Abstract
Background
Despite endometriosis is common estrogen dependent disease afflicting women in reproductive age, the pathogenesis has not been fully elucidated. Retinoic acid has various functions in cells as biologic modulator, and aberrant retinoid metabolism seems to be involved in the lesions of endometriosis. In order to evaluate the potential of all-trans retinoic acid (ATRA) for therapeutic treatment, a transcriptome analysis and estradiol measurements in cultured endometriotic cells and tissues were conducted.
Methods
The mRNA expression levels in ATRA-treated endometriotic stromal cells (ESC) isolated from ovarian endometrial cysts (OEC) were investigated. Estradiol production in OEC tissues was also investigated.
Results
In the isolated ESC culture supplemented with ATRA for four days, total RNA was extracted followed by a transcriptome analysis using GeneChip. Forty-nine genes were upregulated and four genes were down-regulated by the ATRA treatment. Many upregulated genes were associated with the negative regulation of cellular proliferation. In addition, ATRA treatment decreased the mRNA expression of 17-beta-dehydrogenase 2 (HSD17B2) which converts estradiol into estrone in a dose-dependent manner, and the ELISA measurements indicated that estradiol production in the OEC tissue was inhibited by ATRA treatment.
Conclusions
Retinoic acid has the potential to suppress endometriosis development.
Introduction
Endometriosis is a common gynecological disease affecting approximately 10 % of reproductive age females. This condition is characterized by the ectopic localization of endometrial-like tissue in the pelvic cavity. As a result of the development of the disease, chronic inflammation is induced in the pelvic cavity, and symptoms such as chronic pelvic pain and infertility subsequently affect the patient’s health. Although numerous studies have been conducted to elucidate the pathogenesis including the origin, loss of control of cell proliferation and local steroidogenesis, etc., no clear answers have been obtained thus far.
Vitamin A has diverse essential physiological roles in embryonic development, reproduction, vision, immune cell development, and various nervous functions [1–8]. Vitamin A performs these roles through its derivatives called retinoids. After retinol acid is brought into cells by retinol binding protein, which is the principle and specific carrier of retinol in the blood, and is subsequently combined with retinoic acid receptors that form heterodimers with the retinoid X receptor at specific promoters and modulate transcription, it functions as a biologic modulator affecting immunomodulatory, anti-inflammatory and cell developmental activities, etc. All-trans retinoic acid (ATRA) is the active form of the metabolite of vitamin A and produced from the metabolic conversion of retinol.
The uterine endometrium is a retinoic acid accumulated tissue, and has been recognized as being necessary for normal endometrial cell differentiation and functions [9–12]. Recent studies suggest the possibility that aberrant retinoid metabolism is involved in the pathophysiology of endometriosis [13–18]. Our previous study demonstrated many aberrant DNA methylation lesions accompanying an abnormal mRNA expression in isolated endometriotic stromal cells derived from ovarian endometrial cysts (choESC) [19]. Of these genes, the STRA6 and HSD17B2 genes show an abnormally low expression and high level of DNA methylation in cases of ovarian endometriosis. STRA6 is an essential cell surface receptor for retinol binding protein and is necessary for the uptake of retinol into cells. HSD17B2 converts estradiol into estrone. Therefore, a low expression of STRA6 and HSD17B2 results in the enhanced endogenous synthesis of estradiol. An elevated estradiol concentration within the endometriotic tissue promotes the development of endometriosis. Moreover, reduced ATRA levels are observed in clinical endometriotic lesions compared to the eutopic endometrium [18], and ATRA has inhibitory effects on mouse endometriotic implants in an in vivo endometriosis model [20]. Accumulating evidence showns that an aberrant retinoic acid metabolism is a critical factor for the development of endometriosis.
In this study, we examined the effects of ATRA on the gene expression in isolated and cultured choESC. We also evaluated the effect of ATRA on estradiol production, the key modulator of endometriosis development.
Material and methods
The study protocol was reviewed and approved by the Institutional Review Board of Yamaguchi University Graduate School of Medicine. Informed consent was obtained from the participants before the collection of any samples. All experiments involving the handling of human cells and tissues were performed in accordance with the tenets of the Declaration of Helsinki.
ESC isolation, culture and total RNA isolation
Ovarian endometrial cysts (OEC) were obtained from three subjects (aged 24 – 39 years) during the proliferative phase. None of the subjects used any hormonal therapy for at least 3 months prior to operation. ESC was isolated as previously reported [19]. Briefly, OCE were washed with phenol red-free Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Paisley, UK) containing Glutamax (Invitrogen), 50 mg/ml of streptomycin (Invitrogen) and 50 IU/ml of penicillin (Invitrogen) and then minced into small pieces measuring <1 mm3. Then, enzymatic digestion of the minced tissues with 0.2 % collagenase (Sigma, St. Louis, MO, USA) was performed in a shaking incubator for two hours at 37 °C, after which the endometrial stromal cells were separated using filtration through a 70 mm nylon mesh. The filtrates were washed three times. The choESC were seeded in 75 cm2 tissue culture flasks and grown until confluence in phenol red-free DMEM containing Glutamax, antibiotics and 10 % dextran-coated charcoal-stripped fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel) at 37 °C in 95 % air and 5 % CO2. The homogeneity of the isolated ESC preparation was 98 %, which was verified by immunocytochemistry using an antibody against vimentin, a specific marker of stromal cells. If necessary, the cells were subcultured in another 75 cm2 tissue culture flask.
In order to investigate transcriptional changes after ATRA treatment in the cultured choESC, ATRA was added to the media at a final concentration of 10−7 M. Separately, 10−9, 10−8 and 10−7 M of ATRA was added to the media for the HSD17B2-mRNA expression analysis. DMSO was added to the media as a vehicle control. The medium was changed every other day, and after four days of incubation, the cells were harvested and frozen at−80 °C until RNA extraction.
Total RNA was extracted using an RNeasy kit from QIAGEN (Valencia, CA, USA) in accordance with the manufacturer’s instructions.
Transcriptome analysis
In order to evaluate the integrity of the RNA, a microfluidic analysis was performed using an Agilent 2100 Bioanalyzer with the RNA6000 nano kit (Agilent Technologies, Palo Alto, CA, USA). For the microarray analysis, we used only RNA samples whose RNA integrity number (RIN) was greater than 8.5. The gene expression was analyzed using a GeneChip® Human Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA) containing 764,885 probes (and supporting 28,869 genes). Target cDNA was prepared from 200 ng of total RNA with the Ambion® WT Expression kit (Ambion, Austin, TX, USA) and the Affymetrix® GeneChip® WT Terminal Labeling kit (Affymetrix). Hybridization to the microarrays, washing, staining and scanning were performed using the GeneChip® system (Affymetrix) composed of a Scanner 3000 7G Workstation Fluidics 450 and a Hybridization Oven 645. The scanned image data were processed using the Affymetrix® Expression Console™ Version 1.1. The fold-change for each gene was evaluated using a Gene Expression Analysis with the Partek® Genomics Suite 6.5 software program (Partech, Münster, Germany). Genes with an expression level greater than 2-fold or less than 0.5 were recognized as being significantly different.
Quantitative RT-PCR
Among the genes differentially expressed in the ATRA-treated choESC compared to the control cells, we focused on the IRF8, TNFSF13B, WNT4, RARRES1, IGFBP3, PSMB9, RARRES3, IGFBP6, CYP26B1, IDO1 and RARE genes associated with negative cellular proliferation. In order to validate the results of the transcriptome analysis, real-time RT-PCR was conducted on these genes using the same samples. The HSD17B2 mRNA expression was also examined.
The RT reactions were performed with the PrimeScript RT Master Mix (TAKARA, Ohtsu, Japan) according to the manufacturer’s protocol. Briefly, 0.5 μg of total RNA was incubated with 4 μl of 5x PrimeScript RT Master Mix in 20 μl of reaction mixture at 37 °C for 15 min, and reverse transcriptase was inactivated by heating the samples at 85 °C for 5 s. The complementary DNA (cDNA) was immediately used for PCR. All PCR reactions were performed using SYBR Premix Ex Taq (TAKARA) and a LightCycler instrument (Roche Applied Science, Basel, Switzerland). Briefly, 2 μl aliquots containing cDNA were amplified in a total volume of 20 μl containing 4 μl of 5x SYBR PreMix Ex Taq and 0.2 μM of each primer. As an internal control for RT-PCR, TATA box-binding protein (TBP) cDNA was also amplified. The specific primer sets, except for TBP, were designed using the Primer3 software program (frodo.wi.mit.edu), while primers for TBP were synthesized according to a previous report [21]. The primer sequences are described in Table 1. Shuttle PCR was performed in 40 cycles as follows: pre-incubation for 10 s at 95 °C, denaturation for 5 s at 95 °C and annealing/extension for 20 s at 60 °C. All samples were run in duplicate. The melting curves of the products were obtained after cycling with a stepwise increase in temperature from 55 to 95 °C. At the end of the 40 cycles, the reaction products were separated electrophoretically on an agarose gel and stained with ethidium bromide for visual confirmation of the PCR products.
Table 1.
Primer sequences used for quantitative RT-PCR
| Gene ID | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| TNFSF13B | GGAGAAGGCAACTCCAGTCA | GCAATCAGTTGCAAGCAGTC | 92 |
| IRF8 | GTCTTCGACACCAGCCAGTT | GGCCATATCCGGAAACTCTT | 114 |
| RARRES3 | GTGAGCAGGAACTGTGAGCA | CAAAAGAGCATCCAGCAACA | 136 |
| RARRES1 | ACGGCTCATCGAGAAAAAGA | GAAAGCCAAATCCCAGATGA | 151 |
| IGFBP3 | GGGGTGTACACATTCCCAAC | AGGCTGCCCATACTTATCCA | 116 |
| IGFBP6 | GAATCCAGGCACCTCTACCA | GGTAGAAGCCTCGATGGTCA | 173 |
| CYP26B1 | ACACGGTGTCCAATTCCATT | GCCTCCTGGTACACGTTGAT | 172 |
| IDO1 | GGCAAAGGTCATGGAGATGT | TCCAGTTTGCCAAGACACAG | 127 |
| PSMB9 | ACCAACCGGGGACTTACC | GTCAAACTCCACTGCCATGA | 70 |
| RARB | GAAACAGGCCTTCTCAGTGC | TTGCTGGGTCGTCTTTTTCT | 137 |
| WNT4 | GCTGTGACAGGACAGTGCAT | GCCTCATTGTTGTGGAGGTT | 169 |
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 132 |
| HSD17B2 | TGGAACTGTGGAGGTCACAA | CCACTTGGAAAGCTCCAGTC | 178 |
Tissue culture and estradiol assay
The OEC culture was performed as previously reported with modifications [22]. Briefly, the ovarian endometrial cyst wall was surgically removed from the ovary. The tissue was minced into pieces less than 1.5 mm in maximum diameter, and the minced tissue (100 mg wet weight) was randomly aliquoted to the experimental group. Incubation were performed in triplicate in plastic culture dishes with 1 ml phenol red-free DMEM containing Glutamax (Invitrogen), 50 mg/ml of streptomycin (Invitrogen), 50 IU/ml of penicillin (Invitrogen) and 10 % dextran-coated charcoal-stripped fetal calf serum (Biological Industries) at 37 °C in 95 % air and 5 % CO2. ATRA was added to the media at a final concentration of 10−7 M. The tissue was also cultured in control medium containing 0.01 % dimethyl sulfoxide. Two days later, the media were aspirated, centrifuged and frozen at −20 °C until the estradiol assay. The estradiol concentration in the culture medium was measured using a human estradiol ELISA kit (Cusabio Biotech, Wuhan, China).
Statistical analysis
Comparison between groups were performed by ANOVA followed by post hoc comparisons using Turkey-Kramer honestly significant difference test. Values of P < 0.05 was considered to be statistically significant. The statistical analyses were performed using the R version 2.12.0 software program.
Results
Transcriptome analysis and quantitative RT-PCR
Of the 28,869 human genes identified in our gene index, 49 genes were upregulated and only four genes were downregulated in the ATRA-treated choESC compared to the control choESC (Table 2).
Table 2.
Results of the GeneChip microarray. Changes observed in the mRNA levels in the ATRA-treated choESC compared to the control cells
| Gene symbol | Fold-change | P-value |
|---|---|---|
| RARB | 7.77 | 0.0001 |
| RARRES1 | 7.31 | 0.0089 |
| DHRS3 | 6.09 | 0.0006 |
| LXN | 5.30 | 0.0016 |
| ADH1C | 4.21 | 0.0047 |
| CD22 | 4.09 | 0.0210 |
| IRF8 | 3.97 | 0.0168 |
| RARRES3 | 3.83 | 0.0142 |
| TNFSF10 | 3.68 | 0.0169 |
| IDO1 | 3.51 | 0.0369 |
| GBP4 | 3.43 | 0.0032 |
| ALDH1A1 | 3.31 | 0.0260 |
| LGALS9B | 3.06 | 0.0022 |
| ANO3 | 3.04 | 0.0092 |
| LGALS9 | 2.98 | 0.0101 |
| LGALS9C | 2.91 | 0.0034 |
| GALNT12 | 2.89 | 0.0317 |
| IRF1 | 2.87 | 0.0007 |
| IGFBP6 | 2.80 | 0.0001 |
| SLCO4C1 | 2.79 | 0.0021 |
| TRPC4 | 2.76 | 0.0121 |
| IGFBP3 | 2.54 | 0.0135 |
| MX2 | 2.45 | 0.0138 |
| TNFSF13B | 2.44 | 0.0173 |
| CYP26B1 | 2.30 | 0.0066 |
| GNG2 | 2.28 | 0.0108 |
| LOC100287290 | 2.28 | 0.0006 |
| PELO | 2.23 | 0.0459 |
| OAS2 | 2.20 | 0.0198 |
| RTP4 | 2.19 | 0.0176 |
| IFIT2 | 2.19 | 0.0459 |
| C10orf54 | 2.17 | 0.0029 |
| CFI | 2.14 | 0.0255 |
| SAMD9L | 2.12 | 0.0342 |
| ACSL5 | 2.12 | 0.0462 |
| SAMD9 | 2.10 | 0.0025 |
| TMEM140 | 2.08 | 0.0162 |
| APOL6 | 2.08 | 0.0074 |
| PLK2 | 2.06 | 0.0212 |
| CEACAM1 | 2.06 | 0.0235 |
| GNG2 | 2.04 | 0.0018 |
| PARP14 | 2.03 | 0.0113 |
| WNT4 | 2.02 | 0.0405 |
| HS6ST1 | 2.02 | 0.0207 |
| PSMB9 | 2.02 | 0.0122 |
| PTPRJ | 2.01 | 0.0001 |
| ARHGAP20 | 2.00 | 0.0210 |
| GRIA1 | −2.41 | 0.0463 |
| POPDC2 | −2.56 | 0.0401 |
| HAS2 | −2.61 | 0.0323 |
| FMO1 | −3.32 | 0.0262 |
In order to determine the biological relevance of the differentially expressed genes, Gene Ontology and KEGG pathway analyses were performed. Significant related terms were detected as illustrated (Tables 3, 4). The significant highly expressed genes in the ATRA-treated choESC were related to cellular response induced by ATRA stimulation based on the biological process and molecular function ontology analyses. Several terms related to negative regulation of cellular proliferation were also listed. In the KEGG pathway analysis, genes related to retinol metabolism were found to be upregulated (data not shown).
Table 3.
Gene ontology categories using biological process ontology. The top 21 category terms with a gene count over three and p < 0.01 are listed
| Term | Count | P-value |
|---|---|---|
| Response to stimulus | 29 | 0.0035 |
| Immune system process | 20 | p < 0.001 |
| Negative regulation of cellular process | 20 | p < 0.001 |
| Negative regulation of biological process | 20 | 0.0010 |
| Organ development | 17 | 0.0098 |
| Immune response | 16 | p < 0.001 |
| Regulation of cell proliferation | 12 | 0.0016 |
| Defense response | 11 | p < 0.001 |
| Regulation of apoptosis | 11 | 0.0060 |
| Regulation of programmed cell death | 11 | 0.0065 |
| Regulation of cell death | 11 | 0.0066 |
| Negative regulation of cell proliferation | 10 | p < 0.001 |
| Response to other organism | 8 | p < 0.001 |
| Response to biotic stimulus | 8 | 0.0029 |
| Monocarboxylic acid metabolic process | 7 | 0.0037 |
| Blood vessel morphogenesis | 6 | 0.0039 |
| Positive regulation of response to stimulus | 6 | 0.0063 |
| Blood vessel development | 6 | 0.0073 |
| Vasculature development | 6 | 0.0081 |
| Hormone metabolic process | 5 | 0.0019 |
| Regulation of hormone levels | 5 | 0.0067 |
| Positive regulation of protein kinase cascade | 5 | 0.0095 |
| Cellular hormone metabolic process | 4 | 0.0031 |
| Response to lipopolysaccharide | 4 | 0.0066 |
| Secondary metabolic process | 4 | 0.0071 |
| Response to molecule of bacterial origin | 4 | 0.0089 |
Table 4.
Gene ontology categories using molecular function ontology. The top seven category terms with a gene count over three gene count and p < 0.05 are listed
| Term | Count | P-value |
|---|---|---|
| Catalytic activity | 34 | 0.0426 |
| Signal transducer activity | 19 | 0.0231 |
| Molecular transducer activity | 19 | 0.0231 |
| Carbohydrate binding | 7 | 0.0076 |
| Cytokine activity | 6 | 0.0026 |
| Sugar binding | 5 | 0.0152 |
| Carboxylic acid binding | 4 | 0.0334 |
In order to validate the microarray results, quantitative RT-PCR was performed in 11 selected genes. The mRNAs of all genes were highly expressed following treatment with ATRA, and seven genes showed significant differences (Fig. 1).
Fig. 1.
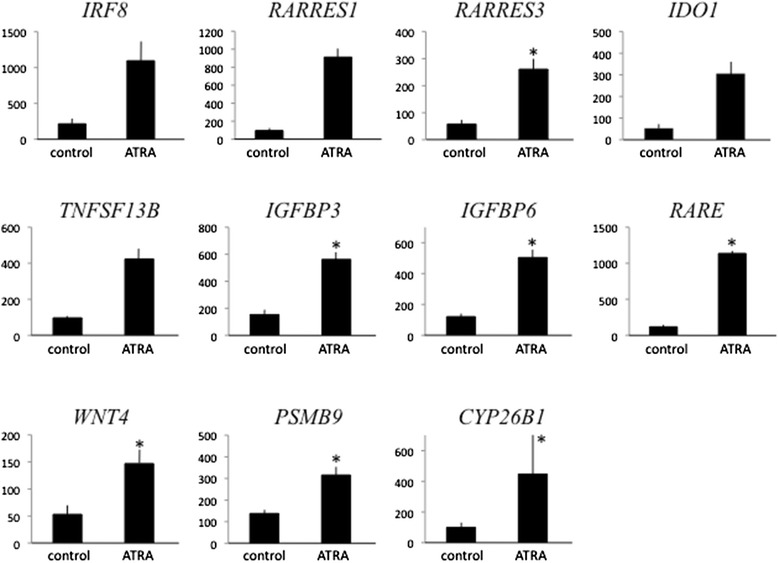
Results of quantitative RT-PCR in the 11 selected genes involved in negative cellular proliferation for validation of the mRNA expression array. The values are shown as mean ± SEM of three experiments. *P < 0.05 vs. control
HSD17B2-mRNA expression
In the choESC culture upon treatment with ATRA, the HSD17B2-mRNA expression was increased in a dose-dependent manner. There was a significant difference at a concentration of 10−7 M (Fig. 2).
Fig. 2.
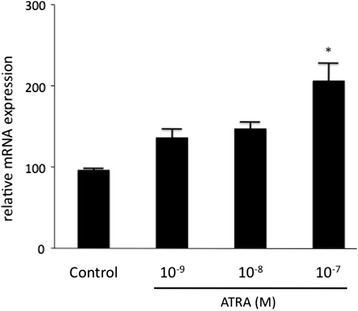
Effect of ATRA on the HSD17B2-mRNA expression in the cultured choESC. Cells were obtained from three different individuals, and the cells from a given individual were cultured in triplicate. The cells were treated with the indicated concentrations of ATRA for four days. The values are shown as mean ± SEM of three experiments. *P < 0.05 vs. control, ATRA: 10−9 M, and 10−8 M
Estradiol levels in the ATRA-treated endometriotic tissue culture
Endometriotic tissue obtained from OEC was cultured with 10−7 M ATRA. The level of estradiol in the medium with ATRA was lower than that observed in the control medium; however, there were no significant differences (Fig. 3).
Fig. 3.
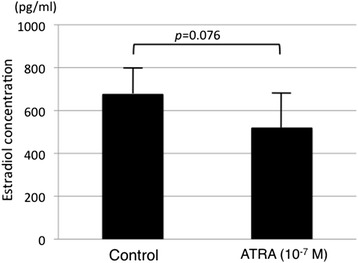
Effect of ATRA on estradiol production in the cultured OEC tissues. Tissues were obtained from four different individuals, and the cells from a given individual were cultured in duplicate. The tissues were treated with 10−7 M ATRA for two days. The values are shown as mean ± SEM of four experiments
Discussion
An association between aberrant retinol metabolism and endometriosis has recently been reported. Pavone M.E. et al. demonstrated that progesterone resistance has an influence on retinol uptake and growth-suppressor actions of retinoic acid in endometriotic stromal cells [14]. These authors also illustrated that an abnormal gene expression is involved in retinol uptake and metabolism in the setting of endometriosis, suggesting that an aberrant retinoic acid signaling pathway may affect endometriosis cell survival [15]. Wieser F. et al. demonstrated that retinoic acid inhibits the development of endometriotic implants in vivo [20]. In our in vitro study followed by a transcriptome analysis using isolated endometriotic stromal cells, genes upregulated by ATRA treatment were associated with the suppression of cell proliferation. We also conducted a cell proliferation study; however, ATRA had no significant anti-proliferative effect on the cultured choESC for up to four days (data not shown). The fact that the environment in the culture is different from the in vivo conditions and the cultured choESC slowly divide indicates the possibility that the anti-proliferative effects of retinoic acid cannot be detected. Wieser F. et al. also reported the same observation suggesting the indirect effects of cell growth inhibition [20]. They postulated the suppressive effect of retinoic acid on IL-6 and MCP-1 production, resulting in peritoneal macrophage differentiation.
Endometriosis is an estrogen-dependent disease. It is well-known that local sex steroid production in endometriotic tissue is upregulated by elevated catalytic enzyme activity, such as that due to aromatase [23–26]. Furthermore, reduced retinoic acid concentrations are observed in endometriotic lesions [18], and we previously reported that the HSD17B2 expression is suppressed in choESC accompanied by aberrant DNA methylation [19]. Retinoic acid insufficiency results in numerous molecular and functional defects, including HSD17B2 deficiency, leading to estradiol excess in endometriotic tissue. The present study demonstrated the direct effects of ATRA on the HSD17B2 expression in choESC. Since ATRA treatment did not alter the DNA methylation status of the HSD17B2 gene (data not shown), the decreased HSD17B2 expression is likely due to negative modulation of transcriptional factors. In the transcriptome analysis, the fold change in the expression of the HSD17B2 gene after treatment with ATRA was +2.22; however, the difference was not significant (p = 0.24). This is due to the limitation of our study resulting from the limited quantity of available samples. It is unclear whether the upregulation of genes related to negative cellular proliferation induced by ATRA treatment is due to the direct function of retinoic acid, reduced estradiol production or any other indirect pathways.
In order to investigate the effects of retinoic acid on steroidogenesis, especially estradiol production, an ovarian endometriotic tissue culture was performed. Since the HSD17B2 expression is much higher in endometrial epithelial cells than in endometrial stromal cells, a tissue culture rather than an endometrial stromal cell culture was conducted in this study. Although the abundance of estradiol slightly decreased with supplementation of ATRA, there were no statistically significant differences. There are some possibilities explaining this result. Retinoic acid has only a weak suppressive effect on the HSD17B2 gene expression, resulting in the ineffective conversion of estradiol. In the setting of endometriosis, it has not been fully clarified whether retinoic acid upregulates or downregulates sex steroid hormone biosynthesis enzymes. Wickenheisser J.K. et al. reported that the gene expressions of STAR, CYP11A and CYP17 are stimulated by ATRA in human ovarian theca cells [27]. Steroid hormone synthesis is increased by retinoids in rodents in other organs [28–30]. These observations intimate that it is too premature to draw conclusions in terms of the relationship between retinoic acid and the local estradiol concentrations in patients with endometriosis. Besides sex steroid hormone biosynthesis, it has been reported that retinoic acid decreases estrogen and progesterone receptor-mediated transcriptional activation [31]. In order to elucidate the association between retinoic acid and aberrant hormonal involvement in the pathogenesis of endometriosis, further research is needed.
In summary, the gene expression related to cell proliferation suppression, etc. was upregulated by ATRA treatment in isolated endometriotic stromal cells derived from ovarian endometriotic lesions in the present study. ATRA treatment also had the potential to suppress estradiol production. These results suggest the therapeutic potential of retinoic acid for the treatment of endometriosis.
Acknowledgement
The authors thank Brian Quinn for correcting the manuscript. This work was supported by Grants-in-Aid 25464560 for Scientific Research from the Ministry of Education, Science and Culture, Japan.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
YY carried out the studies and drafted the manuscript. ET and AN carried out the microarray analysis and statistical analysis. MS, MO, KJ, LL, SS, RM, TT, HA and HT participated in the design of the study and helped to correct samples. NS helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Ross AC, Gardner EM. The function of vitamin A in cellular growth and differentiation, and its roles during pregnancy and lactation. Adv Exp Med Biol. 1994;352:187–200. doi: 10.1007/978-1-4899-2575-6_15. [DOI] [PubMed] [Google Scholar]
- 2.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–54. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 3.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu Rev Nutr. 2002;22:347–81. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 4.Duester G. Alcohol dehydrogenase as a critical mediator of retinoic acid synthesis from vitamin A in the mouse embryo. J Nutr. 1998;128:459S–462S. doi: 10.1093/jn/128.2.459S. [DOI] [PubMed] [Google Scholar]
- 5.Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual process. Photochem Photobiol. 1996;64:613–21. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 6.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Drager UC. Retinoic acid signaling in the functioning brain. Sci STKE. 2006;pe10. [DOI] [PubMed]
- 8.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 9.Zheng WL, Ong DE. Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol Reprod. 1998;58:963–70. doi: 10.1095/biolreprod58.4.963. [DOI] [PubMed] [Google Scholar]
- 10.Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE. Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology. 2000;141:802–8. doi: 10.1210/endo.141.2.7323. [DOI] [PubMed] [Google Scholar]
- 11.Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, Napoli JL, Taylor RN. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–60. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Hansen JM, Hao L, Taylor RN, Sidell N. Retinoic acid stimulation of VEGF secretion from human endometrial stromal cells is mediated by production of reactive oxygen species. J Physiol. 2011;589:863–75. doi: 10.1113/jphysiol.2010.200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawatsri S, Desai N, Rock JA, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril. 2000;73:1012–19. doi: 10.1016/S0015-0282(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 14.Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95:E300–9. doi: 10.1210/jc.2010-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavone ME, Dyson M, Reirstad S, Pearson E, Ishikawa H, Cheng YH, Bulun SE. Endometriosis expresses a molecular pattern consistent with decreased retinoid uptake, metabolism and action. Hum Reprod. 2011;26:2157–64. doi: 10.1093/humrep/der172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierzchalski K, Taylor RN, Nezhat C, Jones JW, Napoli JL, Yang G, Kane MA, Sidell N. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod. 2014;91:84. doi: 10.1095/biolreprod.114.119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidell N, Han SW, Parthasarathy S. Regulation and modulation of abnormal immune responses in endometriosis. Ann N Y Acad Sci. 2002;955:159–73. doi: 10.1111/j.1749-6632.2002.tb02777.x. [DOI] [PubMed] [Google Scholar]
- 18.Sokalska A, Anderson M, Villanueva J, Ortega I, Bruner-Tran KL, Osteen KG, Duleba AJ. Effects of simvastatin on retinoic acid system in primary human endometrial stromal cells and in a chimeric model of human endometriosis. J Clin Endocrinol Metab. 2013;98:E463–71. doi: 10.1210/jc.2012-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagata Y, Nishino K, Takaki E, Sato S, Maekawa R, Nakai A, Sugino N. Genome-wide DNA methylation profiling in cultured eutopic and ectopic endometrial stromal cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieser F, Wu J, Shen Z, Taylor RN, Sidell N. Retinoic acid suppresses growth of lesions, inhibits peritoneal cytokine secretion, and promotes macrophage differentiation in an immunocompetent mouse model of endometriosis. Fertil Steril. 2012;97:1430–7. doi: 10.1016/j.fertnstert.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagata Y, Asada H, Tamura I, Lee L, Maekawa R, Taniguchi K, Taketani T, Matsuoka A, Tamura H, Sugino N. DNA methyltransferase expression in the human endometrium: down-regulation by progesterone and estrogen. Hum Reprod. 2009;24:1126–32. doi: 10.1093/humrep/dep015. [DOI] [PubMed] [Google Scholar]
- 22.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62:904–10. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 23.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, Fushiki S, Osawa Y, Honjo H. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997;57:514–9. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 24.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–9. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulun S, Zeitoun K, Takayama K, Sasano H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum Reprod Update. 2000;6:413–8. doi: 10.1093/humupd/6.5.413. [DOI] [PubMed] [Google Scholar]
- 26.Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87. doi: 10.1186/1477-7827-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF, 3rd, McAllister JM. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4858–65. doi: 10.1210/jc.2005-0330. [DOI] [PubMed] [Google Scholar]
- 28.Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SA, Yamazaki T. Retinoic acid stimulates 17beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150:4260–9. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Yoo MS, Choi HS, Kwon HB, Soh J. Retinoic acids up-regulate steroidogenic acute regulatory protein gene. Mol Cell Endocrinol. 1999;148:1–10. doi: 10.1016/S0303-7207(98)00243-3. [DOI] [PubMed] [Google Scholar]
- 30.Lefevre A, Rogier E, Astraudo C, Duquenne C, Finaz C. Regulation by retinoids of luteinizing hormone/chorionic gonadotropin receptor, cholesterol side-chain cleavage cytochrome P-450, 3 beta-hydroxysteroid dehydrogenase/delta (5–4)-isomerase and 17 alpha-hydroxylase/C17-20 lyase cytochrome P-450 messenger ribonucleic acid levels in the K9 mouse Leydig cell line. Mol Cell Endocrinol. 1994;106:31–9. doi: 10.1016/0303-7207(94)90183-X. [DOI] [PubMed] [Google Scholar]
- 31.Kazmi SM, Plante RK, Visconti V, Lau CY. Comparison of N-(4-hydroxyphenyl)retinamide and all-trans-retinoic acid in the regulation of retinoid receptor-mediated gene expression in human breast cancer cell lines. Cancer Res. 1996;56:1056–62. [PubMed] [Google Scholar]


