Abstract
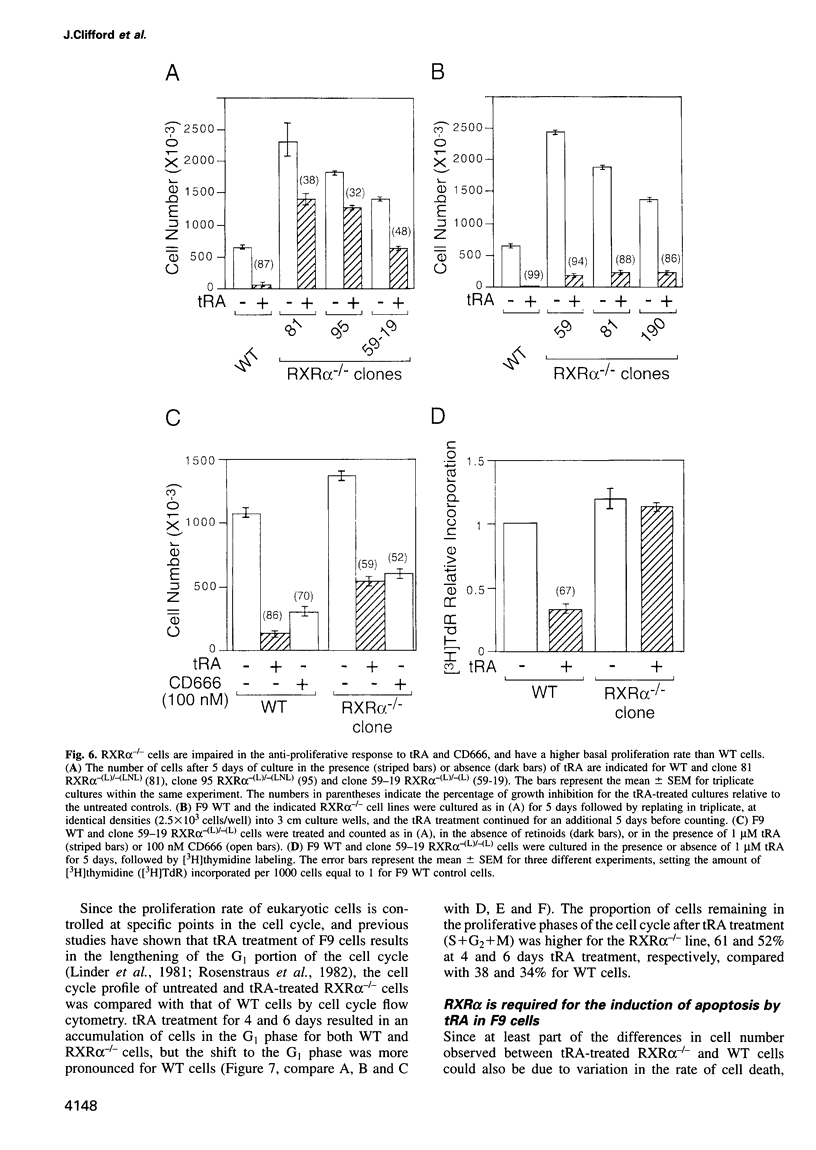
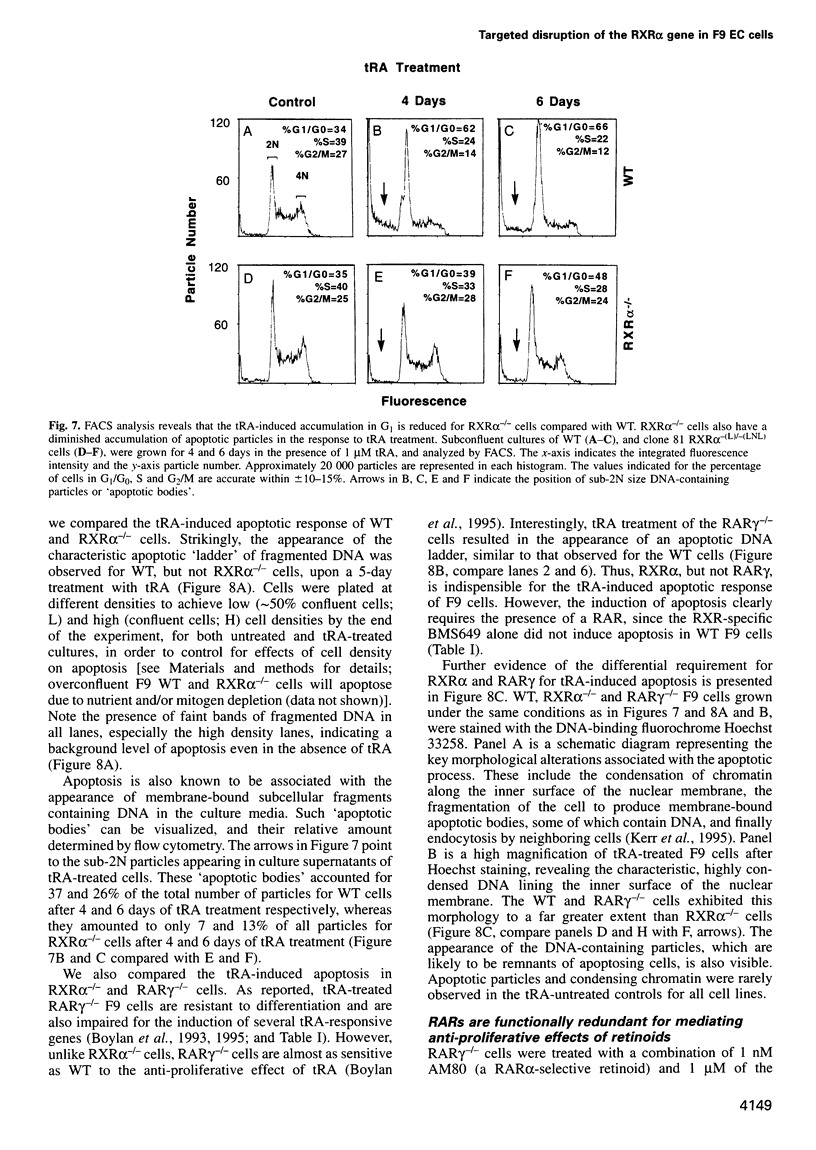
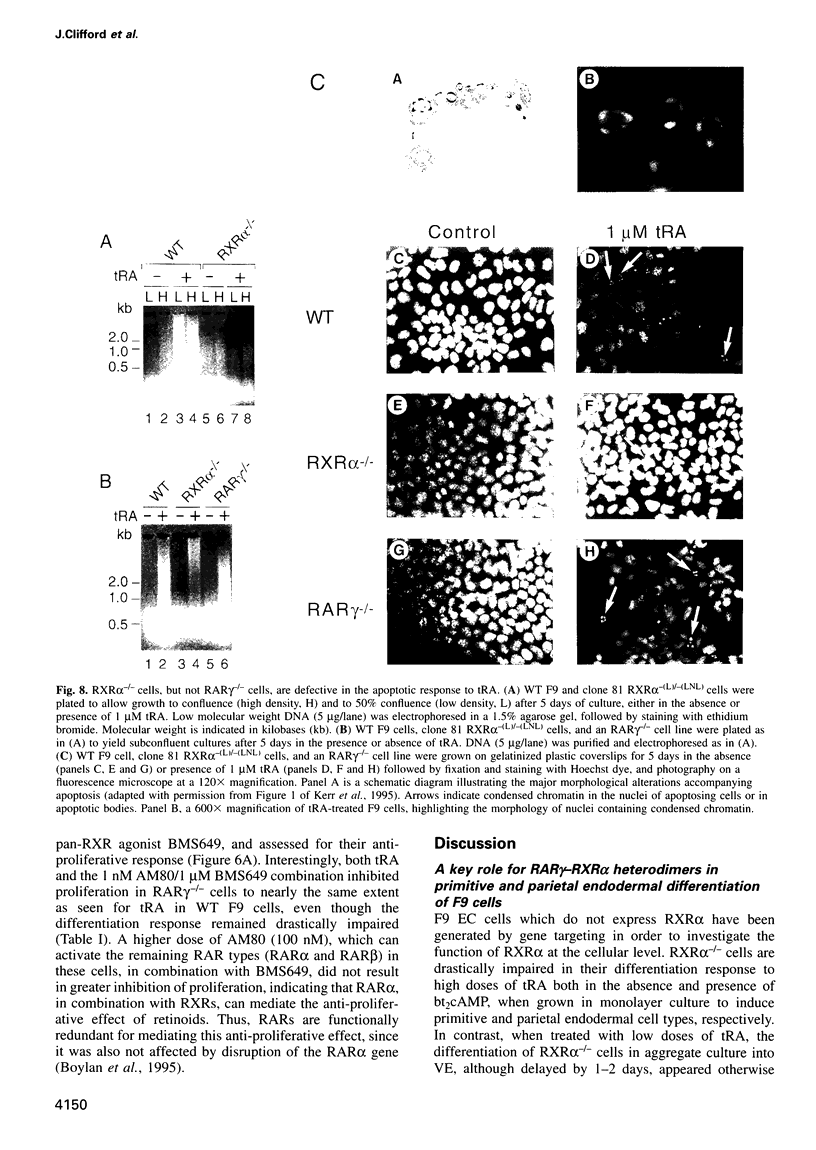
The F9 murine embryonal carcinoma (EC) cell line, a well established model system for the study of retinoic acid (RA)-induced differentiation, differentiates into cells resembling three types of extra-embryonic endoderm (primitive, parietal and visceral), depending on the culture conditions and RA concentration used. A number of previously identified genes are differentially expressed during this process and serve as markers for the different endodermal cell types. Differentiation is also accompanied by a decreased rate of proliferation and an apoptotic response. Using homologous recombination, we have disrupted both alleles of the retinoid X receptor (RXR) alpha gene in F9 cells to investigate its role in mediating these responses. The loss of RXRalpha expression impaired the morphological differentiation of F9 EC cells into primitive and parietal endoderm, but has little effect on visceral endodermal differentiation. Concomitantly the inducibility of most primitive and parietal endoderm differentiation-specific genes was impaired, while several genes upregulated during visceral endodermal differentiation were induced normally. We also demonstrate that RXRalpha is required for both the anti-proliferative and apoptotic responses in RA-treated F9 cells. Additionally, we provide further evidence that retinoic acid receptor (RAR)-RXR heterodimers are the functional units transducing the effects of retinoids in F9 cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos B., Lotan R. Retinoid-sensitive cells and cell lines. Methods Enzymol. 1990;190:217–225. doi: 10.1016/0076-6879(90)90026-w. [DOI] [PubMed] [Google Scholar]
- Atencia R., García-Sanz M., Unda F., Aréchaga J. Apoptosis during retinoic acid-induced differentiation of F9 embryonal carcinoma cells. Exp Cell Res. 1994 Oct;214(2):663–667. doi: 10.1006/excr.1994.1304. [DOI] [PubMed] [Google Scholar]
- Bouillet P., Oulad-Abdelghani M., Vicaire S., Garnier J. M., Schuhbaur B., Dollé P., Chambon P. Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol. 1995 Aug;170(2):420–433. doi: 10.1006/dbio.1995.1226. [DOI] [PubMed] [Google Scholar]
- Boylan J. F., Lohnes D., Taneja R., Chambon P., Gudas L. J. Loss of retinoic acid receptor gamma function in F9 cells by gene disruption results in aberrant Hoxa-1 expression and differentiation upon retinoic acid treatment. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9601–9605. doi: 10.1073/pnas.90.20.9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan J. F., Lufkin T., Achkar C. C., Taneja R., Chambon P., Gudas L. J. Targeted disruption of retinoic acid receptor alpha (RAR alpha) and RAR gamma results in receptor-specific alterations in retinoic acid-mediated differentiation and retinoic acid metabolism. Mol Cell Biol. 1995 Feb;15(2):843–851. doi: 10.1128/mcb.15.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994 Apr;5(2):115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Duprey P., Chowdhury K., Dressler G. R., Balling R., Simon D., Guenet J. L., Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev. 1988 Dec;2(12A):1647–1654. doi: 10.1101/gad.2.12a.1647. [DOI] [PubMed] [Google Scholar]
- Durand B., Saunders M., Gaudon C., Roy B., Losson R., Chambon P. Activation function 2 (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994 Nov 15;13(22):5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus L., Davies P. J., Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991 Dec;56(2):170–177. [PubMed] [Google Scholar]
- Fisher D. E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994 Aug 26;78(4):539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995 May 19;81(4):541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Giguère V., Lyn S., Yip P., Siu C. H., Amin S. Molecular cloning of cDNA encoding a second cellular retinoic acid-binding protein. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6233–6237. doi: 10.1073/pnas.87.16.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994 Feb;15(1):61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- Glass C. K. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994 Jun;15(3):391–407. doi: 10.1210/edrv-15-3-391. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Flanders K. C., Danielpour D., Yuspa S. H., Sporn M. B. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989 Nov;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Cooper D. L., Eiferman F., van de Rijn P., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. I. A comparison of the primary amino acid sequences of mammalian alpha-fetoprotein and albumin. J Biol Chem. 1981 Feb 25;256(4):1954–1959. [PubMed] [Google Scholar]
- Gronemeyer H., Laudet V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2(11):1173–1308. [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Kastner P., Grondona J. M., Mark M., Gansmuller A., LeMeur M., Decimo D., Vonesch J. L., Dollé P., Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994 Sep 23;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark M., Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995 Dec 15;83(6):859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- Kastner P., Mark M., Leid M., Gansmuller A., Chin W., Grondona J. M., Décimo D., Krezel W., Dierich A., Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996 Jan 1;10(1):80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- Kelly D., Campbell W. J., Tiesman J., Rizzino A. Regulation and expression of transforming growth factor type-beta during early mammalian development. Cytotechnology. 1990 Nov;4(3):227–242. doi: 10.1007/BF00563783. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Gobé G. C., Winterford C. M., Harmon B. V. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- Killen P. D., Burbelo P., Sakurai Y., Yamada Y. Structure of the amino-terminal portion of the murine alpha 1(IV) collagen chain and the corresponding region of the gene. J Biol Chem. 1988 Jun 25;263(18):8706–8709. [PubMed] [Google Scholar]
- Krowczynska A. M., Coutts M., Makrides S., Brawerman G. The mouse homologue of the human acidic ribosomal phosphoprotein PO: a highly conserved polypeptide that is under translational control. Nucleic Acids Res. 1989 Aug 11;17(15):6408–6408. doi: 10.1093/nar/17.15.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston A. W., Gudas L. J. Identification of a retinoic acid responsive enhancer 3' of the murine homeobox gene Hox-1.6. Mech Dev. 1992 Sep;38(3):217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Law S. W., Conneely O. M., DeMayo F. J., O'Malley B. W. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992 Dec;6(12):2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Leblanc B. P., Stunnenberg H. G. 9-cis retinoic acid signaling: changing partners causes some excitement. Genes Dev. 1995 Aug 1;9(15):1811–1816. doi: 10.1101/gad.9.15.1811. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Linder S., Krondahl U., Sennerstam R., Ringertz N. R. Retinoic acid-induced differentiation of F9 embryonal carcinoma cells. Exp Cell Res. 1981 Apr;132(2):453–460. doi: 10.1016/0014-4827(81)90120-8. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Love J. M., Gudas L. J. Vitamin A, differentiation and cancer. Curr Opin Cell Biol. 1994 Dec;6(6):825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994 Aug 18;370(6490):567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger D., Clifford J., Chiba H., Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. I., Gruss P. Mouse Cdx-1 expression during gastrulation. Development. 1993 Jan;117(1):191–203. doi: 10.1242/dev.117.1.191. [DOI] [PubMed] [Google Scholar]
- Murphy P., Hill R. E. Expression of the mouse labial-like homeobox-containing genes, Hox 2.9 and Hox 1.6, during segmentation of the hindbrain. Development. 1991 Jan;111(1):61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- Nagy L., Thomázy V. A., Shipley G. L., Fésüs L., Lamph W., Heyman R. A., Chandraratna R. A., Davies P. J. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995 Jul;15(7):3540–3551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T., Glick A. B., Geiser A. G., O'Reilly M. A., Miller J., Roberts A. B., Sporn M. B. Molecular cloning and structure of the human transforming growth factor-beta 2 gene promoter. Growth Factors. 1991;4(4):247–255. doi: 10.3109/08977199109043910. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995 Apr 1;9(7):769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Lutz Y., Pfister V., Heyberger S., Scheuer I., Chambon P., Gaub M. P. Detection of retinoid X receptors using specific monoclonal and polyclonal antibodies. Biochem Biophys Res Commun. 1994 Oct 28;204(2):525–536. doi: 10.1006/bbrc.1994.2491. [DOI] [PubMed] [Google Scholar]
- Rogers M. B., Watkins S. C., Gudas L. J. Gene expression in visceral endoderm: a comparison of mutant and wild-type F9 embryonal carcinoma cell differentiation. J Cell Biol. 1990 May;110(5):1767–1777. doi: 10.1083/jcb.110.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J., Sundell C. L., Liskay R. M. Cell-cycle characteristics of undifferentiated and differentiating embryonal carcinoma cells. Dev Biol. 1982 Feb;89(2):516–520. doi: 10.1016/0012-1606(82)90340-2. [DOI] [PubMed] [Google Scholar]
- Roy B., Taneja R., Chambon P. Synergistic activation of retinoic acid (RA)-responsive genes and induction of embryonal carcinoma cell differentiation by an RA receptor alpha (RAR alpha)-, RAR beta-, or RAR gamma-selective ligand in combination with a retinoid X receptor-specific ligand. Mol Cell Biol. 1995 Dec;15(12):6481–6487. doi: 10.1128/mcb.15.12.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kato S., Kohno K., Martin G. R., Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci U S A. 1987 Feb;84(4):935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Henderson N. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 1990 May;2(5):441–449. [PubMed] [Google Scholar]
- Sleigh M. J. Differentiation and proliferation in mouse embryonal carcinoma cells. Bioessays. 1992 Nov;14(11):769–775. doi: 10.1002/bies.950141109. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Parkinson D. R., Cheson B. D., Friedman M. A. Retinoids in cancer therapy. J Clin Oncol. 1992 May;10(5):839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Dyson E., Gumeringer C. L., Price J., Chien K. R., Evans R. M. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994 May 1;8(9):1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Taneja R., Bouillet P., Boylan J. F., Gaub M. P., Roy B., Gudas L. J., Chambon P. Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. J., Wang L., Wu T. C. The expression of retinoid X receptor genes is regulated by all-trans- and 9-cis-retinoic acid in F9 teratocarcinoma cells. Exp Cell Res. 1994 Jan;210(1):56–61. doi: 10.1006/excr.1994.1009. [DOI] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zelent A., Krust A., Petkovich M., Kastner P., Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989 Jun 29;339(6227):714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]