Abstract
It has been hypothesized that the relatively rare autosomal dominant Alzheimer disease (ADAD) may be a useful model of the more frequent, sporadic, late-onset AD (LOAD). Individuals with ADAD have a predictable age at onset and the biomarker profile of ADAD participants in the preclinical stage may be used to predict disease progression and clinical onset. However, the extent to which the pathogenesis and neuropathology of ADAD overlaps with that of LOAD is equivocal. To address this uncertainty, two multicenter longitudinal observational studies, the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN), leveraged the expertise and resources of the existing Knight Alzheimer Disease Research Center (ADRC) at Washington University School of Medicine, St. Louis, Missouri, USA, to establish a Neuropathology Core (NPC). The ADNI/DIAN-NPC is systematically examining the brains of all participants who come to autopsy at the 59 ADNI sites in the USA and Canada and the 14 DIAN sites in the USA (8), Australia (3), UK (1), and Germany (2). By 2014, 41 ADNI and 24 DIAN autopsies (involving 9 participants and 15 family members) had been performed. The autopsy rate in the ADNI cohort in the most recent year was 93% (total since NPC inception: 70%). In summary, the ADNI/DIAN NPC has implemented a standard protocol for all sites to solicit permission for brain autopsy and to send brain tissue to the NPC for a standardized, uniform, and state-of-the-art neuropathologic assessment. The benefit to ADNI and DIAN of the implementation of the NPC is very clear. The NPC provides final ‘gold standard’ neuropathological diagnoses and data against which the antecedent observations and measurements of ADNI and DIAN can be compared.
Keywords: Autosomal dominant, Alzheimer disease, Late-onset Alzheimer disease, neuropathologic diagnostic criteria, neuropathologic heat map, PET-PiB amyloid imaging
INTRODUCTION
The Alzheimer Disease Neuroimaging Initiative (ADNI) (http://www.adni-info.org/) was established to determine the relationships among the clinical, cognitive, imaging, genetic, and biochemical biomarker characteristics of sporadic, predominantly late-onset, Alzheimer disease (LOAD) (1,2). A complementary longitudinal observational study, the Dominantly Inherited Alzheimer Network (DIAN) focuses on the rarer, mainly early-onset, autosomal dominant AD (ADAD) (http://dian-info.org/) with known gene defects (3,4). Both ADNI and DIAN are informing the neuroscience of AD, identifying diagnostic, prognostic, and potentially theranostic biomarkers to be used in clinical trials, and helping to develop the most effective clinical trial protocols. Both projects are funded as public-private collaborations involving the National Institute of Aging of the National Institutes of Health (NIH) (ADNI through NIH Grant U01 AG0249049 and DIAN through U01 AG032438), foundations, associations, and multiple partners in industry (http://www.adni-info.org/Scientists/ADNISponsorsAndPartners.aspx).
To achieve the goals of both ADNI and DIAN, the Neuropathology Core is essential to validate the clinical and biomarker classifications and diagnoses; otherwise, the data generated by the different clinical assessments, imaging modalities, and biomarkers obtained from participants believed to have AD may be contaminated by the effects of individuals who may not have AD or, more commonly, by the effects of common co-morbidities such as vascular disease and non-AD neurodegenerative disorders. A single Neuropathology Core site is advantageous because processing and staining methods vary across neuropathology laboratories, and neuropathologists may differ in their application and interpretation of diagnostic criteria, even for the neuropathologic diagnosis of AD. The literature has extensive data showing variability between different neuropathologists, sites, and countries (5). A single Neuropathology Core ensures uniformity and fidelity of staining and application of diagnostic criteria to all ADNI and DIAN participants who come to autopsy.
The ADNI/DIAN-NPC capitalizes on the existing infrastructure of the Washington University Knight Alzheimer Disease Research Center (WU ADRC; P50-AG05681, JC Morris, PI), funded continuously by the National Institute on Aging since 1985. The ADRC's Administrative (Dr. Morris) and Neuropathology (Dr. Cairns) Cores provide the framework for both the ADNI-NPC and DIAN-NPC, and will continue to do so during the period of renewed funding. Fidelity of data between ADNI, DIAN and the National Alzheimer Coordinating Center (NACC; U01 AG016976, W. Kukull, PI) is maintained by using the same NACC Neuropathology Data Form. This data collection instrument is used by all NIH-funded Alzheimer Disease Centers (ADCs) to report neuropathological findings from autopsied cases and remains the primary data collection instrument. In this way, the ADNI/DIAN-NPC uses standard criteria for neuropathologic diagnoses of dementing illness and existing protocols and procedures to achieve these diagnoses (6–14). Importantly, the ADNI/DIAN-NPC does not interfere with or supersede neuropathologic activities at any ADNI or DIAN site. The ADNI/DIAN-NPC uses brain tissue obtained at the participating sites to provide a uniform neuropathologic assessment to support the clinical classifications and research aims of both ADNI and DIAN.
NIH funding of the NPC started on September 1, 2007 for ADNI, and on July 1, 2014 for DIAN (prior to this date, the Core received philanthropic support). The NPC serves all ADNI sites (n = 59) in the USA and Canada, and all DIAN sites (n = 14) in the USA (8), Australia (3), UK (1), and Germany (2). Now in its third phase (ADNI, ADNI GO and ADNI 2), ADNI 2 is studying the rate of change of cognition, function, brain structure, and biomarkers in 150 elderly controls, 450 participants with mild cognitive impairment, 150 with mild to moderate AD and a new group of 100 people with significant, yet subtle, memory complaints, referred to as the significant memory concern cohort.
In comparison, DIAN in its initial funding period established an international registry of biological adult children of a parent with AD caused by an APP, PSEN1, or PSEN2 mutation, including symptomatic and asymptomatic mutation carriers (MCs) and non-carriers (NCs). Participants are assessed every 2 years with the uniform DIAN protocol. In order to accommodate the attrition of the cohort caused by recruitment to the DIAN Trials Unit DIAN aims to recruit to the registry 50 new participants, both MCs and NCs, in Year 1 of the next budget period to maintain the total DIAN cohort at ~250 individuals. These new participants will include those who are more than 15 years younger than the estimated age at symptomatic onset (EAO) in order to explore the earliest observable biomarker changes of preclinical AD.
The ADNI/DIAN-NPC is achieving its stated goals. It has: (1) provided and implemented training materials and protocols to assist clinicians at ADNI and DIAN sites in obtaining voluntary consent for brain autopsy in participants; (2) established a central laboratory to provide uniform neuropathologic assessments in all autopsied participants in accordance with standard criteria [4–18] and has thereby promoted clinical-neuroimaging-neuropathologic correlations; (3) established a state-of-the-art resource for fixed and frozen brain tissue obtained from autopsied ADNI and DIAN participants to support biomarker studies; (4) developed a process wherein investigators may request and receive access to the tissue and data for research purposes; and (5) established an ongoing interaction with the Data Coordinating Centers of ADNI and DIAN to support the fidelity and exchange of NPC data with all ADNI and DIAN components, in support of synergistic progress toward common research goals.
METHODS
Provision of training materials and protocols to assist clinicians at participating sites in obtaining voluntary consent for brain autopsy in ADNI and DIAN participants
As there may be personnel changes over time, there is a continuing need to monitor each site to ensure that training and protocols for obtaining autopsies are in place, so it is essential to maintain a dedicated coordinator within the NPC to ensure these functions are performed over the period of the grant. At each site, to obtain consent for an autopsy, the physician leads a discussion about the autopsy with all participants (demented and non-demented) at their initial assessment (study partners and families are welcomed in the discussion and required for participants with dementia). There are three objectives of the discussion: (1) to convey information about the value of brain autopsy in confirming the clinical diagnosis and advancing knowledge regarding pre-symptomatic and symptomatic AD; (2) to initiate consideration of the individual's wishes concerning an autopsy; and (3) to answer questions, misconceptions, or concerns about autopsy. The involvement of the physician in these discussions emphasizes the importance of the autopsy. The discussions are repeated on an annual basis if the individual remains undecided about autopsy, but are terminated once a decision is reached. There is no pressure on an individual to decide; they are encouraged to involve family members, clergy, physicians, or other appropriate persons in their decision-making. Participants are assured that a decision to decline autopsy does not in any way jeopardize their research participation or any other patient rights. The protocols used at each site are approved by the local Institutional Review Board, or Ethics Committee, and conform to the provisions of the Declaration of Helsinki (as revised in Brazil, 2013; http://www.wma.net/en/30publications/10policies/b3/index.html).
When voluntary consent is granted, more detailed information is provided about procedures to follow at the time of death including telephone numbers to call and other guidelines (sample forms are available online at http://dian-info.org/ and http://www.adni-info.org/). A Participant is strongly encouraged to share this information with the next-of-kin, a Durable Power of Attorney (DPOA), and personal physician. In many states, final legal authorization by the Legally Authorized Representative (LAR) or next-of-kin must be obtained at the time of death. Each ADNI and DIAN site is encouraged to establish an autopsy coordinator (typically a research nurse or coordinator) who processes the autopsy consent, provides information as needed, and monitors the need to update any information (e.g., change of address) at the ADNI or DIAN participant's longitudinal assessments. The site coordinator also develops local procedures to facilitate autopsies outside of usual office hours (e.g., at evenings and weekends). The actual procedures vary in accordance with specific needs and resources of each site (one model used by many ADCs is to provide 24-hour telephone access).
At the time of death, the site autopsy coordinator (or a suitable representative) facilitates arrangements to ensure the completion of the autopsy. The site coordinator notifies the NPC, which in turn verifies that the site neuropathologist has the dissection protocol and necessary materials to send the requisite tissue to the NPC in St. Louis, Missouri. The NPC, in addition to instructing site personnel at each ADNI and DIAN Steering Committee Meeting in these procedures, is available at any time to answer questions. Contact information, including a 24-hour pager, is available. At sites that already have ADRC/ADC neuropathology services these continue to follow their own existing protocols. For sites that do not have established neuropathology services, transportation costs from the point of death to the autopsy location, costs of the autopsy procedure, and shipment of specimens are covered by the NPC so that the decedent's family and the individual site do not incur extra expense. Once the participant has given consent (provisional or otherwise), the Acknowledgement of Autopsy Authorization letter and supporting documentation are sent to the following: participant and/or family and/or applicable other (e.g., DPOA), nursing home, funeral home/transport service, and the participant's private physician (as requested).
Maintaining a central laboratory to provide uniform neuropathologic assessments
Where possible, each center is encouraged to undertake its own brain assessment and forward a standard set of fixed-tissue blocks or sections and frozen tissue to the NPC. Non-standard blocks and sections (e.g. whole coronal sections) may also be handled by the NPC. For sites that do not routinely undertake neuropathologic studies, a separate brain removal protocol is available (sample protocols are available online at http://dian-info.org/ and http://www.adni-info.org/). When requested, the Neuropathology Core makes available financial assistance with the autopsy, block sampling, tissue preservation, and shipping costs. The Neuropathology Core funds all costs in shipping frozen and fixed tissue samples to St. Louis, Missouri. To assist participating centers and neuropathologists with the costs of obtaining frozen tissue blocks and/or formalin-fixed paraffin wax-embedded tissue, costs are reimbursed, if requested. To minimize the burden on participating centers, formalin-fixed paraffin wax embedded tissue blocks, representing the following 16 areas, are taken from coronal slices of the left cerebrum and forwarded to the NPC: middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobe (angular gyrus), occipital lobe (to include the calcarine sulcus and peristriate cortex), anterior cingulate gyrus at the level of the genu of the corpus callosum, posterior cingulate gyrus and precuneus at the level of the splenium, amygdala and entorhinal cortex, hippocampus and parahippocampal gyrus at the level of the lateral geniculate nucleus, striatum (caudate nucleus and putamen) at the level of the anterior commissure, lentiform nuclei (globus pallidus and putamen), thalamus and subthalamic nucleus, midbrain, pons, medulla oblongata, cerebellum with dentate nucleus, and spinal cord. In the unusual situation where it is impractical to forward a tissue block (e.g., if the entire hippocampus is used for stereology), 10 paraffin wax sections (4–8 µm) from each block may be sent to the NPC for systematic neuropathology and diagnosis. To provide tissue for biochemical studies and to advance the aims of the biomarker studies, unfixed brain tissue is dissected, snap frozen by contact with Teflon®-coated aluminum plates maintained in liquid N2 vapor in a cryogenic vessel according to the method described by Dr. Vonsattel and colleagues (15), and sent to the NPC. We have found that this method produces good quality frozen tissue with good anatomical preservation and minimal ice crystal artefacts (Figure 1). The following minimal number of coronal hemibrain slices (0.5 to 1cm thick), where possible, are taken for freezing: (1) frontal lobe to include striatum; (2) frontal and temporal lobe at the level of the mammillary body; (3) temporal and parietal lobes at the level of the lateral geniculate nucleus; and (4) occipital lobe to include the calcarine sulcus. In the event that a frozen hemibrain is received intact, this is coronally sliced at 0.5 – 1 cm intervals using an electric band saw within a fume hood in a Biohazard Level II laboratory – appropriate personal protective clothing is worn. All frozen slices are placed in Ziploc® plastic bags to prevent desiccation and stored at −80°C.
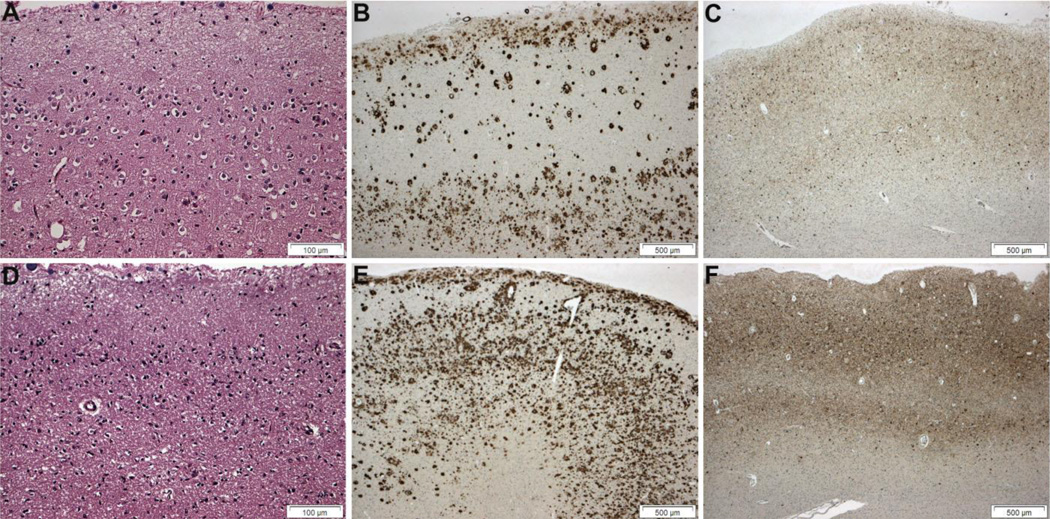
Figure 1.
Comparison of histology and immunohistochemistry in formalin-fixed, paraffin-embedded (A–C) and snap-frozen brain tissue (D–F). Mild to moderate neuronal loss and gliosis in the right frozen occipital lobe (D) are comparable in the left formalin-fixed occipital lobe (A) (hematoxylin and eosin; Brodmann area 18). Amyloid burden (Aβ plaques and cerebral amyloid angiopathy; Aβ(10D5) immunohistochemistry) and tauopathy (neurofibrillary tangles, neuropil threads and neuritic plaques; PHF1 immunohistochemistry) appear more severe in the frozen right occipital lobe (E and F) than in the formalin-fixed left lobe (B and C). Bars: A and D, 500 µm; B, C, E, F, 100 µm.
Quality of frozen brain tissue
The NPC has eleven −80°C freezers to store unfixed, frozen brain tissue. Each freezer is alarmed and connected to the Barnes-Jewish Hospital, St. Louis, Missouri, electronic equipment monitoring system which is connected to an automated paging system; the on-duty neuropathologist and laboratory technician each carry a pager at all times in case of freezer failure. There is always at least one empty freezer available as back-up in the event of freezer failure. Routinely, the NPC preserves the left hemibrain in 10% buffered formalin, allowing detailed anatomical studies (e.g. language processing areas) and subsequent histology. Where there is evidence of a lesion, such as an infarct, in the right hemibrain, the coronal slice containing the lesion is preserved in formalin for histological assessment. One in twenty brains is received fixed whole from out-of-town sites and these permit bilateral sampling. To date, we have found no bilateral differences in molecular pathology but we continue to sample bilaterally when appropriate cases become available. The mean postmortem interval (PMI; mean time from death to tissue snap freezing) to date has been 17.2 h (range: 4.5 – 38 h) for DIAN cases and 12.5 h (range: 2 – 43.1 h) for ADNI cases. Although the postmortem interval correlates with mRNA quality, the integrity of mRNA is more reliably assessed using the RNA integrity number (RIN) (16,17). The RIN for frozen brain is calculated using an Agilent BioAnalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA, USA) and is available as an additional measure of mRNA quality for cases where frozen tissue is available; these data are made available to investigators in addition to the agonal state and PMI.
Genotyping
For all cases without blood available, frozen brain samples (cerebellum) are used for DNA extraction and genotyping (e.g. for APOE) by the Knight ADRC Genetics Core using established methods (18).
Histology
In all cases, multiple histological stains (see Table 1) are performed at the NPC laboratory on sections cut from the blocks indicated above, and/or as requested by the neuropathologist. These include hematoxylin and eosin, a modified Bielschowsky silver impregnation, and immunohistochemistry using the following primary antibodies: phosphorylated tau (PHF1, a gift of Dr. Peter Davies, Albert Einstein College of Medicine, Yeshiva University, New York, NY); β-amyloid (10D5, Eli Lilly, Indianapolis, IN); phosphorylated α-synuclein (Cell Applications, San Diego, CA), and phosphorylated TDP-43 (Cosmo Bio, Carlsbad, CA). Additional stains and immunohistochemistry may be used when appropriate.
Table 1.
Immunohistochemistry, antibodies, and antigen retrieval methods used in ADNI and DIAN protocols
| Antigen | Antibody name (Source) | Clone | Mode of staining |
Pretreatment | Dilution | Incubation time |
|---|---|---|---|---|---|---|
| Aβ | 10D5 (Eli Lilly, Indianapolis, IN, USA) | 10D5 | Manual | MW 98% FA | 1:100 000 | 4°C overnight |
| Phospho-tau | PHF1 (Dr. P. Davies, Albert Einstein College of Medicine, NY, USA) | PHF1 | Manual | MW | 1:10 000 | 4°C overnight |
| Phospho-α-synuclein | Phospho-α-synuclein (Ser129) (Cell Applications, Inc., San Diego, CA, USA) | Rabbit polyclonal | Manual | MW 98% FA | 1:10 000 | 4°C overnight |
| Phospho-TDP-43 | (Cosmo Bio USA, Inc., Carlsbad, CA, USA) | pS409/410 | Manual | MW 98% FA | 1:40 000 | 4°C overnight |
ADNI, Alzheimer Disease Neuroimaging Initiative; DIAN, Dominantly Inherited Alzheimer Network; FA, formic acid; MW, microwave.
Neuropathologic assessment
The operational criteria for the classification of AD and other pathologies, defined by NACC, are applied to all ADNI/DIAN-NPC cases [6–14]. Consensus neuropathologic diagnoses are determined by Dr. Cairns in collaboration with Dr. Perrin and other neuropathologists within the Division of Neuropathology at Washington University using consensus neuropathologic criteria for AD and for non-AD disorders. The NACC Neuropathology Form includes an entry for the diagnosis of AD by several sets of criteria: Khachaturian, CERAD, NIA-Reagan, and the NIA-AA [6–9]. NPC cases are thus diagnosed in accordance with each of these criteria as no consensus currently exists in favor of one set in relation to the others (particularly for the incipient stages of AD addressed by the ADNI and DIAN studies). This practice allows for historical comparisons with older cases that pre-date newer diagnostic consensus criteria, and also affords investigators maximal utility in applying the neuropathologic diagnoses most appropriate to their research aims. The neuropathologic data are entered into the NACC Neuropathology Data Form and transmitted to the Biostatistics and Informatics Cores at the ADNI and DIAN Coordinating Centers. The final neuropathologic diagnosis and neuropathology report are forwarded to ADNI and DIAN for entry into the central database and to the center that made available the tissue.
RESULTS
The ADNI/DIAN-NPC Research Coordinator, Erin Franklin, has contacted all participating ADNI and DIAN sites to implement the protocols established for obtaining autopsy consent and performing neuropathology services. The coordinator continuously monitors the sites to encourage and facilitate autopsy consent in ADNI and DIAN participants. Additionally, for reference, all ADNI-NPC documentation is available to ADNI and DIAN site personnel at the Alzheimer’s Disease Cooperative Study (ADCS) website: http://www.adcs.org/Resource/studyResources.aspx. Where autopsy procedures do not exist locally, arrangements have been put in place with the site PI and local hospital to collect brain tissue for delivery to the NPC in St Louis, Missouri. To promote the goals of the NPC and to inform participating sites, meetings have been held concurrently at the meetings of the American Association of Neuropathologists and the American Academy of Neurology.
Autopsy rate
During the period of funding of ADNI, there have been 65 participant deaths (Table 2). In the initial phase of ADNI (September 1st, 2005 to August 31st, 2007), when no resources were available for neuropathology and prior to the establishment of the NPC, there were six participant deaths and no autopsies (autopsy rate = 0%). The cumulative autopsy rate since the inception of the ADNI NPC is 70% (Table 2). Although the overall numbers to date are small, these data demonstrate that the Neuropathology Core has established the administrative organization with the participating sites to collect brains from ADNI participants who come to autopsy. As expected, the number of ADNI participants who come to autopsy is increasing as the period of the study lengthens and participants age. As of October 1, 2014, there were forty-one autopsies. The mean age at death was 80.7 years. Seventy-seven percent were male. At least one APOE ε4 allele was present in 57% of cases (5 cases pending). At the time of the final clinical assessment, dementia of the Alzheimer’s type (DAT) was reported in all cases examined. To preserve anonymity of ADAD participants, demographic data from DIAN cases are not provided.
Table 2.
ADNI autopsy rates (9-1-05 to 7-1-14)†
| Funding period | ADNI- NPC |
Deaths | Autopsies | Annual autopsy rate (%) |
|---|---|---|---|---|
| 9-1-05 to 8-31-07 | No | 6 | 0 | 0 |
| 9-1-07 to 8-31-08 | YES | 7 | 2 | 28 |
| 9-1-08 to 8-31-09 | YES | 8 | 8 | 100 |
| 9-1-09 to 8-31-10 | YES | 4 | 1 | 25 |
| 9-1-10 to 8-31-11 | YES | 13 | 6 | 46 |
| 9-1-11 to 8-31-12 | YES | 3 | 3 | 100 |
| 9-1-12 to 8-31-13 | YES | 10 | 8 | 80 |
| 9-1-13 to 8-31-14 | YES | 14 | 13 | 93 |
| Total (2005–2014) | — | 65 | 41 | 65 |
| Total since NPC established | — | 59 | 41 | 70 |
The ADNI-NPC (Alzheimer Disease Neuroimaging Initiative Neuropathology Core) was established on September 1, 2007.
Neuropathologic assessment of ADNI and DIAN participants at autopsy
Brain samples from 41 ADNI and 24 DIAN cases (9 DIAN participants and 15 DIAN family members) have been received and neuropathologically assessed by the NPC (Tables 3 and 4). Preliminary studies indicate that total Aβ load is greater (~50%) in DIAN participants compared with LOAD cases, similar to that observed in a previous study (19). Lewy body pathology (dementia with Lewy bodies and amygdala predominant Lewy bodies) was the most frequent comorbidity in both cohorts. Additional comorbidities (TDP-43 proteinopathy, argyrophilic grain disease [AGD], hippocampal sclerosis [HS], and infarcts) were present in the LOAD cases but these comorbidities were absent from the DIAN cases (Table 3). The presence of cases with an additional molecular pathology in the ADNI cohort is representative of other larger series, indicating that the contributions of vasculopathy/infarction, tauopathy, α-synucleinopathy, TDP-43 proteinopathy, and possibly other proteinopathies, will need to be assessed in the ADNI series as more cases come to autopsy. If the neuropathologic sample is representative of the total ADNI cohort of dementia patients, these preliminary data indicate widespread co-morbidity which may contribute to variance in the antemortem data obtained from different clinical and biomarker modalities. Likewise, if these data are also representative of the total DIAN cohort, a more intrinsic role for Lewy body pathology in Alzheimer disease might be considered.
Table 3.
Comorbidities in AD in ADNI and DIAN participants
| Neuropathologic diagnoses | LOAD (ADNI) | ADAD (DIAN) | |||
|---|---|---|---|---|---|
| Primary | Comorbidities† | N‡ | % | N§ | % |
| AD | 1Comorbidity | 33 | 100 | 22 | 100 |
| AD | None | 14 | 42.4 | 11 | 50 |
| AD | DLB/ALB | 14 | 42.4 | 11‡ | 50 |
| AD | TDP-43 | 7 | 21.2 | 0 | 0 |
| AD | AGD | 6 | 18.2 | 0 | 0 |
| AD | Hippocampal sclerosis | 2 | 6.1 | 0 | 0 |
| AD | Infarcts | 1 | 3.0 | 0 | 0 |
| AGD | None | 1 | 3.0 | 0 | 0 |
More than one comorbidity may be present in a single case.
One case had additional glioblastoma multiforme.
Seven cases pending.
Two cases pending.
ADNI, Alzheimer Disease Neuroimaging Initiative; DIAN, Dominantly Inherited Alzheimer Network; ALB, amygdala Lewy bodies; DLB, dementia with Lewy bodies; TDP-43, trans-activation response DNA protein 43; AGD, argyrophilic grain disease.
Table 4.
Neuropathologic assessment of seven DIAN participants and 15 family members
| Mutation | P/F | PMI (h) |
Brain wt. (g) |
Clin. Dx.† |
Npath. Dx. |
A‡ (Aβ) |
B‡ (NFT) |
C‡ (NP) |
SYN§ |
|---|---|---|---|---|---|---|---|---|---|
| PSEN1 I143T | P | 18 | 1330 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN1 M146L | P | 38 | 1070 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN1 H163R | P | 9 | 1130 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 H163R | F | 4.5 | 1300 | AD | AD + ALB | 3 | 3 | 3 | ALB |
| PSEN1 H163R | F | 9 | 1490 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 H163R | F | 6 | 1210 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN1 G206A | F | na | na | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 G206V | P | 15 | 1095 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 G217R | F | 15 | 1040 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN1 L226R | F | 16 | 1124 | AD | AD + ALB | 3 | 3 | 3 | ALB |
| PSEN1 I229F | P | 23 | 1220 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 I229F | P | 24.5 | 1080 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 S290C | F | 60 | 1144 | AD | AD | 3 | 3 | 3 | 0 |
| PSEN1 C410Y | F | 21 | 1224 | AD | AD | 3 | 3 | 1 | 0 |
| PSEN1 A431E | F | 5 | 720 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN1 T245p | P | 6.5 | 1050 | AD | AD + DLB | 3 | 3 | 3 | 6 |
| PSEN2 A141I | F | 6 | 1100 | AD | AD + ALB | 3 | 3 | 3 | ALB |
| APP K670N, M671L | F | 6 | 1210 | AD | AD | 3 | 3 | 3 | 0 |
| APP V717I | F | 15 | 1150 | AD | AD | 3 | 3 | 3 | 0 |
| APP V717I | F | 26.5 | 1370 | AD | AD | 3 | 3 | 3 | 0 |
| APP V717I | F | 10 | 1110 | AD | AD + ALB | 3 | 3 | 3 | ALB |
| APP V717I | F | na | 980 | AD | AD + ALB | 3 | 3 | 3 | ALB |
| Mean | 7P, 15F | 16.7 | 1150 | AD (100%) | |||||
| Range | 4.5–60 | 720–1490 | AD + DLB/ALB (50%) | ||||||
Symptomatic AD.
NIA-AA criteria for AD neuropathologic change: A, (Aβ plaque score); B, (Braak neurofibrillary tangle score); and C, (CERAD neuritic plaque score).
SYN, α-synucleinopathy; Braak Parkinson’s disease Lewy body stage.
All cases also had small vessel disease with moderate to severe cerebral amyloid angiopathy (CAA) and arteriolosclerosis; but none had infarcts. TDP-43 proteinopathy was not detected in any case. ALB, amygdala Lewy bodies; DLB, dementia with Lewy bodies; F, family member; na, not available; P, participant; PMI, post mortem interval (hours).
Neuropathologic lesions of pre-clinical AD
The NPC has addressed the key question of what neuropathologic lesion best defines AD as a disease process and distinguishes it from normal aging (20–25). Even in cognitively normal children and young adults, evidence of neurofibrillary tangle formation may be present, so it is unusual to find an aged brain, even one with a clinical dementia rating (CDR) of 0, without some molecular pathology. Thus, neuropathological assessment of every case is necessary. The NPC applies four different neuropathologic diagnostic criteria (Khachaturian, CERAD, NIA-Reagan Institute, and the NIA-Alzheimer’s Association). The widely-used CERAD, NIA-Reagan, and the NIA-AA criteria emphasize lesions (neuritic plaques and tangles) that correlate with overt dementia. In contrast, the most recent set of neuropathologic criteria acknowledges the fact that established AD biomarkers (notably CSF Aβ42, CSF tau, and radiographic Aβ imaging with positron emission tomography [PET]) and neuropathology identify a preclinical stage of AD that precedes symptomatic AD. The new NIA-AA criteria underscore the importance of Aβ plaques as a key morphological lesion that best discriminates AD pathology from non-AD. We continue to use all four sets of criteria for each ADNI and DIAN participant who comes to autopsy, thus enabling other investigators to readily compare their data with the NPC data.
Multimodal studies
Both the longitudinal ADNI and DIAN studies facilitate multimodal correlations (neuropathology, neuroimaging, biomarkers, neuropsychology, genetics and clinical assessment). Toledo and Cairns and colleagues have undertaken a multimodal study of structural imaging (MRI), functional imaging (PET-FDG), CSF biomarkers (Aβ42, total tau, phosphorylated tau, alpha-synuclein), genetics, clinical, and neuropsychological assessments of the first 22 ADNI autopsies (Figure 2 and [26]). Clinical diagnosis was either probable DAT or Alzheimer disease (AD)-type mild cognitive impairment (MCI) at last evaluation prior to death. All patients had a pathological diagnosis of AD, but only four had pure AD. A coincident pathological diagnosis of dementia with Lewy bodies (DLB), medial temporal lobe pathology (MTL; e.g., TDP-43 proteinopathy, argyrophilic grain disease and hippocampal sclerosis), or vascular pathology was present in 45.5%, 40.0% and 22.7% of these cases, respectively. Hallucinations were a strong predictor of coincident DLB (100% specificity) and a more severe dysexecutive profile was also a useful predictor of coincident DLB (80.0% sensitivity and 83.3% specificity). Occipital FDG-PET hypometabolism accurately classified coincident DLB (80% sensitivity and 100% specificity). Participants with coincident MTL showed lower hippocampal volume (26). This preliminary study indicates that biomarkers can be used to independently predict coincident AD and DLB pathology, a common finding in amnestic MCI and DAT patients. The DIAN cohort of participants (n = 7 of 9) has similar comprehensive neuropathological assessments, allowing neuroanatomic comparisons of histological and radiological biomarker data (Figure 3). Such multimodal biomarker data will help to characterize independent predictors of the different neuropathological substrates of cognitive impairment in these cohorts.
Figure 2.
Heatmaps summarizing the semiquantitative neuropathologic grading of the left hemibrain of ADNI participants. AD, Alzheimer disease; DLB, dementia with Lewy bodies. Heatmaps summarizing the semiquantitative neuropathological grading of molecular pathologies from left to right: Diffuse Plaques, diffuse Aβ plaques; Tau NFT, phospho-tau immunoreactive neurofibrillary tangles; Lewy Bodies, phospho-α-synuclein immunoreactive Lewy bodies; and TDP Dep., phospho-TDP-43-immunoreactive neuronal cytoplasmic inclusions. Adapted from [26].
Figure 3.
Semiquantitative neuropathologic heat maps facilitate PET-PiB amyloid imaging – Aβ plaque correlations. Upper panel: Coronal view (left) and lateral view (right) show PET-PiB binding in arbitrary units in a DIAN participant. Lower panel: Semiquantitative histopathologic data of diffuse plaques (left) and cored/compact plaques (right) from the left hemibrain; data are mirrored to generate a whole coronal slice at the same level as in the upper panel. Areas in gray not assessed.
SUMMARY
The establishment of a centralized neuropathology laboratory (ADNI/DIAN-NPC) has permitted a standardized and uniform neuropathologic assessment of participants who come to autopsy. A central NPC provides the gold standard validation of clinical diagnoses and imaging and biofluid surrogates. Even with the advances in CSF and neuroimaging biomarkers, neuropathology is still required to determine the presence of co-morbidities where currently no robust biomarker exists. For example, no biomarker is currently available to reliably determine the presence of TDP-43 proteinopathy or AGD, and improved biomarkers are needed for the detection of Lewy body pathology. Retrospective analysis of biomarker data following neuropathological assessment may allow the identification of new antemortem biomarkers for these non-AD co-morbidities. The importance of such work is underscored by the high prevalence of Lewy body pathology in both LOAD and ADAD cases. The contribution of additional pathologies to the clinical, neuroimaging, and biomarker phenotypes will be the subject of future studies.
Previous studies indicate that a single NPC site is necessary because different neuropathologists use diverse processing and staining methods, as well as different antibodies, and interpret diagnostic criteria differently [2,3]. A single NPC ensures uniform staining and application of diagnostic criteria to all ADNI and DIAN participants who come to autopsy [2,3]. The ADNI/DIAN-NPC is built on the already established infrastructure of the Washington University Alzheimer Disease Research Center. The ADRC's Administrative (Dr. Morris) and Neuropathology (Dr. Cairns) Cores provide the framework for the ADNI/DIAN-NPC and continue to do so during the period of project funding. Compatibility of data between ADNI and DIAN and the NACC has been maintained by using the same NACC Neuropathology Data Form as is used by all ADCs to report neuropathologic findings from autopsied cases. In this way, the ADNI/DIAN-NPC uses standard criteria for neuropathologic diagnoses of dementing illness and existing protocols and procedures to achieve these diagnoses. Importantly, the NPC does not interfere with or supersede neuropathologic activities at any ADNI or DIAN site, but receives brain tissue obtained at the participating sites to provide a uniform neuropathologic assessment.
ADNI funding of the Neuropathology Core started on September 1st, 2007. Since that time the NPC has become fully operational, serving all 59 ADNI sites. Funding from DIAN began in 2014. During the initial period of funding, the ADNI/DIAN-NPC achieved its stated goals. It has: (1) provided and implemented training materials and protocols to assist clinicians at ADNI and DIAN sites in obtaining voluntary consent for brain autopsy in participants; (2) established a central laboratory to provide uniform neuropathologic assessments in all autopsied participants in accordance with standard criteria [4–18]; (3) established and maintained a state-of-the-art resource for fixed and frozen brain tissue from autopsied participants; (4) developed a process wherein investigators may have access to the tissue and data for research purposes; and (5) established a workflow with the ADNI and DIAN Data Coordinating Centers to ensure appropriate entry of the Core's data into the database. Collectively, these achievements promote data sharing and collaborative research efforts in support of the research aims of ADNI and DIAN.
Acknowledgments
This work was supported by grants P50 AG05681, P01 AG03991, P01 AG26276, and DIAN (U01 AG032438) from the National Institute on Aging, and P30-NS048056 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, and by the Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Alzheimer's Disease Research Center. The ADNI (National Institute of Health Grant U01 AG024904) is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Pfizer Inc., Wyeth Research, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck and Co. Inc., AstraZeneca AB, Novartis Pharmaceuticals Corporation, Alzheimer's Association, Eisai Global Clinical Development, Elan Corporation plc, Forest Laboratories, and the Institute for the Study of Aging, with participation from the U.S. Food and Drug Administration. Industry partnerships are coordinated through the Foundation for the National Institutes of Health.
REFERENCES
- 1.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers. Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. PMCID:PMC3329969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA, et al. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers. Dement. 2010;6:202–211. doi: 10.1016/j.jalz.2010.03.007. PMCID:PMC2927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JC, Aisen PS, Bateman RJ, Benzinger TL, Cairns NJ, Fagan AM, Ghetti B, Goate AM, Holtzman DM, Klunk WE, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin. Investig. (Lond) 2012;2:975–984. doi: 10.4155/cli.12.93. PMCID:PMC3489185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. PMCID:PMC3474597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, Bodi I, Budka H, Capetillo-Zarate E, Ferrer I, et al. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115:533–546. doi: 10.1007/s00401-008-0358-2. [DOI] [PubMed] [Google Scholar]
- 6.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch. Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 7.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol. Aging. 1997;18(Suppl):S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 8.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol. Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 9.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. PMCID:PMC3268003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 11.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J. Alzheimers. Dis. 2006;9(3 Suppl):417–23. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 12.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, III, Schneider JA, Grinberg LT, Halliday G, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. PMCID:PMC2827877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. PMCID:PMC2799633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 15.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115:509–532. doi: 10.1007/s00401-007-0311-9. PMCID:PMC2292479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birdsill AC, Walker DG, Lue L, Sue LI, Beach TG. Postmortem interval effect on RNA and gene expression in human brain tissue. Cell Tissue Bank. 2011;12:311–318. doi: 10.1007/s10561-010-9210-8. PMCID:PMC3343031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. PMCID:PMC1995236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. PMCID:PMC2830375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann DM, Iwatsubo T, Ihara Y, Cairns NJ, Lantos PL, Bogdanovic N, Lannfelt L, Winblad B, Maat-Schieman ML, Rossor MN. Predominant deposition of amyloid-beta 42(43) in plaques in cases of Alzheimer's disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am. J. Pathol. 1996;148:1257–1266. PMCID:PMC1861527. [PMC free article] [PubMed] [Google Scholar]
- 20.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol. Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 21.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann. Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Price JL, McKeel DW, Jr, Morris JC. Synaptic loss and pathological change in older adults--aging versus disease? Neurobiol. Aging. 2001;22:351–352. doi: 10.1016/s0197-4580(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 23.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging. 2009;301026 doi: 10.1016/j.neurobiolaging.2009.04.002. PMCID:PMC2737680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in "normal" aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, Householder E, Ayutyanont N, Roontiva A, Bauer RJ, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol. Commun. 2013;1:65. doi: 10.1186/2051-5960-1-65. PMCID:PMC3893373. [DOI] [PMC free article] [PubMed] [Google Scholar]