Abstract
Background
Epidemiologic studies have reported various results relating phytoestrogens to prostate cancer (PCa). The aim of this study was to provide a comprehensive meta-analysis on the extent of the possible association between phytoestrogens (including consumption and serum concentration) and the risk of PCa.
Methods
Eligible studies were retrieved via both computer searches and review of references. The summary relative risk ratio (RR) or odds ratio (OR) and 95 % confidence interval (CI) were calculated with random effects models.
Results
A total of 11 studies (2 cohort and 9 case–control studies) on phytoestrogen intake and 8 studies on serum concentration were included in the meta-analysis. The pooled odds ratio (OR) showed a significant influence of the highest phytoestrogens consumption (OR 0.80, 95 % CI 0.70–0.91) and serum concentration (OR 0.83, 95 % CI 0.70–0.99) on the risk of PCa. In stratified analysis, high genistein and daidzein intake and increased serum concentration of enterolactone were associated with a significant reduced risk of PCa. However, no significant associations were observed for isoflavone intake, lignans intake, or serum concentrations of genistein, daidzein, or equol.
Conclusions
The overall current literature suggests that phytoestrogen intake is associated with a decreased risk of PCa, especially genistein and daidzein intake. Increased serum concentration of enterolactone was also associated with a significant reduced risk of PCa. Further efforts should be made to clarify the underlying biological mechanisms.
Keywords: Phytoestrogens, Prostate cancer, Meta-analysis, Observational studies, Serum concentration
Background
Prostate cancer (PCa) was the second most frequently diagnosed cancer and the sixth leading cause of death from cancer among men worldwide in 2008 according to the estimate of the International Agency for Research on Cancer [1]. The worldwide PCa burden is expected to grow to 1.7 million new cases and 499,000 new deaths by 2030 simply due to the growth and aging of the global population [2]. Given the 25-fold variation in disease incidence between population at the highest and lowest risk [1], lifestyle, diet, environmental, and genetic factors have been suggested to play a role in the etiology of the disease [3, 4]. The association between dietary factors and PCa has been investigated and one explanation for the low incidence of the cancer in Asia might be high consumption of soybeans and its products [5, 6], which are rich in one class of phytoestrogens known as isoflavones.
Phytoestrogens, which have structural and functional similarities to 17b-oestradiol, are believed to have a prophylactic effect on PCa [7]. There are 3 main classes of phytoestrogens: isoflavones, lignans, and coumestans. In Western populations with a low intake of isoflavones, phytoestrogen intake is predominantly derived from intake of plant lignans. It is reported that isoflavones, lignans, and their metabolites have anticarcinogenic properties [8, 9]. Isoflavones principally include genistein, daidzein, and glycitein. Equol is a metabolite of daidzein produced by the intestinal microflora [10] that has higher oestrogenic activity than its parent isoflavone. The most abundant lignan in human subjects is enterolactone, which is produced by certain types of intestinal microflora from plant lignan glycosides. Variation in individual metabolism of phytoestrogens due to differences in gut microflora [11] may influence the serum concentration of phytoestrogens and their biologic effects. It is reported that the capacity to produce equol has been found be to lower among American than Japanese and Korean men [12]. So, it is important to quantify the association between serum concentration of phytoestrogens and risk of PCa.
According to 2 previous meta-analyses [13, 14], consumption of soy products rich in isoflavones are inversely associated with PCa risk. However, both of them focused on the soy consumption neglecting the intake of plant lignans (which is the primary phytoestrogen intake in Western populations). Meanwhile, neither one evaluated the association between serum concentration of phytoestrogens and risk of PCa.
Therefore, we performed a meta-analysis to address this gap. We updated and assessed quantitatively the association between intake of isoflavones and lignans and risk of PCa from the cohort and case–control studies. We also investigated the association between serum concentration of phytoestrogens and their metabolites and the risk of PCa.
Methods
Search strategy
We identified relevant publications in the MEDLINE database using PubMed, Web of Science, and the Cochrane Library up to June 2014. Search terms included “phytoestrogens,” “isoflavones,” “lignans,” “flavonoids,” “genistein” or “daidzein,” “glycitein,” “equol,” “enterolactone,” and “enterodiol,” combined with “prostate cancer” or “prostatic carcinoma”. Two of the authors (SW and JH) reviewed the titles and abstracts independently to exclude any clearly irrelevant studies. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. Any disagreements were resolved by discussion. Furthermore, references in the retrieved publications, as well as those in previous reviews [15, 16], were checked for any other pertinent studies.
Study selection
To be included, studies had to fulfill all of the following inclusion criteria: (i) case–control or cohort study published as an original article reported in English between 1980 and February 2014, (ii) estimated the relationship between phytoestrogens (intake or serum concentration) and the risk of PCa, (iii) provided a risk estimate relative risk (RR) [or odds ratio (OR)] and its 95 % confidence intervals (CI) or sufficient information allowing us to compute them, and (iv) adjustment made for age and potential risk factors. In studies with overlapping patients or controls, only the latest or the most informative were included. Any study with inconsistent or erroneous data was excluded. Meeting abstracts with insufficient data or unpublished reports were not considered. We included all methods for measuring exposure to phytoestrogens such as questionnaires, interviews, and serum level or urinary excretion. We did not include studies that used tumor-related biomarkers (such as PSA) as outcome. We also exclude data concerning phytoestrogens and the risk of recurrent PCa.
Data extraction
For each study, the following characteristics were extracted: last name of first author, publication year, country in which the study was conducted, study design, population type and sample size, adjustment for potential confounders, definition of phytoestrogens exposure status, and estimates of associations. The levels of phytoestrogens exposure varied considerably among the studies, so we extracted the most adjusted risk estimate of the highest reported category of phytoestrogens exposure relative to the lowest from these studies for comparison.
Statistical analyses
The ORs were used as the common measure of association across studies by considering the RRs as ORs. The data from individual studies were pooled by use of the random effects model with the DerSimonian-Laird method [17], which considers within-study and between-study variation. We performed subgroup analyses based on different kinds of phytoestrogens. Certain items, such as biochanin A, coumestrol, secoisolariciresinol, or matairesinol, were seldom assessed in individual reports; these analyses were not performed. Meta-regression analysis was used to assess the heterogeneity in publication year, study conducted area, study design, and sample size. The Q-statistic and I2 score were used to assess the between-study heterogeneity of results [18, 19]. Publication bias assessment was done using the Egger regression asymmetry test [20] and the Begg-adjusted rank correlation test [21]. If publication bias was observed, the “trim and fill” method [22] was used to calculate an estimate of the effect size after considering publication bias (adjusted effect size). Possible outliers were visually identified and tested for their effect on the significance of the effect size. The statistical software used was Stata/SE 11.0 (Stata Corporation, College Station, TX), and the significance level was set to P < 0.05.
Results
Phytoestrogen intake
The detailed steps of our literature search are shown in Fig. 1. We identified 13 studies that had investigated the association between on phytoestrogen intake and the PCa risk. We excluded two studies [23, 24] from the analysis because they were updated by Hedelin et al. [25] and Word et al. [26]. The remaining 11 studies selected for analysis are presented in Table 1. Two were cohort studies [27, 28], and the other nine were case–control studies. All of them used quantitative food frequency questionnaire (FFQ) to measure phytoestrogen intake. Two of these studies were quintile comparisons [27, 29], six were quartile comparisons [25, 28, 30–33], and three reported comparison between populations with low and high intakes [26, 34, 35]. Of the studies, four were conducted in North America, four in Europe, and three in Asia. Because different kinds of phytoestrogens were evaluated in these studies, some of which assessed more than one kind of phytoestrogen, we chose the risk estimate for the phytoestrogen kind that was representative of their phytoestrogen intake in overall analysis. These phytoestrogen kinds were prioritized in descending order of total phytoestrogens or isoflavones, genistein, daidzein, and lignans. Subsequently, we did the stratified analysis of individual types of phytoestrogens.
Fig. 1.
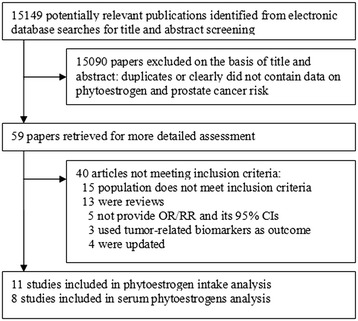
Study selection process
Table 1.
Epidemiologic studies on phytoestrogen intake in association with prostate cancer risk
| Reference | Study site/race | Design | Cases/controls or cohort size | Dietary assessment | Phytoestrogens | Contrast | Adjusted OR (95 % CI) | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Park et al. [27] 2008 | USA/multiethnic | Cohort | 4404/82,483 | QFFQ (118 items) | Genistein | <0.7 vs. ≥3.1 mg/1000 kcal | 0.94 (0.84–1.04) | Time since cohort entry, ethnicity, family history of prostate cancer, education level, BMI, smoking status, and energy intake |
| Daidzein | <0.7 vs. ≥3.2 mg/1000 kcal | 0.92 (0.82–1.02) | ||||||
| Total isoflavones | <1.6 vs. ≥7.2 mg/1000 kcal | 0.93 (0.83–1.04) | ||||||
| Kurahashi et al. [28] 2007 | Japan/Japanese | Cohort | 307/43,509 | FFQ (147 items) | Genistein | <13.2 vs. ≥32.8 mg/day | 0.71 (0.48–1.03) | Age, area, smoking status, drinking frequency, marital status, BMI, intake of total fatty acids, dairy, vegetables, and fruits |
| Daidzein | <8.5 vs. ≥20.4 mg/day | 0.77 (0.52–1.13) | ||||||
| Nagata et al. [31] 2007 | Japan/Japanese | HCC | 200/200 | Semi-quantitative FFQ | Isoflavones | <30.5 vs. ≥89.9 mg/day | 0.48 (0.25–0.93) | Smoking, energy, and PUFA intake |
| Genistein | <1.1 mg/day vs. ≥ 2.5 mg/day | 0.68 (0.39–1.20) | ||||||
| Daidzein | <0.8 mg/day vs. ≥1.9 mg/day | 0.64 (0.36–1.17) | ||||||
| Heald et al. [32] 2007 | Scotland/Scottish | PCC | 433/483 | SCG-FFQ | Isoflavones | ≤581.1 μg/day vs. ≥1982.8 μg/day | 1.18 (0.79–1.75) | Age, total energy intake, family history of PCa and BrCa, Carstairs Deprivation Index, smoking and energy intake: BMR ratio |
| Bosetti et al. [29] 2006 | Italy/Italian, | HCC | 1294/1451 | FFQ | Isoflavones | ≤14.7 vs. ≥32.2 μg/day | 0.98 (0.76–1.26) | Terms for age, study center, education, body mass index, family history of prostate cancer, and total calorie intake |
| Hedelin et al. [25] 2006 | Sweden/Swedish | PCC | 1499/1130 | FFQ (261 items) | Phytoestrogens | ≤1.18 vs. >4.71 μg/day | 0.74 (0.57–0.95) | Age, intake of antibiotics, zinc, animal fat, total energy intake, alcohol, vegetable fat, red meat during the last year |
| Lignans | ≤113 vs. >213 μg/day | 0.85 (0.65–1.12) | ||||||
| Isoflavonoids | ≤1.0 vs. >2.6 μg/day | 0.99 (0.77–1.28) | ||||||
| Genistein | ≤0.27 vs. >1.08 μg/d | 0.97 (0.75–1.26) | ||||||
| Daidzein | ≤0.49 vs. >1.11 μg/d | 1.22 (0.92–1.62) | ||||||
| Lee et al. [30] 2003 | China/Chinese | HCC | 133/265 | FFQ | Genistein | <17.9 vs. >62.0 mg/day | 0.53 (0.29–0.97) | Age and total calories |
| Daidzein | <10.0 vs. >36.3 mg/day | 0.56 (0.31–1.04) | ||||||
| Strom et al. [34] 1999 | USA/American white | HCC | 83/107 | FFQ (modified block) | Genistein | Low vs. high | 0.71 (0.39–1.30) | Age, family history of prostate cancer, alcohol intake, and total caloric intake |
| Daidzein | 0.57 (0.31–1.05) | |||||||
| McCann et al. [33] 2005 | USA/American | PCC | 433/538 | FFQ (172 items) | Lignans | <335.4 vs. >603.9 μg/day | 0.66 (0.47–0.94) | Age, education, body mass index, cigarette smoking status, and total energy |
| Word et al. [26] 2010 | UK/British Caucasians | Nested C-C | 203/800 | FFQ and 7-day food diaries | Daidzein | Low vs. high | 0.88 (0.72–1.09) | Age, height, weight, physical activity, social class, family history of prostate cancer, and daily intake of energy, fat, zinc, selenium, dairy products, and lycopene |
| Genistein | 0.89 (0.72–1.09) | |||||||
| Total isoflavones | 0.87 (0.70–1.09) | |||||||
| Total lignans | 0.96 (0.71–1.31) | |||||||
| Lewis et al. [35] 2009 | USA/American | HCC | 478/382 | Block FFQ (100 items) | Genistein | ≤196.0 vs. >196.0 mcg | 0.54 (0.33–0.89) | Age, education, BMI, smoking history, family history of prostate cancer in first-degree relatives, and total caloric intake |
| Daidzein | ≤77.0 vs. >77.0 mcg | 0.54 (0.33–0.89) |
QFFQ quantitative food frequency questionnaire, PUFA polyunsaturated fatty acid, SCG-FFQ Scottish Collaborative Group-FFQ, EPIC European Prospective Investigation into Cancer and Nutrition, HCC hospital-based case–control, PCC population-based case–control, BMI body mass index, PCa prostate cancer
We found that phytoestrogen intake (OR 0.80, 95 % CI 0.70–0.91) was statistically significantly associated with reduced risk of PCa with significant heterogeneity (I2 = 47.7, P = 0.039). We used meta-regression analysis to explore the influence of publication year, study design, sample size, and study conducted area. However, none was identified as a possible source of heterogeneity among all the included studies (data not shown). Further scrutiny found that the heterogeneity was reduced when the analysis was stratified by geographical region (see Fig. 2). The association was stronger among Asian (OR 0.62, 95 % CI 0.46–0.82) than American (OR 0.74, 95 % CI 0.56–0.98) and European (OR 0.90, 95 % CI 0.76–1.06). The funnel plot showed some asymmetry. Begg’s test (P = 0.043) and Egger’s test (P = 0.021) for publication bias were significant. The trim and fill analysis yielded the same conclusions without evidence of any potentially missed unpublished studies.
Fig. 2.
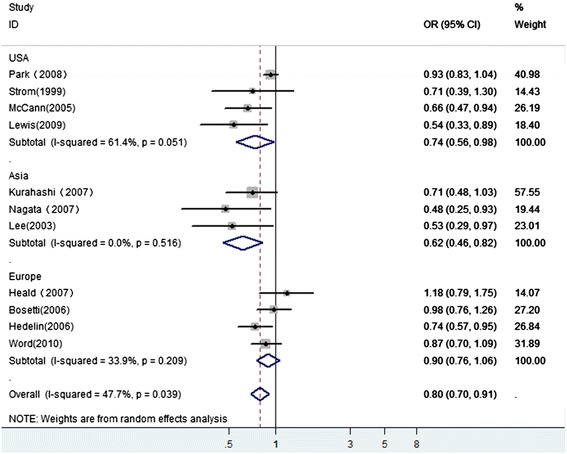
A forest plot showing pooled data for the association between phytoestrogen intake and prostate cancer risk in a variety of geographical region
In stratified analysis of individual types of phytoestrogens (see Fig. 3), eight studies investigated the genistein and daidzein, three studies tested lignans, and six studies tested the isoflavones. The risk of PCa decreased significantly in association with high consumption of genistein (OR 0.83, 95 % CI 0.72–0.95) and daidzein (OR 0.82, 95 % CI 0.70–0.97), but high consumption of lignans (OR 0.87, 95 % CI 0.69–1.09) and isoflavones (OR 0.93, 95 % CI 0.84–1.04) were not significantly associated with the risk of PCa. Heterogeneity was detected (P = 0.035) among the eight studies evaluating daidzein intake and the risk of PCa. In contrast, there was no evidence of heterogeneity among studies of genistein, lignans, and isoflavones.
Fig. 3.
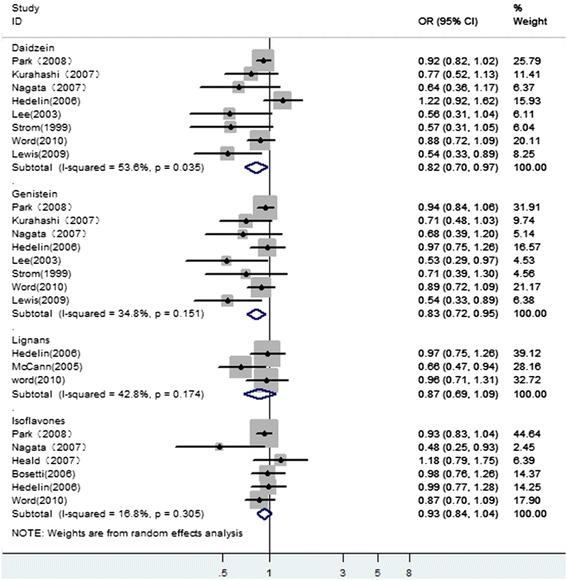
A forest plot showing the pooled risk estimates of prostate cancer for different types of phytoestrogen intake
The Begg’s tests and Egger’s test provided no evidence of publication bias for daidzein and isoflavones (data not shown). However, for genistein analysis, the Begg’s tests indicated no substantial publication bias (P = 0.083), while the Egger’s test provided evidence for publication bias (P = 0.005). We used the trim and fill method to estimate the missing studies. The result yielded the same conclusions without evidence of any potentially missed unpublished studies.
Serum phytoestrogens
We identified 10 studies that had investigated the association between serum phytoestrogen concentration and the risk for PCa. We excluded two studies [26, 36] because they presented results identical to those of a later publication. One study [37] contained data from three countries that were recently updated for two of these countries [38, 39]. So, we included all these three studies and extracted the latest data. The remaining 8 studies selected for analysis are presented in Table 2. All of them are case–control studies. Five of these studies are quartile comparisons [25, 32, 37–39], two are tertile comparison [40, 41], and one is a quintile comparison [42]. Four studies investigated genistein, daidzein, and equol [32, 40–42]. Six studies investigated the plasma enterolactone [25, 32, 37–39, 42]. Only one study provided the relationship between serum isoflavones and PCa risk [32]. In overall combination of serum phytoestrogens, these serum phytoestrogen kinds were prioritized in descending order of total isoflavones, genistein, daidzein, equol, and enterolactone. The summary OR was 0.83 (95 % CI 0.70–0.99), and the P value for heterogeneity was 0.525. The Begg funnel plots were symmetric, and the Egger’s tests provided no evidence of publication bias (P = 0.497). When stratified analysis was conducted of individual types of serum phytoestrogens, only serum enterolactone was inversely associated with the risk of PCa with no heterogeneity (P = 0.183) (see Fig. 4). High serum concentration of genistein, daidzein, and equol were not associated with the risk of PCa (see Fig. 4). There was no heterogeneity among these subgroup studies. No publication bias was detected either by Begg’s test or by Egger’s test in all subgroups (data not shown).
Table 2.
Epidemiologic studies on serum phytoestrogens concentrations in association with prostate cancer risk
| Reference | Study site/race | Design | Cases/controls | Serum phytoestrogens | Contrast | Adjusted OR (95 % CI) | Adjustment |
|---|---|---|---|---|---|---|---|
| Heald et al. [25] 2007 | Scotland/Scottish | PCC | 249/205 | Equol | 0 vs. ≥0.10 nmol/l | 1.07 (0.71–1.61) | Age, total energy intake, family history of PCa and BrCa, Carstairs Deprivation Index, smoking and energy intake: BMR ratio. |
| Daidzein | ≤8.26 vs. >29.11 nmol/l | 1.34 (0.76–2.38) | |||||
| Genistein | ≤14.23 vs. >64.53 nmol/l | 1.36 (0.76–2.43) | |||||
| Enterolactone | ≤8.41 vs. >28.90 nmol/l | 0.40 (0.22–0.71) | |||||
| Hedelin et al. [32] 2006 | Sweden/Swedish | PCC | 1499/1130 | Enterolactone | ≤15.2 vs. >37.8 nmol/l | 0.74 (0.41–1.32) | Age, intake of antibiotics, zinc, animal fat, total energy intake, alcohol, vegetable fat, red meat during the last year |
| Kurahashi et al. [40] 2007 | Japan/Japanese | NCC | 307/43,509 | Genistein | <57 vs. ≥151.7 ng/ml | 0.66 (0.40–1.08) | Smoking status, alcohol intake, marital status, and intake of green tea, protein, fiber, and green or yellow vegetables. |
| Daidzein | <22 vs. ≥61.5 ng/ml | 0.78 (0.49–1.25) | |||||
| Equol | <1.0 vs. ≥15.0 ng/ml | 0.60 (0.36–0.99) | |||||
| Ozasa et al. [41] 2004 | Japan/Japanese | NCC | 52/151 | Genistein | <239 vs. >682 nM | 0.76 (0.32–1.82) | Age |
| Daidzein | <89 vs. >239 nM | 0.74 (0.31–1.76) | |||||
| Equol | <1.9 vs. >56.1 nM | 0.39 (0.15–0.98) | |||||
| Travis et al. [42] 2009 | EPIC | NCC | 950/1042 | Genistein | <0.30 vs. ≥7.00 ng/ml | 0.74 (0.54–1.00) | Smoking, education, BMI, physical activity, alcohol intake, and marital status |
| Daidzein | <0.30 vs. ≥4.10 ng/ml | 0.80 (0.60–1.07) | |||||
| Equol | <0.05 vs. ≥0.80 ng/ml | 0.99 (0.70–1.39) | |||||
| Enterolactone | <0.05 vs. ≥0.80 ng/ml | 0.77 (0.57–1.04) | |||||
| Kilkkinen et al. [38] 2003 | Finland | NCC | 214/214 | Enterolactone | <5.9 vs. ≥24.4 nmol/l | 0.71 (0.42–1.21) | Age match |
| Stattin et al. [37] 2002 | Norway, Finland, Sweden | NCC | 794/2550 | Enterolactone | <8.9 vs. ≥27.89 nmol/l | Finland 1.21 (0.91–1.60) | Age match |
| <3.49 vs. ≥11.58 nmol/l | Norway 1.02 (0.59–1.76) | ||||||
| <7.15 vs. ≥25.14 nmol/l | Sweden 0.87 (0.45–1.67) | ||||||
| Stattin et al. [39] 2004 | Sweden | NCC | 265/525 | Enterolactone | <9.38 vs. ≥28.31 nmol/l | 1.05 (0.65–1.69) | Age, BMI, smoking, and fasting |
EPIC European Prospective Investigation into Cancer and Nutrition (include 23 centers in 10 European countries), BMI body mass index, NCC nested case–control, PCC population-based case–control, PCa prostate cancer
Fig. 4.
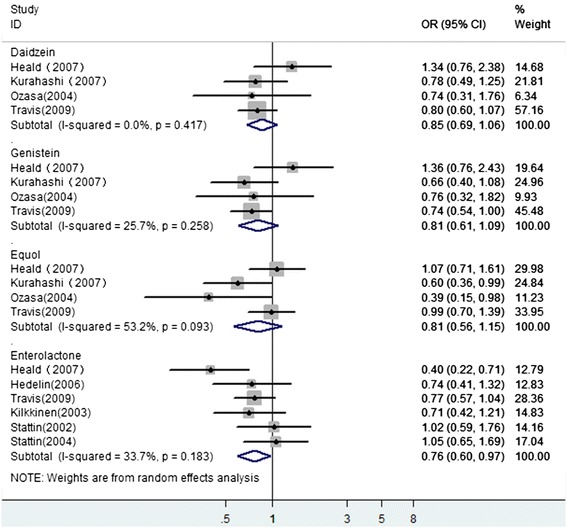
A forest plot depicting the pooled risk estimates on the association between serum phytoestrogen concentration and prostate cancer risk
Discussion
This meta-analysis demonstrated that consumption of phytoestrogens was associated with a reduction in PCa risk of 20 % in men when the highest reported intake was compared with the lowest reported intake. The results of our separate analysis based on the type of phytoestrogens showed inverse associations for the consumption of genistein and daidzein and with increased serum concentrations of enterolactone. However, no significant associations were observed for isoflavone intake, lignan intake, or serum level of genistein, daidzein, and equol.
It has been suggested that phytoestrogens may prevent cancer by a variety of mechanisms, including sex and/or growth hormone regulation, antioxidant properties, apoptosis of PCa cells, and/or inhibition of angiogenesis, invasion, and metastasis [43, 44]. These bioavailable metabolites can be estimated from dietary intake data by using in vitro data from incubation of foods with human feces [45, 46], but this assessment does not take into account interindividual variations in microbial synthesis. Moreover, the quantification of the phytoestrogen content of food can vary threefold to fourfold, depending on variety, environmental factors, growth, harvesting time, and processing [47]. So, the associations between phytoestrogen intake and risk of PCa were not so convincing. Measurement of metabolites in blood and urine is considered to be more objective and precise [48]. As we all know, human gut microflora have been shown to exert metabolic activities on phytoestrogens [49]. As the gut microflora may differ by its concentration and composition from one person to the other, antimicrobials (i.e., antibiotics) may lead to intra- and interindividual variations in amounts of intestinal phytoestrogen metabolites that are converted from consumed phytoestrogens by the gut bacteria. In comparison to the metabolism of isoflavones, biotransformation of lignans has been found less variable [50]. This may explain why we found a significant association with increased serum concentrations of enterolactone but did not find significant associations with serum concentrations of genistein, daidzein, and equol. Of course, that may be also related to other bioactive isoflavone metabolites that were not researched yet.
Due to the potential benefits, there have been six randomized controlled trials [51–56] that used phytoestrogens in man already diagnosed with PCa. Most of them had small sample sizes and were of short duration. These studies assessed the effect of soy/isoflavones on tumor marker (prostate-specific antigen, PSA) or hormonal markers levels in men with PCa. So far, no study was reported on 5-year survival or metastasis. A search of ClinicalTrials.gov reveals numerous currently ongoing trials that are investigating the role of phytoestrogens in the treatment of PCa (i.e., NCT01126879, NCT01325311, NCT01682941, NCT01036321, NCT00345813). The results of those studies will provide more evidence for the role of phytoestrogens.
It is worth noting that in our analysis, stratification by region yielded a significant inverse association with PCa risk for studies in Asian populations, a marginally significant inverse association with risk for studies in US populations but no significant association with risk for those in Europe populations. This difference may, in part, be due to differences in environment and dietary patterns in these regions. Characterization of the individual variability as defined by the gut microflora composition and gene polymorphisms [23] may also help to explain the discrepancies observed so far.
As a meta-analysis of previously published observational studies, our study has several limitations that need to be taken into account. First, only English language articles were included. We did not attempt to uncover unpublished observations and did not include studies with insufficient information to estimate an adjusted OR, which could bring publication bias, even though the trim and fill analysis yielded the same conclusions without evidence of any potentially missed unpublished studies. Second, for now, the two available cohort studies could not provide sufficient data for meta-analysis, so we have to choose case–control studies as the data resource for analysis. In addition, because some items were combined only from three studies, the total numbers of cases remained low. Third, the intake levels of phytoestrogens in the lowest and highest categories and the range of consumption level varied across studies. Moreover, food frequency questionnaires which assess dietary habits may lead to measurement errors. It is not only due to recall bias but also to the estimation by using different food composition databases, which may not be complete for the whole range of foods consumed. On the other hand, variation in individual metabolism of phytoestrogens due to differences in gut microflora and measurement in a single blood or urine sample may only reflect recent dietary intake. These differences may have contributed to the heterogeneity among studies. To be honest, phytoestrogen spectrum and content varies between the plant species, sort, and origin. Even the same molecule arising from the different sources can exert various effects. It may not be excluded that synthetic phytoestrogens with desirable structure and activity could be an easier and safer alternative of the traditional plant product of variable origin, phytoestrogen content, and activity. Several studies are going on developing novel and more selective synthetic phytoestrogens.
Conclusions
Our findings support the hypotheses that serum enterolactone and consumption of genistein and daidzein protect against PCa risk. Interestingly enough, an association between PCa risk and isoflavone intake or serum concentrations of its metabolites was not found. The complexity of phytoestrogen composition and its metabolism make the evaluation of the effect of phytoestrogen on PCa very difficult. In the light of these findings, further prospective epidemiological studies using improved food databases and experimental studies are needed to identify the specific compounds that provide protection, to determine precisely how the complex metabolism of phytoestrogens may interact with other mechanisms to prevent cancer. Synthetic phytoestrogens with desirable structure and activity could be an easier and safer alternative of the traditional plant product.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Zhejiang (Grant No. Q14H050007).
Abbreviations
- PCa
prostate cancer
- RR
risk ratio
- OR
odds ratio
- CI
confidence interval
- PSA
prostate-specific antigen
- FFQ
quantitative food frequency questionnaire
Footnotes
Jinjing He and Shuai Wang contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SW and JH conceived the study concept and participated in its design, data extraction, statistical analysis, and manuscript drafting and editing. SW and MZ participated in the literature research and manuscript drafting and editing. WY participated in design and data extraction. YZ participated in manuscript drafting and editing and statistical analysis. XH conceived the study concept and participated in data analysis. All authors read and approved the final manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. p. 29. [Google Scholar]
- 3.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl. 2012;14(3):365–74. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekman P. Genetic and environmental factors in prostate cancer genesis: identifying high-risk cohorts. Eur Urol. 2012;35(5–6):362–9. doi: 10.1159/000019910. [DOI] [PubMed] [Google Scholar]
- 5.Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. 1999;70(3):439s–50. doi: 10.1093/ajcn/70.3.439s. [DOI] [PubMed] [Google Scholar]
- 6.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case–control study. Cancer Epidemiol Biomarkers Prev. 2000;9(8):795–804. [PubMed] [Google Scholar]
- 7.Yildiz F. Phytoestrogens in functional foods. Florida: CRC Press; 2005. [Google Scholar]
- 8.Hsu A, Bray TM, Helferich WG, Doerge DR, Ho E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp Biol Med. 2010;235(1):90–7. doi: 10.1258/ebm.2009.009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azrad M, Vollmer RT, Madden J, Dewhirst M, Polascik TJ, Snyder DC, et al. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J Med Food. 2013;16(4):357–60. doi: 10.1089/jmf.2012.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132(12):3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 11.Turner NJ, Thomson BM, Shaw IC. Bioactive isoflavones in functional foods: the importance of gut microflora on bioavailability. Nutr Rev. 2003;61(6):204–13. doi: 10.1301/nr.2003.jun.204-213. [DOI] [PubMed] [Google Scholar]
- 12.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34(2):86–9. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 13.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61(5):598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89(4):1155–63. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 15.Ganry O. Phytoestrogens and prostate cancer risk. Prev Med. 2005;41(1):1–6. doi: 10.1016/j.ypmed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Miller PE, Snyder DC. Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract. 2012;27(5):599–612. doi: 10.1177/0884533612456043. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Zintzaras E, Ioannidis J. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Hedelin M, Balter KA, Chang ET, Bellocco R, Klint A, Johansson JE, et al. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate. 2006;66(14):1512–20. doi: 10.1002/pros.20487. [DOI] [PubMed] [Google Scholar]
- 24.Low YL, Taylor JI, Grace PB, Mulligan AA, Welch AA, Scollen S, et al. Phytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer risk. Nutr Cancer. 2006;56(1):31–9. doi: 10.1207/s15327914nc5601_5. [DOI] [PubMed] [Google Scholar]
- 25.Hedelin M, Klint A, Chang ET, Bellocco R, Johansson JE, Andersson SO, et al. Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden) Cancer Causes Control. 2006;17(2):169–80. doi: 10.1007/s10552-005-0342-2. [DOI] [PubMed] [Google Scholar]
- 26.Ward HA, Kuhnle GG, Mulligan AA, Lentjes MA, Luben RN, Khaw KT. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. Am J Clin Nutr. 2010;91(2):440–8. doi: 10.3945/ajcn.2009.28282. [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN, Multiethnic CS. Legume and isoflavone intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. 2008;123(4):927–32. doi: 10.1002/ijc.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S, et al. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16(3):538–45. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 29.Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, Negri E, et al. Flavonoids and prostate cancer risk: a study in Italy. Nutr Cancer. 2006;56(2):123–7. doi: 10.1207/s15327914nc5602_1. [DOI] [PubMed] [Google Scholar]
- 30.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12(7):665–8. [PubMed] [Google Scholar]
- 31.Nagata Y, Sonoda T, Mori M, Miyanaga N, Okumura K, Goto K, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr. 2007;137(8):1974–9. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 32.Heald CL, Ritchie MR, Bolton-Smith C, Morton MS, Alexander FE. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br J Nutr. 2007;98(2):388–96. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 33.McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, et al. Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York. Nutr Cancer. 2005;53(1):33–41. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 34.Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, et al. Phytoestrogen intake and prostate cancer: a case–control study using a new database. Nutr Cancer. 1999;33(1):20–5. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JE, Soler-Vila H, Clark PE, Kresty LA, Allen GO, Hu JJ. Intake of plant foods and associated nutrients in prostate cancer risk. Nutr Cancer. 2009;61(2):216–24. doi: 10.1080/01635580802419756. [DOI] [PubMed] [Google Scholar]
- 36.Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Lack of prospective associations between plasma and urinary phytoestrogens and risk of prostate or colorectal cancer in the European Prospective into Cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2891–4. doi: 10.1158/1055-9965.EPI-08-0335. [DOI] [PubMed] [Google Scholar]
- 37.Stattin P, Adlercreutz H, Tenkanen L, Jellum E, Lumme S, Hallmans G, et al. Circulating enterolactone and prostate cancer risk: a Nordic nested case–control study. Int J Cancer. 2002;99(1):124–9. doi: 10.1002/ijc.10313. [DOI] [PubMed] [Google Scholar]
- 38.Kilkkinen A, Virtamo J, Virtanen MJ, Adlercreutz H, Albanes D, Pietinen P. Serum enterolactone concentration is not associated with prostate cancer risk in a nested case–control study. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1209–12. [PubMed] [Google Scholar]
- 39.Stattin P, Bylund A, Biessy C, Kaaks R, Hallmans G, Adlercreutz H. Prospective study of plasma enterolactone and prostate cancer risk (Sweden) Cancer Causes Control. 2004;15(10):1095–102. doi: 10.1007/s10552-004-1480-7. [DOI] [PubMed] [Google Scholar]
- 40.Kurahashi N, Iwasaki M, Inoue M, Sasazuki S, Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case–control study: the Japan Public Health Center. J Clin Oncol. 2008;26(36):5923–9. doi: 10.1200/JCO.2008.16.8807. [DOI] [PubMed] [Google Scholar]
- 41.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Serum phytoestrogens and prostate cancer risk in a nested case–control study among Japanese men. Cancer Sci. 2004;95(1):65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100(11):1817–23. doi: 10.1038/sj.bjc.6605073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lampe JW. Emerging research on equol and cancer. J Nutr. 2010;140(7):1369S–72. doi: 10.3945/jn.109.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91(04):513–31. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 45.Coward L, Barnes NC, Setchell KD, Barnes S. Genistein, daidzein, and their. beta.-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41(11):1961–7. doi: 10.1021/jf00035a027. [DOI] [Google Scholar]
- 46.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. 1991. [DOI] [PubMed] [Google Scholar]
- 47.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the isoflavone content of selected foods release 2.0. Maryland: US Department of Agriculture; 2008. [Google Scholar]
- 48.Horn-Ross PL, Barnes S, Lee M, Coward L, Mandel JE, Koo J, et al. Assessing phytoestrogen exposure in epidemiologic studies: development of a database (United States) Cancer Causes Control. 2000;11(4):289–98. doi: 10.1023/A:1008995606699. [DOI] [PubMed] [Google Scholar]
- 49.Akaza H. Prostate cancer chemoprevention by soy isoflavones: role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012;103(6):969–75. doi: 10.1111/j.1349-7006.2012.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36(1):27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 51.Dalais FS, Meliala A, Wattanapenpaiboon N, Frydenberg M, Suter DA, Thomson WK, et al. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64(3):510–5. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 52.deVere WRW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62(8):1036–43. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar NB, Cantor A, Allen K, Riccardi D, Besterman‐Dahan K, Seigne J, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59(2):141–7. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 54.Kumar NB, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, et al. Results of a randomized phase I dose-finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. J Soc Integr Oncol. 2010;8(1):3. [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar NB, Krischer JP, Allen K, Riccardi D, Besterman-Dahan K, Salup R, et al. A Phase II randomized, placebo-controlled clinical trial of purified isoflavones in modulating steroid hormones in men diagnosed with localized prostate cancer. Nutr Cancer. 2007;59(2):163–8. doi: 10.1080/01635580701432678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr Cancer. 2011;63(6):889–98. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]


