Abstract
Plant defence compounds occur in floral nectar, but their ecological role is not well-understood. We provide the first evidence that plant compounds pharmacologically alter pollinator behaviour by enhancing their memory of reward. Honeybees rewarded with caffeine, which occurs naturally in nectar of Coffea and Citrus species, were three times more likely to remember a learned floral scent than those rewarded with sucrose alone. Caffeine potentiated responses of mushroom body neurons involved in olfactory learning and memory by acting as an adenosine receptor antagonist. Caffeine concentrations in nectar never exceeded the bees’ bitter taste threshold, implying that pollinators impose selection for nectar that is pharmacologically active but not repellent. By using a drug to enhance memories of reward, plants secure pollinator fidelity and improve reproductive success.
Many drugs commonly consumed by humans are produced by plants as a form of toxic defence against herbivores (1, 2). While plant-derived drugs like caffeine or nicotine are lethal in high doses (3-5), they have pharmacological effects at low doses that affect mammalian behaviour. For example, low doses of caffeine are mildly rewarding and enhance cognitive performance and memory retention (6). Interestingly, caffeine has been detected in low doses in the floral nectar and pollen of Citrus (7), but whether it has an ecological function is unknown.
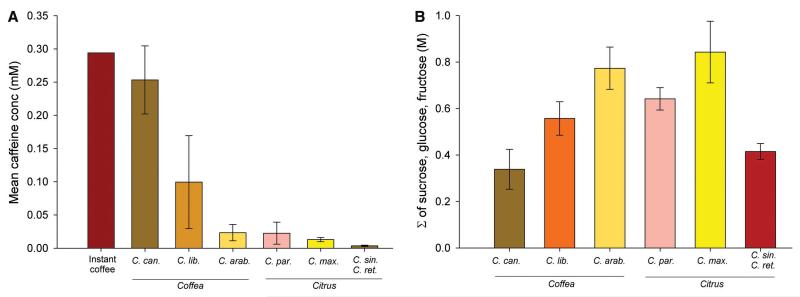
Two caffeine-producing plant genera, Citrus and Coffea, have large floral displays with strong scents and produce more fruits and seeds when pollinated by bees (8, 9). If caffeine confers a selective advantage when these plants interact with pollinators, we might expect it to be commonly encountered in nectar. We measured caffeine in the nectar of 3 species of Coffea (C. canephora, C. arabica, and C. liberica) and 4 species of Citrus (C. paradisi, C. maxima, C. sinesis, C. reticulata) using liquid chromatography-mass spectrometry (10, Fig. S1A). When caffeine was present, its concentration ranged from 0.003 - 0.253 mM. The median caffeine concentration in both genera was not significantly different (Fig. 1A, Mann-Whitney, Z = −1.09, P = 0.272). Caffeine was more common in the nectar of C. canephora than in C. arabica or C. liberica (Coffea: logistic regression χ22 = 11.1, P = 0.004); it was always present in Citrus nectar. The mean total nectar sugar concentration ranged from 0.338-0.843 M (Fig. 1B, see Fig S1B for individual sugars). Caffeine concentration in nectar did not correlate with total sugar concentration (Pearson’s r = 0.063, P = 0.596).
Figure 1.
(A) Caffeine concentration in Coffea and Citrus sp. and a cup of instant coffee. Caffeine concentration depended on species within each genus (Coffea: Kruskal-Wallis, χ22 = 28.1, P < 0.001; Citrus: Kruskal-Wallis, χ22 = 6.98, P = 0.030); C. canephora had the highest mean concentration of all species sampled. (B) The sum of the concentration of sucrose, glucose and fructose (total nectar sugars) depended on species (1-way ANOVA: F5, 161 = 4.64, P < 0.001) and was greatest in Citrus maxima and hybrids (citron, lemons, clementines). (C. can. = Coffea canephora, N = 34; C. lib. = Coffea liberica, N = 31; C. arab. = Coffea arabica, N = 27; C. par. = Citrus paradisi and hybrids, Ncp = 17; C. max. = Citrus maxima and hybrids, N = 5; C. sin. and C. ret. = Citrus sinensis and Citrus reticulata, NCS = 7, NCR = 5 – data for these two species was pooled).
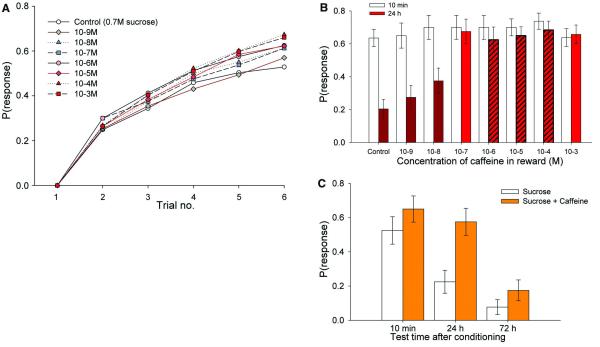
We hypothesized that caffeine could affect the learning and memory of foraging pollinators. To test this, we trained individual honeybees to associate floral scent with 0.7 M sucrose and 7 different concentrations of caffeine and tested their olfactory memory. Using a method for classical conditioning of feeding responses (proboscis extension reflex, 11), bees were trained for 6 trials with 30 s between each pairing of odour with reward. This inter-trial interval approximated the rate of floral visitation exhibited by honeybees foraging from multiple flowers on a single Citrus tree (see methods). The presence of low doses of caffeine in reward had a weak effect on the rate of learning (Fig. 2A), but it had a profound effect on long-term memory. When rewarded with solutions containing nectar-levels of caffeine, three times as many bees remembered the conditioned scent 24 h later and to responded as if it predicted reward (Fig. 2B, logistic regression, χ72 = 41.9, P < 0.001). Twice as many bees remembered it 72 h later (Fig. 2C). This improvement in memory performance was not due to a general increase in olfactory sensitivity resulting from caffeine consumption (Fig. S2A). In fact, the effect of caffeine on long-term olfactory memory in bees was greater than that produced by high concentrations of sucrose when the same experimental methods were used (e.g. 2.0 M, Fig. S2B).
Figure 2.
(A) The rate of learning of bees conditioned with an odour stimulus paired with a 0.7 M sucrose reward containing caffeine. The rate of learning was slightly greater for the bees fed caffeine in reward during conditioning (logistic regression, χ12 = 4.85, P = 0.028). N ≥ 79 for all groups. (B) Memory recall test for odours at 10 min (clear bars) or 24 h (red bars) after bees had been trained as in (A). Bright red bars indicate that the response at 24 h was significantly different from the control (0.7 M sucrose) (least-squares contrasts: P < 0.05); dark red bars were not significantly different. Nectar-levels of caffeine are indicated by hatching. N > 79 for each group. (C) Bees fed 0.1 mM caffeine in sucrose (red bars) were more likely to remember the conditioned odour sucrose alone (white bars) (logistic regression, χ12 = 9.04, P < 0.003) at 24 h and 72 h after conditioning. N = 40 per group.
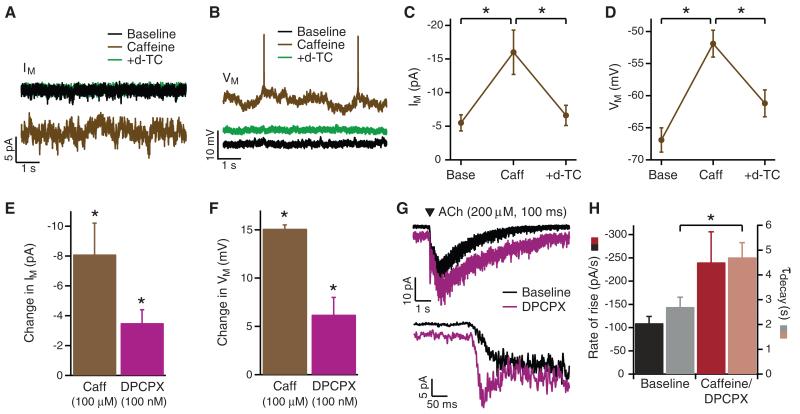
Caffeine’s influence on cognition in mammals is in part mediated by its action as an adenosine receptor antagonist (11). In the hippocampal CA2 region, inhibition of adenosine receptors by caffeine induces long-term potentiation (12), a key mechanism of memory formation (13). The Kenyon cells (KCs) in mushroom bodies of the insect brain are similar in function to hippocampal neurons: they integrate sensory input during associative learning, exhibit long-term potentiation and are involved in memory formation (14-16). To test whether nectar-caffeine doses affect mushroom body function, we made whole-KC recordings in the intact honeybee brain. Caffeine (100 μM) evoked a small increase in the holding current (IM) and depolarized KC membrane potential (VM) towards the action potential firing threshold, by increasing nicotinic ACh receptor (nAChR) activation (Fig. 3A-D). To test whether the observed effects of caffeine were due to interactions with adenosine receptors, we applied the adenosine receptor antagonist, DPCPX, and observed that it similarly increased IM and depolarized VM but to a lesser extent (Fig. 3E,F). Both caffeine and DPCPX affected KC response kinetics evoked by brief, local application of ACh, increasing the activation rate and slowing the decay (Fig.3G,H). Our data show that caffeine modulates cholinergic input via a postsynaptic action, but could act via presynaptic adenosine receptors to potentiate ACh release (17). The resulting increase in KC excitability should lead to an increased probability of action potential firing in response to sensory stimulation (18), thereby facilitating the induction of associative synaptic plasticity in KCs (19). The enhanced activation of KCs may also facilitate plasticity at synapses with mushroom body extrinsic neurons (20), which exhibit spike-timing-dependent plasticity (21). In this way, a ‘memory trace’ could be formed for the odour associated with reward during and after conditioning (22-23).
Figure 3.
The effect of caffeine on Kenyon cells. (A, B) Example traces from a KC in intact honeybee brain recorded under voltage-clamp (A, VH = −73 mV) and current-clamp (B; at resting VM), showing the increase in IM and depolarization evoked by bath application of caffeine (100 μM), and subsequent reversal by the nAChR antagonist d-TC (500 μM). (C, D) Mean data showing the reversal by d-TC (500 μM) of the effect of caffeine (Caff; 100 μM) on IM (C; N = 6, t5 = 4.03, P = 0.010; t5 = 4.07, P = 0.010) and VM (D; N = 6, t5 = 34.1, P < 0.001; t5 = 12.0, P < 0.001). (E, F) Comparison of the mean effects of caffeine and DPCPX on IM (E, Caff: N = 10, t9 = 3.84, P = 0.004; DPCPX: N = 6, t5 = 4.04, P = 0.010) and VM (F, Caff: N = 6, t5 = 34.1, P < 0.001; DPCPX: N = 6, t5 = 3.39, P = 0.019). (G, H) Example traces (G; rising phase shown on an expanded time-scale below) and mean data (H, Rate of rise: N = 6, t5 = 2.20, P = 0.079; τdecay: N = 9, t8 = 3.54, P = 0.008) showing that DPCPX (100 nM) and caffeine (100 μM) slowed the decay and, in 6 of 9 KCs, potentiated the fast component of the response evoked by exogenous ACh. (Student’s paired t-test used in all comparisons).
Caffeine is bitter tasting to mammals and is both toxic (24) and repellent to honeybees at high concentrations (25, 26). If bees can detect caffeine, they might learn to avoid flowers offering nectar containing it (27). We found that honeybees were deterred from drinking sucrose solutions containing caffeine at concentrations greater than 1 mM (Fig. 4); they also have neurons that detect caffeine in sensilla on their mouthparts (Fig. S3). However, nectar concentrations never exceeded 0.3mM (i.e. 0.058 mg/ml), even though levels of caffeine in vegetative and seed tissues of Coffea have been reported to be as great as 24mg/ml (28). This implies that pollinators drive selection towards concentrations of caffeine that are not repellent but still pharmacologically active.
Figure 4.
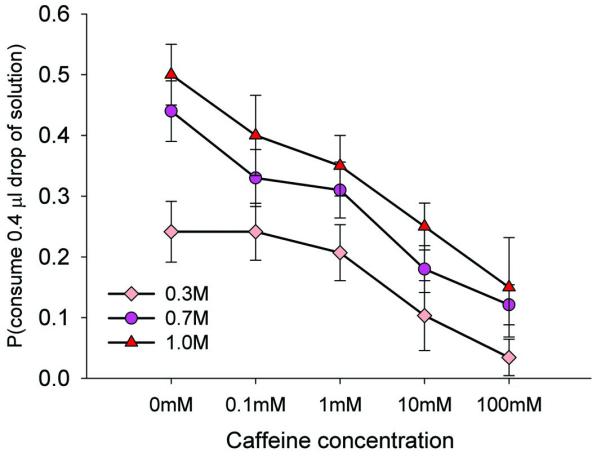
Bees are more likely to reject sucrose solutions containing caffeine of concentrations greater than 1 mM (logistic regression, χ42 = 23.4, P < 0.001; for 0.7M and 1.0M, 1 mM caffeine vs sucrose post hoc, P < 0.05; for 0.3M, 100 mM caffeine vs sucrose post hoc, P < 0.05). Bees were less likely to drink 0.3 M sucrose (pale pink diamonds) than 0.7M (pink circles) or 1.0M solutions (red triangles) (logistic regression, χ22 = 8.69, P = 0.013). Mean responses + SE. N0.3M = 29, N0.7M = 100, N1.0M = 20.
Our data show that plant-produced alkaloids like caffeine have a role in addition to defence: they can pharmacologically manipulate a pollinator’s behaviour. When bees and other pollinators learn to associate floral scent with food while foraging (29), they are more likely to visit flowers bearing the same scent signals. Such behaviour increases their foraging efficiency (30) while concomitantly leading to more effective pollination (31, 32). Our experiments suggest that by affecting a pollinator’s memory, plants reap the reproductive benefits arising from enhanced pollinator fidelity.
Supplementary Material
Acknowledgments
The authors wish to thank staff at CATIE for access to the Coffea collections and at TEI for access to Citrus orchards; Mitch Thomson, Karen Smith, Frederic Marion-Poll, Alexandra Popescu for help with the data collection; Malcolm Thompson for beekeeping; and Jenni Harvey and Chris Connolly for project support. This work was funded in part by the Linnean Society of London, and by a UK government Insect Pollinators Initiative grant BB/I000968/1 to GAW and a separate grant to Chris Connolly (BB/1000313/1). Methods and additional data are available in the online supplementary materials. All data are archived on the NERC Environmental Information Data Centre.
References
- 1.Revealing the paradox of drug reward in human evolution. P R Soc B. 2008 Jun 7;275:1231. doi: 10.1098/rspb.2007.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harborne JB. Introduction to ecological biochemistry. 4th ed Academic Press; London: 1993. p. 318. [Google Scholar]
- 3.Hollingsworth RG, Armstrong JW, Campbell E. Pest control: Caffeine as a repellent for slugs and snails. Nature. 2002 Jun 27;417:915. doi: 10.1038/417915a. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of Aging on Acute Toxicity of Nicotine in Rats. Pharmacol Toxicol. 1994 Jul;75:1. doi: 10.1111/j.1600-0773.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph T, Knudsen K. A case of fatal caffeine poisoning. Acta Anaesth Scand. 2010 Apr;54:521. doi: 10.1111/j.1399-6576.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 6.Nehlig A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci Biobehav R. 1999 Mar;23:563. doi: 10.1016/s0149-7634(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmar JA, Baumann TW. Caffeine in Citrus flowers. Phytochemistry. 1999 Sep;52:19. [Google Scholar]
- 8.Ricketts TH, Daily GC, Ehrlich PR, Michener CD. Economic value of tropical forest to coffee production. P Natl Acad Sci USA. 2004 Aug 24;101:12579. doi: 10.1073/pnas.0405147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roubik DW. Tropical agriculture - The value of bees to the coffee harvest. Nature. 2002 Jun 13;417:708. doi: 10.1038/417708a. [DOI] [PubMed] [Google Scholar]
- 10.Materials and methods are available in the supplementary materials in Science online.
- 11.Ribeiro JA, Sebastiao AM. Caffeine and Adenosine. J Alzheimers Dis. 2010;20:S3. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 12.Simons SB, Caruana DA, Zhao ML, Dudek SM. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci. 2012 Jan;15:23. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris RGM, Anderson E, Lynch GS, Baudry M. Selective Impairment of Learning and Blockade of Long-Term Potentiation by an N-Methyl-D-Aspartate Receptor Antagonist, Ap5. Nature. 1986 Feb 27;319:774. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 14.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003 Apr;4:266. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 15.Oleskevich S, Clements JD, Srinivasan MV. Long-term synaptic plasticity in the honeybee. J Neurophysiol. 1997 Jul;78:528. doi: 10.1152/jn.1997.78.1.528. [DOI] [PubMed] [Google Scholar]
- 16.Strausfeld NJ, Hansen L, Li YS, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Memory. 1998 May-Jun;5:11. [PMC free article] [PubMed] [Google Scholar]
- 17.Sperlagh B, Vizi ES. The Role of Extracellular Adenosine in Chemical Neurotransmission in the Hippocampus and Basal Ganglia: Pharmacological and Clinical Aspects. Curr Top Med Chem. 2011 Apr;11:1034. doi: 10.2174/156802611795347564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002 Jul 19;297:359. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 19.Szyszka P, Galkin A, Menzel R. Associative and non-associative plasticity in kenyon cells of the honeybee mushroom body. Frontiers in systems neuroscience. 2008;2:3. doi: 10.3389/neuro.06.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menzel R, Manz G. Neural plasticity of mushroom body-extrinsic neurons in the honeybee brain. The Journal of experimental biology. 2005 Nov;208:4317. doi: 10.1242/jeb.01908. [DOI] [PubMed] [Google Scholar]
- 21.Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012 Feb 2;482:47. doi: 10.1038/nature10776. [DOI] [PubMed] [Google Scholar]
- 22.Galili DS, Lude A, Galizia CG, Szyszka P, Tanimoto H. Olfactory Trace Conditioning in Drosophila. J Neurosci. 2011 May 18;31:7240. doi: 10.1523/JNEUROSCI.6667-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szyszka P, et al. Mind the Gap: Olfactory Trace Conditioning in Honeybees. J Neurosci. 2011 May 18;31:7229. doi: 10.1523/JNEUROSCI.6668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detzel A, Wink M. Attraction, deterrance, or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology. 1993;4:8. [Google Scholar]
- 25.Mustard JA, Dews L, Brugato A, Dey K, Wright GA. Consumption of an acute dose of caffeine reduces acquisition but not memory in the honey bee. Behav Brain Res. 2012 Jun 15;232:217. doi: 10.1016/j.bbr.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Singaravelan N, Nee’man G, Inbar M, Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J Chem Ecol. 2005 Dec;31:2791. doi: 10.1007/s10886-005-8394-z. [DOI] [PubMed] [Google Scholar]
- 27.Wright GA, et al. Parallel Reinforcement Pathways for Conditioned Food Aversions in the Honeybee. Curr Biol. 2010 Dec 21;20:2234. doi: 10.1016/j.cub.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashihara H, Crozier A. Caffeine: a well known but little mentioned compound in plant science. Trends Plant Sci. 2001 Sep;6:407. doi: 10.1016/s1360-1385(01)02055-6. [DOI] [PubMed] [Google Scholar]
- 29.Wright GA, Schiestl FP. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol. 2009 Oct;23:841. [Google Scholar]
- 30.Chittka L, Gumbert A, Kunze J. Foraging dynamics of bumble bees: Correlates of movements within and between plant species. Behav Ecol. 1997 May-Jun;8:239. [Google Scholar]
- 31.Hopkins R, Rausher MD. Pollinator-Mediated Selection on Flower Color Allele Drives Reinforcement. Science. 2012 Mar 2;335:1090. doi: 10.1126/science.1215198. [DOI] [PubMed] [Google Scholar]
- 32.Kunin WE. Sex and the Single Mustard - Population-Density and Pollinator Behavior Effects on Seed-Set. Ecology. 1993 Oct;74:2145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.