi: 10.1016/0196-9781(95)00067-t. [DOI] [PubMed] [Google Scholar]
- 31.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyVitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–12. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 32.Firouzabadi Rd, Aflatoonian A, Modarresi S, Sekhavat L, MohammadTaheri S. Therapeutic effects of calcium & Vitamin D supplementation in women with PCOS. Complement Ther Clin Pract. 2012;18:85–8. doi: 10.1016/j.ctcp.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Osendarp SJ, van Raaij JM, Arifeen SE, Wahed M, Baqui AH, Fuchs GJ. A randomized, placebo-controlled trial of the effect of zinc supplementation during pregnancy on pregnancy outcome in Bangladeshi urban poor. Am J Clin Nutr. 2000;71:114–9. doi: 10.1093/ajcn/71.1.114. [DOI] [PubMed] [Google Scholar]
- 34.De-Regil LM, Palacios C, Ansary A, Kulier R, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term Vitamin D (3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 36.Hatzistavri LS, Sarafidis PA, Georgianos PI, Tziolas IM, Aroditis CP, Zebekakis PE, et al. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am J Hypertens. 2009;22:1070–5. doi: 10.1038/ajh.2009.126. [DOI] [PubMed] [Google Scholar]
- 37.Farvid MS, Jalali M, Siassi F, Saadat N, Hosseini M. The impact of Vitamins and/or mineral supplementation on blood pressure in type 2 diabetes. J Am Coll Nutr. 2004;23:272–9. doi: 10.1080/07315724.2004.10719370. [DOI] [PubMed] [Google Scholar]
- 38.Goel RK, Lal H. Role of Vitamin d supplementation in hypertension. Indian J Clin Biochem. 2011;26:88–90. doi: 10.1007/s12291-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90 doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]


