Abstract
Background:
Cetuximab is a monoclonal antibody which acts against the epidermal growth-factor receptor. Randomized controlled trials show that the addition of cetuximab to folinic acid, 5-flourouracil, irinotecan (FOLFIRI), folinic acid, 5-flourouracil, oxaliplatin (FOLFOX) and capecitabin + oxaliplatin (CAPOX) regimens, as the first-line treatment for metastatic colorectal cancer (CRC), increases the overall survival (OS) and progression-free survival (PFS) compared to FOLFIRI, FOLFOX and CAPOX regimens alone. The aim of this study was to analyze the cost-effectiveness of different treatment programs for managing metastatic CRC with and without cetuximab in the first-line treatment of unresectable metastatic CRC in Iran.
Methods:
A systematic search of the literature was performed in PubMed, Centre for Reviews and Dissemination Databases and Cochrane Library to assess the effectiveness of the drug in the context of PFS, OS and the adverse events. The incremental cost-effectiveness ratio of each treatment program was calculated. An extensive sensitivity analysis was conducted on the results regarding the effectiveness.
Results:
The addition of cetuximab to FOLFIRI, FOLFOX and CAPOX programs increased PFS by 0.1, 0.042 and 0.042 years, respectively. Similarly, the addition of cetuximab to FOLFIRI, FOLFOX and CAPOX increased OS by 0.325, 0.442 and 0.442 years and also cost $212825, $202484 and $204198 individually. Whereas, based on the World Health Organisation (WHO) suggested threshold for cost-effectiveness analysis, even FOLFOX + cetuximab was very higher than the threshold in Iran (37.4 times higher).
Conclusions:
The FOLFOX regimen + cetuximab provides lower costs per additional life years gained (more cost-effective) compared with its alternatives in the treatment of patients with unresectable metastatic CRC. However, according to the WHO indicator, none of the cetuximab regimens could be considered as cost effective for the Iranian health care market.
Keywords: Cetuximab, colorectal cancer, cost-effectiveness, effectiveness, kristen rat sarcoma
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer-related deaths in the world.[1] The incidence of this cancer has increased in past years steadily.[2,3] In 2008, the average lifetime cost of managing a case of CRC was reported to be 39,607 Euros in Ireland.[4] Various health care services including chemotherapy, radiotherapy and surgery are available for managing different stages of CRC. Biological agents such as cetuximab and bevacizumab are usually used in Stage IV treatments of (metastatic) CRC.[5]
The epidermal growth-factor receptor (EGFR) is known as a clinically validated anticancer molecular target. This factor is usually expressed in colorectal tumors.[6,7,8] Cetuximab is an immunoglobulin G1 monoclonal antibody, specifically targeting the EGFR, and competitively inhibiting ligand binding and ligand-dependent downstream signaling.[9,10,11] Randomized controlled trials (RCTs) show that the addition of cetuximab to folinic acid, 5-flourouracil, irinotecan (FOLFIRI), folinic acid, 5-flourouracil, oxaliplatin (FOLFOX) and capecitabin + oxaliplatin (CAPOX) regimens, as the first line treatment for metastatic CRC increases the overall survival (OS) and progression-free survival (PFS) compared with FOLFIRI, FOLFOX and CAPOX regimens alone. Multiple studies have shown that while patients with wild-type (normal or nonmutated) sequence of v-k-ras 2 kristen rat sarcoma viral oncogene homolog (KRAS) have a favorable response to cetuximab, patients with mutations in codons 12 or 13 do not benefit from cetuximab.[12,13,14,15,16,17] These findings strongly suggest that patients without KRAS mutations are regarded as candidates of treatment with cetuximab.[18] Several RCTs have shown that cetuximab increases PFS and OS in patients with wild-type KRAS gene.[17,19] Nonetheless, as there are limited resources for pharmaceutical services, it is crucial to evaluate the costs and the consequences of cetuximab in managing metastatic CRC. This evaluation could help healthcare decision makers to set priorities and allocate scarce resources more efficiently.
The aim of this study was to undertake a comparative cost-effectiveness analysis of different treatment programs for managing metastatic CRC with and without cetuximab in the first-line treatment of unresectable metastatic CRC in Iran.
METHODS
Common chemotherapy regimens in the treatment of metastatic CRC are FOLFIRI, FOLFOX, and CAPOX. All of these regimens could be used with or without cetuximab.[20] We selected these therapeutic regimens to evaluate and compare the effectiveness, cost, and incremental cost-effectiveness ratio (ICER) of these treatment programs.
Measuring effectiveness
A systematic search of the literature was performed in PubMed, Cochrane Library, and Centre for Review and Dissemination database on April 17, 2011, to identify all studies which would meet our inclusion criteria. The search was conducted using keywords such as cetuximab, metastatic CRC, KRAS, and effectiveness.
We established as inclusion criteria all RCTs which evaluated cetuximab in combination with chemotherapy regimens of FOLFIRI, FOLFOX, and CAPOX in the first-line treatment of metastatic CRC considering the role of KRAS in their treatment. The RCTs, which evaluated cetuximab in cases other than Stage IV (metastatic) in the subsequent line treatment of metastatic CRC without considering the role of KRAS in their treatment, were excluded from this study.
OS and PFS were considered as the main and positive outcomes of the treatments. OS and PFS are defined as the time period of randomization to death and as the time period of randomization to free disease progression or death, respectively.[21]
Measuring costs
In order to evaluate the costs of treatment programs with or without cetuximab, the total direct medical costs were considered in this study. Activity-based costing (ABC) was used to calculate the cost of medical services. That is the number of medical services and their costs in each treatment programs (with and without cetuximab) including medication, outpatient injections, required tests before chemotherapy, drugs administration and managing adverse events of chemotherapy were calculated. Similar medical services and their costs (i.e. hospitalization and oncologist visits) in two groups were ignored to avoid unnecessary calculations.[22,23] Similarly, only high prevalent and important adverse effects of the chemotherapies were considered. The total prices of physician visits and prescribed medicines were calculated to evaluate the cost of managing adverse events.
To calculate the cost of chemotherapy in patients with unresectable metastatic CRC, the required amounts of medicine were estimated based on the mean body surface area of a normal human (1.73 m2).[24]
All the prices of medicines and medical services at the end of the Iranian financial year of 1388 were considered in the calculation of the costs of treatments. The prices were extracted from the Food and Drug Deputy and the Therapeutic Deputy of the Ministry of Health and Medical Education.[25]
Measuring cost-effectiveness
Incremental cost per OS (life years gained [LYG]) and incremental cost per progression-free LYG (PFLYG) for FOLFIRI, FOLFOX, and CAPOX regimens compared with these treatment programs + cetuximab in the first-line treatment of metastatic CRC are reported in this study. The recommended World Health Organisation (WHO) threshold[26] was considered to conclude which treatment programs are cost effective for the Iranian health care market.
Sensitivity analysis
Because of limitations in the number of RCTs, an extensive sensitivity analysis was conducted on the effectiveness results to evaluate whether the conclusion of the study is sensitive to the results extracted from the RCTs.
RESULTS
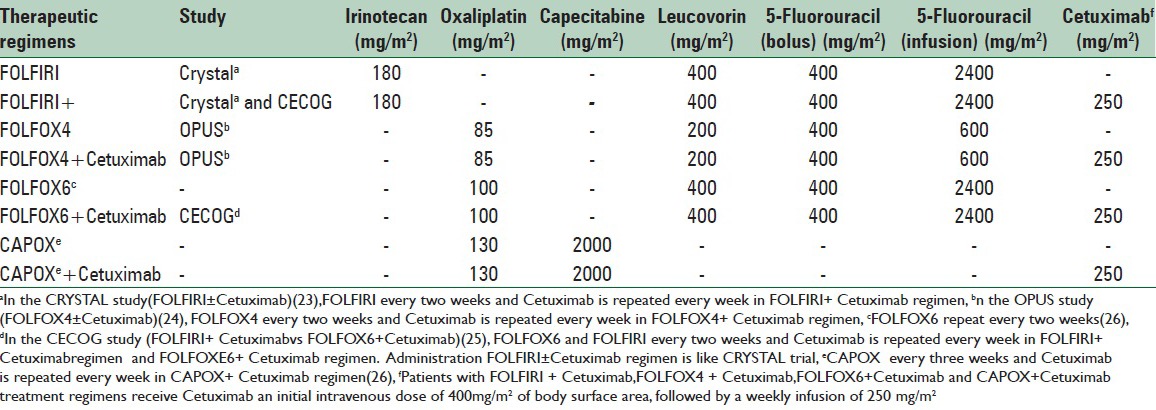
Only three of the RCTs met the inclusion and exclusion criteria of this study; which were CRYSTAL,[27] OPUS,[28] and CECOG[29] RCTs. The review of the literature showed that each of the obtained trials have used different doses to administer drug regimens. Table 1 shows the summary of different doses of drug regimens administrations.
Table 1.
The administration of chemotherapy for treatment regimens
Effectiveness
The details of the effectiveness results are summarized in Table 2. This table shows that the median of PFS in wild-type KRAS patients who received FOLFIRI plus and without cetuximab were 9.9 and 8.7 months, respectively (P = 0.017, hazard ratio = 0.68). This means that the addition of cetuximab increased the PFS of the patients for 1.2 months. Similarly, cetuximab also increased the OS of the patients by 3.9 months (hazard ratio = 0.84). However, the addition of cetuximab in mutant KRAS patients decreased the median PFS of the patients by 0.5 months (P = 0.47, hazard ratio = 1.07). Similarly adding cetuximab to FOLFIRI for these patients reduced the median OS of the patients by 0.2 months (hazard ratio = 1.03).[27]
Table 2.
Summary of the results of randomized controlled trials in the field of efficacy of Cetuximab
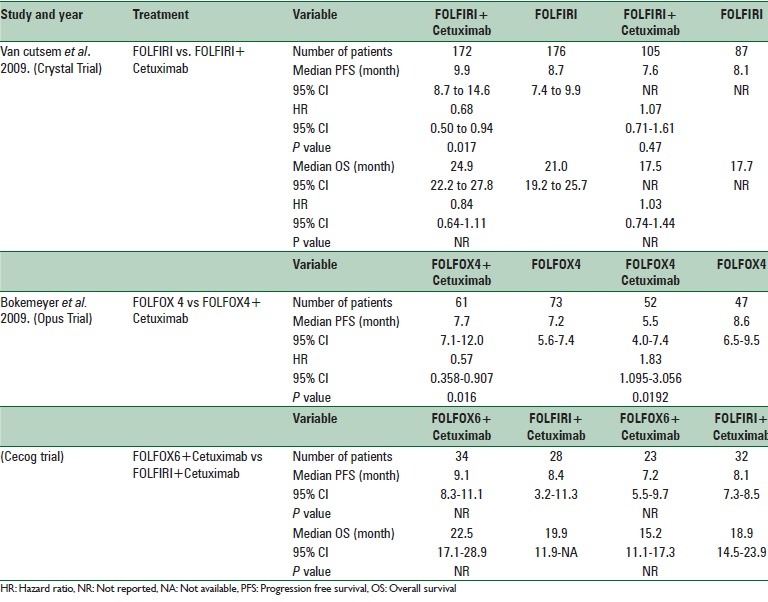
The results of the OPUS trial illustrated that adding cetuximab to the FOLFOX4 regimen in wild-type KRAS patients increased the median PFS of the patients by merely 0.5 months (hazard ratio = 0.57, P = 0.016). However, the addition of cetuximab to the FOLFOX4 regimen for mutant KRAS patients decreased the median PFS by 3.1 months (P = 0.0192, hazard ratio = 1.83).[28]
In the CECOG study, median PFS with FOLFOX6 + cetuximab in the wild-type KRAS patients was 0.7 months higher compared to FOLFIRI + cetuximab; but in mutant KRAS patients the median PFS was 0.9 months lower in FOLFIRI + cetuximab regimen.[29]
Cost
The total costs of treatment programs with and without cetuximab were calculated based on ABC method. The summary results of the cost of various treatment programs are presented in Table 3. As it is clear from the table, chemotherapy was the major cost driver in total costs of the therapies.
Table 3.
The cost of treatment programs with and without Cetuximab
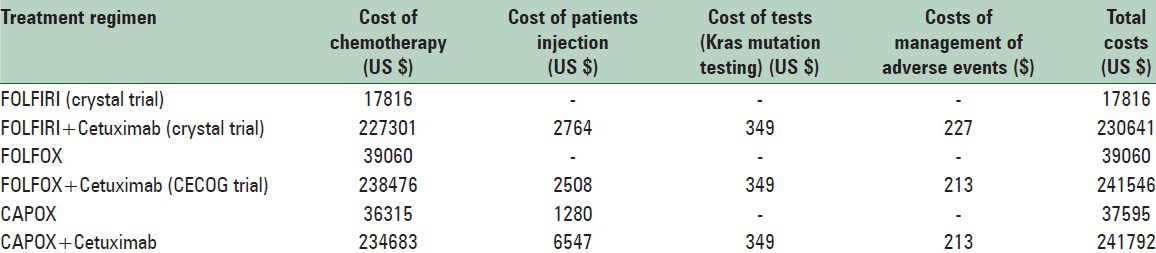
The most common adverse event which was statistically significant in the treatment groups with and without cetuximab was acne like rash.[27,28] Thus, the costs of managing acne-like rash for all cetuximab regimens were calculated.
As Table 3 clearly shows the CAPOX + cetuximab treatment program, with $241792, was the most expensive treatment program. Similarly, FOLFIRI regimen with $17816 was the cheapest treatment program.
Cost-effectiveness
To determine the ICER of each treatment program, the difference between the costs of two treatment programs were divided by the differences in their effectiveness.[22,23]
The Zhao et al. study[30] showed that there were no statistically significant differences between PFS and OS in CAPOX and FOLFOX regimens. In this study, the median PFS was 7.1 months for the CAPOX group and 8.6 months for the FOLFOX group, and there was no significant difference in PFS between the two groups (P = 0.19). While the median OS was 17.2 months in the CAPOX group, it was 18.8 months in the FOLFOX group, but there was no significant difference between the two groups (P = 0.47). The results of this study show that CAPOX is equivalent to FOLFOX in terms of PFS and OS in the first-line treatment for patients with metastatic CRC.[30] Furthermore, the result of OS in FOLFOX regimens, considering our inclusion and exclusion criteria, were not available in the literature, therefore we used the results of OS in CAPOX regimens as 17.2 months,[30] instead of FOLFOX and then estimated the cost per LYG accordingly. Similarly, given that the result of OS in CAPOX + cetuximab regimens were not available in the literature, we used the results of OS in FOFOX + cetuximab regimens instead of CAPOX + cetuximab as 22.5 months[29] and estimated the cost per LYG accordingly. These assumptions enable us to estimate the ICER of these four regimens mathematically.
For calculating the incremental cost per PFLYG of CAPOX ± cetuximab treatment programs, we also used the efficacy results (PFS) of the OPUS study (FOLFOX4 ± cetuximab).
As Table 4 shows, FOLFOX + cetuximab with $458113 per additional LYG provide the lowest (best) ICER compared to the other two alternatives. Similarly, FOLFIRI + cetuximab with $654846 per additional LYG offers the highest (worst) ICER compared to the other two alternatives. However, based on the WHO threshold for the cost-effectiveness analysis, less than 3 times GDP per capita,[26] it is obvious that even the cost of FOLFOX + cetuximab regimen is much higher than the threshold of Iran (37.4 times higher). The GDP per capita for Iran was $12258.[31]
Table 4.
The ICER of the treatment programmes and sensitivity analysis
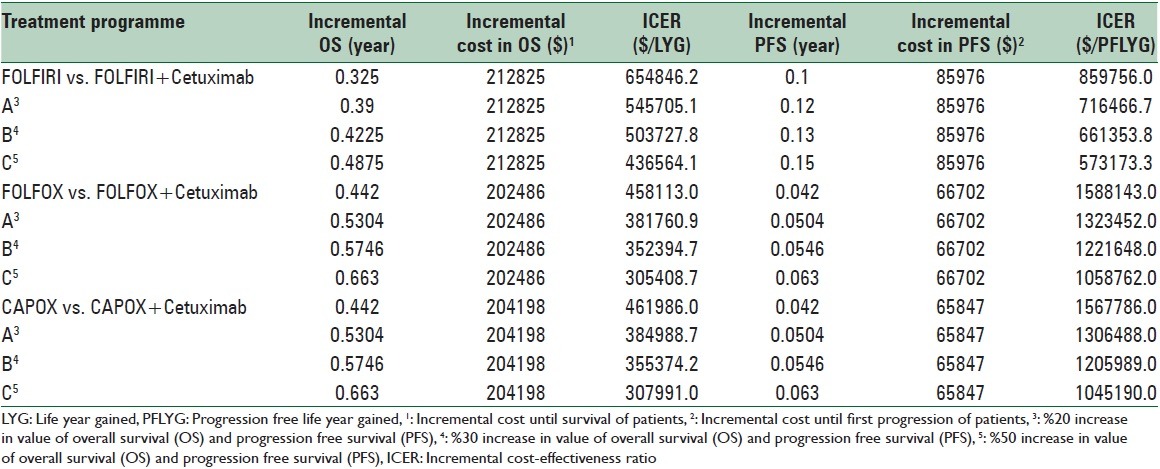
The results of the extensive sensitivity analysis showed that, although a %50 increase in OS would decrease the ICER to $305408.7 per additional LYG, the results are still much higher than the WHO suggested threshold (24.9-fold higher). The summary of the ICER of the treatment programs and the results of sensitivity analysis are presented in Table 4.
DISCUSSION
The aim of this study was to analyze the cost-effectiveness of different therapeutic regimens of cetuximab in the first-line treatment of metastatic CRC in Iran.
CRYSTAL,[27] OPUS,[28] and CECOG studies show that the application of the FOLFIRI, FOLFOX and CAPOX therapeutic regimens in combination with cetuximab as the first-line treatment for metastatic CRC would increase both OS and PFS in patients with wild-type KRAS. These studies also confirmed that adding cetuximab to the basic therapeutic regimens of patients with tumors carrying KRAS mutations do not increase the OS and PFS of the CRC patients, and also cause considerable reductions in the efficacy of the basic regimens.[27,28,29] Even though the cost of detecting KRAS mutation before administration of cetuximab is fairly high at US$349, this test is placed among the necessary services in the utilization of cetuximab for patients with metastatic CRC.
Comparing the cost and the consequence of cetuximab combined with FOLFIRI, FOLFOX and CAPOX treatment programs in terms of PFS, represents that FOLFIRI + cetuximab treatment program provides a better value for money with the cost of $859756 per additional PFLYG [Table 4]. CAPOX and FOLFOX programs plus cetuximab provide higher cost per additional PFLYG, respectively, (less cost effective) in the treatment of patients with unresectable metastatic CRC.
The ICER of FOLFOX + cetuximab treatment program against the basic FOLFOX regimen confirms a positive but very high cost per additional LYG, with US$ 458113 per LYG; which is 37.4 times higher than the recommended WHO threshold. The results of extensive sensitivity analysis showed that, although a %50 increase in OS would decrease the ICER to $305408.7 per additional LYG, the result is still very high for Iran by considering the suggested threshold by the WHO (24.9-fold higher). The same scenario is repeated for CAPOX and FOLFIRI treatment programs with higher ICER (less cost-effective).
The findings of this study confirm that the result of ICER would differ when considering the cost per PFLYG or cost per LYG. Nonetheless, it seems that focusing on cost per LYG may offer clearer insights into the cost-effectiveness of the therapeutic regimens.
It may also be worth noting that although the prescribing cetuximab is repeated every week, the CAPOX regimen is repeated every three weeks and FOLFIRI and FOLFOX regimens are iterated every 2 weeks. Therefore, the CAPOX regimen may provide a better compliance for patients than other alternative regimens. In addition to less frequency, it is important to note that the administration route of capecitabine in the CAPOX regimen is oral while the components of the other two regimens are taken by injection. These differences may increase the patient's compliance with CAPOX regimen. Putting all these together with considering a fairly small difference in cost per additional LYG by CAPOX + cetuximab and FOLFOX + cetuximab treatment programs it may be implied that CAPOX + cetuximab provides a better compliance with almost the same cost per additional LYG for CRC patients.
The results of the extensive sensitivity analysis illustrated that, although a %50 increase in OS would decrease the value of ICER (more cost effective), conclusions are not sensitive to the variation of the results; even with the %50 changes.
Limitation
The main limitation of this study is that it relies only on the results of three RCTs. The second limitation of this study is the lack of RCT for assessing the clinical effectiveness of CAPOX + cetuximab regimen in managing CRC. The last limitation which is worth mentioning is the small size of the sample in CECOG RCT.
CONCLUSIONS
In summary, the results of this study confirm that the administration of FOLFOX in combination with cetuximab provides a better ICER compared to its alternatives in terms of LYG. However, according to the WHO suggested threshold, none of the cetuximab treatment programs could be considered cost-effective for the Iranian health care market.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–65. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Emami MH, Fatemi AM, Farajzadegan Z, Abtahi SM. Epidemiology of colorectal cancer in isfahan province. Govaresh. 2005;10:134–9. [Google Scholar]
- 3.Shafayan B, Keyhani M. Epidemiological evaluation of colorectal cancer. Acta Med Iran. 2003;41:156–60. [Google Scholar]
- 4.Tilson L, Sharp L, Usher C, Walsh C, SW, O’Ceilleachair A, et al. Cost of care for colorectal cancer in Ireland: A health care payer perspective. Eur J Health Econ. 2012;13:511–24. doi: 10.1007/s10198-011-0325-z. [DOI] [PubMed] [Google Scholar]
- 5.Devita VT, Lawrence TS, Weinberg RA. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2009. DeVita, Hellman and Rosenberg's Cancer: Principle and Practice of Oncology. [Google Scholar]
- 6.Folprecht G, Lutz MP, Schöffski P, Seufferlein T, Nolting A, Pollert P, et al. Cetuximab and irinotecan/5-fluorouracil/folinic acid is a safe combination for the first-line treatment of patients with epidermal growth factor receptor expressing metastatic colorectal carcinoma. Ann Oncol. 2006;17:450–6. doi: 10.1093/annonc/mdj084. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: Implications for a standardized scoring system. Cancer. 2001;92:1331–46. doi: 10.1002/1097-0142(20010901)92:5<1331::aid-cncr1455>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–8. [PubMed] [Google Scholar]
- 10.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–65. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 12.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 13.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 14.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–7. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 16.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 17.Lièvre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 18.Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, Nikiforova MN. The role of KRAS mutation testing in the management of patients with metastatic colorectal cancer. Arch Pathol Lab Med. 2009;133:1600–6. doi: 10.5858/133.10.1600. [DOI] [PubMed] [Google Scholar]
- 19.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Colon Cancer (version 2.) 2011. [Last cited on 2011 Jan 11]. Available from: http://www.Nccn.org/professional/physician-gls/PDF/colon.Pdf .
- 21.Halabi S, Vogelzang NJ, Ou SS, Owzar K, Archer L, Small EJ. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27:2766–71. doi: 10.1200/JCO.2008.18.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drummond MF, Sculpher MJ, Torrance GW, O’ Brien BJ, Storddart GL. 3rd ed. Oxford: Oxford University Press; 2005. Methods of Economic Evalluation of Health Care Programmes. [Google Scholar]
- 23.Gold MR, Russell LB, Siegel JE, Weinstein MC. Oxford: Oxford University Press; 1996. Cost-Effectiveness in Health and Medicine. [Google Scholar]
- 24.Rocco MV. Body surface area limitations in achieving adequate therapy in peritoneal dialysis patients. Perit Dial Int. 1996;16:617–22. [PubMed] [Google Scholar]
- 25.Food and Drug Department, Ministry of Health and Medical Education. [Last accessed on 2010 Mar]. Available from: http://www.fdo.behdasht.gov.ir .
- 26.Switzerland: Cost-effectiveness Thresholds; [Last cited on 2014 Jul 15]. WHO. Available from: http://www.who.int/choice/costs/CER_thresholds/en/ [Google Scholar]
- 27.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 28.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 29.Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol. 2010;16:3133–43. doi: 10.3748/wjg.v16.i25.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao G, Gao P, Yang KH, Tian JH, Ma B. Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: A meta-analysis. Colorectal Dis. 2010;12:615–23. doi: 10.1111/j.1463-1318.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Central Bank of Iran, the Rank of Iran Between 183 Countries. [Last cited on 2014 Jul 15]. Available from: http://www.cbi.ir/showitem/8918.aspx .


