Abstract
Objective
To describe prevalence trends in hospitalized live births affected by placental transmission of alcohol and drugs, as well as prevalence trends among parturient women hospitalized for liveborn delivery and diagnosed with substance abuse problems in the United States from 1999 to 2008. Comparison of the two sets of trends helps determine whether the observed changes in neonatal problems over time were caused by shifts in maternal substance abuse problems.
Methods
This study independently identified hospitalized live births and maternal live born deliveries from discharge records in the Nationwide Inpatient Sample, one of the largest hospital administrative databases. Substance-related diagnosis codes on the records were used to identify live births affected by alcohol and drugs and parturient women with substance abuse problems. The analysis calculated prevalence differences and percentage changes over the 10 years, with Loess curves fitted to 10-year prevalence estimates to depict trend patterns. Linear and quadratic trends in prevalence were simultaneously tested using logistic regression analyses. The study also examined data on costs, primary expected payer, and length of hospital stays.
Results
From 1999 to 2008, prevalence increased for narcotic- and hallucinogen-affected live births and neonatal drug withdrawal syndrome but decreased for alcohol- and cocaine-affected live births. Maternal substance abuse at delivery showed similar trends, but prevalence of alcohol abuse remained relatively stable. Substance-affected live births required longer hospital stays and higher medical expenses, mostly billable to Medicaid.
Conclusions
The findings highlight the urgent need for behavioral intervention and early treatment for substance-abusing pregnant women to reduce the number of substance-affected live births.
Keywords: Alcohol, drug, live birth, neonatal drug withdrawal syndrome, trend analysis
Introduction
According to the National Survey on Drug Use and Health (NSDUH) in 2007 and 2008 (1), about 14 million women of reproductive age (15–44 years) in the United States (23.4%) consumed 5 or more drinks on 1 occasion at least once in the past 30 days. Moreover, 6 million women in this age group (9.6%) currently use illicit drugs. Although pregnant women were less likely than non-pregnant women to be current users of alcohol and illicit drugs, 1 of every 10 pregnant women still consumed at least one drink in the past 30 days, and 1 of every 20 still used illicit drugs (1).
Maternal substance abuse during pregnancy is well known for its potential harmful effects on the fetus. Prenatal exposure to alcohol has been linked to a variety of impairments, ranging from neurobehavioral or cognitive abnormalities called fetal alcohol spectrum disorders (2) to a characteristic pattern of facial anomalies, growth restriction, and central nervous system (CNS) dysfunction known as fetal alcohol syndrome (3). Prenatal exposure to cocaine can result in a higher risk of low birth weight, preterm birth, small for gestational age, and cardiac and circulatory congenital anomalies (4–7). A recent systematic review suggested an association between prenatal amphetamine exposure and poor fetal growth (8). Reviews of neuroimaging studies indicate that prenatal exposure to alcohol and drugs can potentially cause structural and functional abnormalities of fetal brain (9, 10). Infants born to substance-abusing mothers may develop neonatal withdrawal syndromes and have varied symptoms, including CNS irritability, tremors, gastrointestinal dysfunction, tight muscle tone, unstable temperature, and prematurity (11). Any of these symptoms could complicate neonatal care and prolong hospital stays for the affected infants.
These fetal disorders caused by maternal substance abuse during pregnancy are preventable. The goal is therefore to encourage abstinence from alcohol and illicit drugs during pregnancy, as stated in the U.S. Federal government's objectives for the Healthy People 2010 and 2020 programs. Proper allocation of prevention resources and funding requires an understanding of the seriousness of the problem nationwide. In 2006, Robbins and colleagues reported a reduction in the prevalence of alcohol-affected live births from 7.3 per 10,000 live births in 1993 to 1.7 per 10,000 live births in 2002 (12). No updated analysis has been published to re-examine whether this declining trend has been sustained or reached a plateau since 2002, given that the prevalence of maternal alcohol drinking during pregnancy remained unchanged from 2002 to 2008 (13). As for the prevalence of drug-affected newborns, Dicker and Leighton observed an increasing trend from 1979 to 1990 (14). To our knowledge, no other published studies have updated this information since. In Australia, O'Donnell and colleagues reported an increase in the prevalence of neonatal withdrawal syndrome from 1980 to 2005 (15). The United States has lacked such national-level estimates for the past decade.
Our main objective was therefore to use the Nationwide Inpatient Sample (NIS), one of the largest nationally representative hospital administrative databases in the United States, to examine trends in the prevalence of hospitalized live births diagnosed as affected by placental transmission of alcohol or drugs. The study also looked at neonatal drug withdrawal syndrome from 1999 to 2008 and examined trends in the prevalence of maternal substance abuse diagnosed at time of delivery. Because not all exposed fetuses develop an adverse outcome detectable at birth, the maternal data served as a proxy measure for substance-exposed live births. It can help determine whether the observed changes in neonatal problems over time were caused by shifts in maternal substance abuse problems. Costs and length of hospital stays were also estimated as a measure of the healthcare burden posed by these hospitalizations.
Methods
Data Sources
Data were obtained from inpatient discharge records in the NIS from 1999 to 2008. The NIS is part of the Healthcare Cost and Utilization Project (HCUP), funded by the Agency for Healthcare Research and Quality (AHRQ). The NIS contains a 20% sample of U.S. community hospitals as defined by the American Hospital Association. The sampling strata cover five hospital characteristics: U.S. region, urban or rural location, teaching status, ownership and control, and hospital size. All inpatient stays of the sampled hospitals were included in the NIS data, roughly 8 million discharges each year. In 1999, 24 states participated in the NIS, gradually increasing to 42 in 2008, which covered 65% of the U.S. population in 1999 and 95% in 2008 (16). With sampling weights, the NIS data can be used to estimate national statistics on hospitalizations. These data are publicly accessible and contain de-identifiable hospital administrative information, which exempted this study from the human subject research review. To comply with the AHRQ confidentiality policy, we have followed the NIS data use agreement throughout the study.
Case Identification
Live births
Each record in the NIS has 1 principal diagnosis and up to 14 secondary diagnoses using codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Inpatient discharges with a principal diagnosis code of V30–V39 were defined as live births. According to the ICD-9-CM Official Guidelines for Coding and Reporting, codes V30–V39 can only be assigned once at birth. These codes should not be used for transferred newborns at the receiving sites (17), which prevent double counting in our analysis. Live births affected by placental transmission of substances were identified by any listed secondary diagnosis codes of 760.71 (newborn affected by alcohol), 760.72 (by narcotics), 760.75 (by cocaine), 760.73 (by hallucinogenic agents), 760.70 (by unspecified noxious substances), and 779.5 (drug withdrawal syndrome in newborn). According to the ICD-9-CM coding guidelines, live births prenatally exposed to maternal substance use had to be actually affected by it to be coded as 760.7× (17). Because an inpatient discharge record can have multiple diagnoses, a live birth can be assigned to more than one substance group.
Parturient women with a liveborn delivery
Most maternal and neonatal discharges in the NIS were reported on separate records without data linkages between them. Parturient women hospitalized for a liveborn delivery were identified independently by any listed diagnosis codes of V27.0, V27.2, V27.3, V27.5 and V27.6. Focusing on liveborn deliveries instead of all pregnancy-related discharges avoided double counting of pregnant women who were admitted multiple times in pregnancy. This also made maternal estimates (based on liveborn deliveries) comparable with neonatal estimates (based on live births). The analysis identified and classified maternal substance abuse problems based on the following diagnosis codes: abuse of alcohol (303 and 305.0); opioids (304.0, 304.7, and 305.5); cocaine (304.2 and 305.6); cannabis and hallucinogens (304.3, 304.5, 305.2, and 305.3); amphetamines, including other psychostimulants (304.4 and 305.7); sedatives, including hypnotics and anxiolytics (304.1 and 305.4); and other and unspecified drugs (304.6, 304.8, 304.9, 305.8, and 305.9). The substance classification was not mutually exclusive. Parturient women with diagnosis codes of alcohol- or drug-induced mental disorders (291 or 292) or poisoning (960–980) but without the substance abuse diagnosis codes were not identified as substance abusing, because they might have exhibited different substance use behavior during pregnancy. There were relatively few of these cases (e.g., in 2008, fewer than 50 for alcohol and 244 for drugs).
Estimated Cost and Length of Hospital Stay
How much each hospital charged for the entire hospital stay was reported for each record. This amount can be converted into estimated cost by multiplying total charge by hospital-specific cost-to-charge ratios (CCRs) derived by the HCUP (18). Because CCRs were only available since 2001, trends in costs were analyzed for 2001–2008 instead of the whole study period of 1999–2008. When the sampled hospitals had no hospital-specific CCRs (ranging from 12% to 29% in 2001–2008), the group-weighted average CCRs were used. When NIS-participating states were not part of the NIS CCR project, the CCRs of the sample hospitals in these states were missing, ranging from 0% in 2006 and 2007 to 14% in 2001–2003. A 3% downward adjustment was made to 2004 CCRs, as suggested by HCUP (18). In order to adjust for inflation, all costs of 2001–2007 were adjusted to 2008 U.S. dollars using the overall Consumer Price Index (19). Estimated mean costs were reported to the nearest hundred dollars. Total cost was calculated as mean cost multiplied by number of cases. The expected primary payer noted on discharge records was checked to determine if Medicaid had been the payment source.
The HCUP processed and provided data on length of stay (LOS) per hospitalization in the NIS. LOS was calculated as the admission date subtracted from the discharge date. When dates were missing or invalid, the HCUP supplemented the information with the LOS supplied by the original sources (20).
Statistical Analysis
All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) and SAS-callable SUDAAN10.0 (Research Triangle Institute, Research Triangle Park, NC) to take into account sampling design effect and sampling weights. Since neonatal discharges were reported separately from maternal discharges in the NIS, we estimated the neonatal prevalence and maternal prevalence independently. Neonatal prevalence was estimated as the number of live births affected by placental transmission of substances, divided by the total number of hospitalized live births in each year. The result was presented as per 10,000 live births. Maternal prevalence was estimated as the number of parturient women diagnosed with substance abuse problems at delivery, divided by the total number of hospitalized parturient women with a liveborn delivery. It was presented as per 10,000 liveborn deliveries. Prevalence estimates were considered reliable when the relative standard error (i.e., standard error divided by estimate) was less than 0.30, a reliability guideline recommended by HCUP for the NIS data.
The difference in prevalence between 1999 and 2008, calculated as Prev2008 - Prev1999 (where Prev denotes prevalence), represented the absolute measure of change between the beginning and end time points. The percentage change was calculated as (Prev2008 - Prev1999)/Prev1999 × 100% to show the magnitude of the change over the 10 years relative to the prevalence in 1999.
To depict the 10-year trend patterns, we used SAS statistical graphic procedures to fit the locally weighted scatterplot smoothing (Loess) curves to annual prevalence estimates for the 10 years (as 10 data points). The results are presented in two sets of graphs for neonatal prevalence and maternal prevalence, respectively.
To efficiently test trends over the 10 years, we conducted survey logistic regression models (SAS SURVEYLOGISTIC procedure) with the 10-year data combined. In these models, a linear term and a quadratic term of calendar year were treated as independent variables. Our analysis included the quadratic term to allow for a non-linear change over time. Race (Black, other, and unknown vs. White) and U.S. Census geographic regions (Northeast, Midwest, and West vs. South) were also included in the models. This controlled for changes in the underlying population characteristics and in diagnostic coding practices across regions. The analytical model is therefore:
When Prev is small (as in the outcomes of this study), logit(Prev) is close to ln(Prev). Two-sided significance tests were conducted at an a priori alpha level of 0.05. Statistical significance of the linear term of calendar year indicates the prevalence was increasing (for positive coefficient) or decreasing (for negative coefficient) at a constant annual percent change. Statistical significance of the quadratic term of calendar year reflects inconstant annual percent changes and/or a changing direction in the trend over the 10 years. When a result indicated a non-significant linear term but a statistically significant quadratic term, the trend was referred to as a “non-linear change” over time.
We used weighted least squares linear regression models to assess the statistical significance of the trends from 2001 through 2008 in mean cost, in percentage of hospitalizations paid by Medicaid, and in mean LOS. In the models, year was the independent variable, and the cost or percentage of hospitalizations or LOS was the dependent variable. The inverse of the square of standard errors of the estimates in each year was used as the weight. The slope represents the estimated annual average change in costs/percentage/LOS.
Results
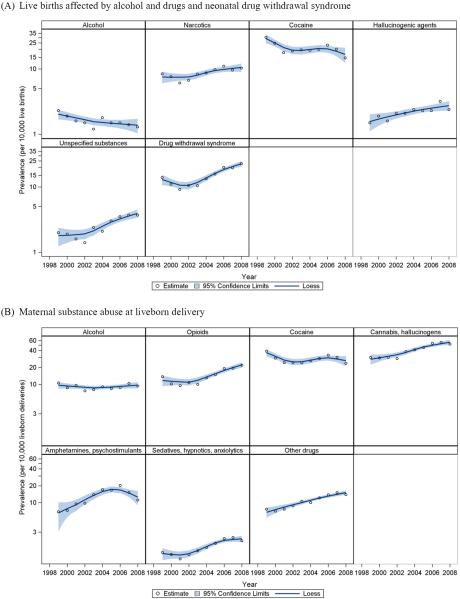
In 1999 and 2008, the United States had an estimated 3,842,837 and 4,253,656 hospitalized live births, respectively. In 1999, cocaine was the most common substance noted on neonatal records as affecting newborns via placental transmission, with 11,689 such live births reported (Table 1). Its prevalence decreased by 51.3% over the 10 years, from 30.4 to 14.8 per 10,000 live births. As shown in Figure 1A, the decrease plateaued around 2001–2005, followed by another decline in 2006–2008. Live births affected by alcohol also decreased, with a 43.5% reduction in prevalence, from 2.3 to 1.3 per 10,000 live births (Table 1). In contrast, the prevalence of live births affected by narcotics, hallucinogenic agents, and unspecified noxious substances rose statistically significantly over the 10-year period, with increases of 22.6%, 60.0%, and 85.0%, respectively. Neonatal drug withdrawal syndrome increased overall by 63.3% between 1999 and 2008, from 13.9 to 22.7 per 10,000 live births (Table 1). However, Figure 1A shows this prevalence initially decreased in 1999–2001, followed by an increase in 2002–2008 (p-values of both linear and quadratic terms < 0.01). In 1999, 51.7% of alcohol-affected live births were also diagnosed as drug affected. This proportion dropped to 38.0% in 2008 (data not shown in table).
Table 1.
Estimated number, prevalence, and trend pattern of hospitalized live birth affected by alcohol and drugs transmitted via the placenta and of parturient women diagnosed with substance abuse at liveborn delivery: United States, 1999–2008.
| 1999 |
2008 |
Difference in prevalence (% change)b between 1999 and 2008 | Trend patternc 1999–2008 (on logit scale) | |||||
|---|---|---|---|---|---|---|---|---|
| Diagnosisa | No. | Prev. | 95% CI | No. | Prev. | 95% CI | ||
| Live births (95% CI) | 3,842,837 | (3,577,779–4,107,894) | 4,253,656 | (3,931,169–4,576,143) | ||||
| Newborns affected by | ||||||||
| Alcohol | 880 | 2.3 | 1.8–3.0 | 548 | 1.3 | 1.0–1.6 | −1.0 (−43.5%) | Linear decrease |
| Narcotics | 3,222 | 8.4 | 6.5–10.7 | 4,401 | 10.3 | 8.3–12.9 | 1.9 (22.6%) | Linear increase |
| Cocaine | 11,689 | 30.4 | 25.0–37.0 | 6,302 | 14.8 | 13.2–16.7 | −15.6 (−51.3%) | Non-linear decrease |
| Hallucinogenic agents | 588 | 1.5 | 1.1–2.1 | 1,001 | 2.4 | 1.8–3.1 | 0.9 (60.0%) | Linear increase |
| Unspecified noxious substances | 781 | 2.0 | 1.6–2.7 | 1,569 | 3.7 | 3.0–4.5 | 1.7 (85.0%) | Linear increase |
| Drug withdrawal syndrome | 5,355 | 13.9 | 9.9–19.6 | 9,638 | 22.7 | 18.5–27.7 | 8.8 (63.3%) | Non-linear increase |
| Women with a liveborn delivery (95% CI) | 3,675,794 | (3,415,387–3,936,201) | 4,154,797 | (3,842,966–4,466,628) | ||||
| Maternal substance abuse | ||||||||
| Alcohol | 3,895 | 10.6 | 8.9–12.6 | 3,885 | 9.4 | 8.0–10.9 | −1.2 (−11.3%) | Non-linear change |
| Opioids | 5,022 | 13.7 | 9.7–19.2 | 9,153 | 22.0 | 17.9–27.1 | 8.3 (60.6%) | Non-linear increase |
| Cocaine | 14,281 | 38.9 | 32.6–46.3 | 9,902 | 23.8 | 21.3–26.6 | −15.1 (−38.8%) | Non-linear change |
| Cannabis and hallucinogens | 11,110 | 30.2 | 25.5–35.9 | 21,632 | 52.1 | 45.2–59.9 | 21.9 (72.5%) | Linear increase |
| Amphetamines | 2,537 | 6.9 | 5.5–8.7 | 4,657 | 11.2 | 9.2–13.6 | 4.3 (62.3%) | Non-linear increase |
| Sedatives | 489 | 1.3 | 1.1–1.7 | 859 | 2.1 | 1.7–2.6 | 0.8 (61.5%) | Linear increase |
| Other and unspecified drugs | 2,847 | 7.7 | 6.3–9.5 | 5,814 | 14.0 | 12.2–16.0 | 6.3 (81.8%) | Linear increase |
No, number; Prev, prevalence (per 10,000 live births or per 10,000 liveborn deliveries); CI, confidence interval.
A live birth and a liveborn delivery can be assigned to more than one group if more than one substance-specific diagnosis was coded.
% change was calculated as difference in prevalence between 1999 and 2008 divided by the prevalence in 1999.
Trend pattern was tested by multivariable logistic regression with a linear and a quadratic term for calendar year (centered at 2003) and with adjustment for race and geographic region. The description of “increase” versus “decrease” was based on the statistical significance of the linear term, and the description of “linear” versus “non-linear” was based on the statistical significance of the quadratic term. “Non-linear change” was used to describe results in which the linear term was not significant but the quadratic term was statistically significant. All p-values were less than 0.01, except that p-values for the quadratic term in the model for newborns affected by cocaine and in the model for maternal alcohol abuse were close to 0.05.
Figure 1.
Prevalence (per 10,000) on a natural log scale and Loess trend curves with 95% confidence interval for (A) live births affected by alcohol and drugs and neonatal drug withdrawal syndrome, and (B) maternal substance abuse at liveborn delivery: United States, 1999–2008.
In 1999 and 2008, an estimated 3,675,794 and 4,154,797 parturient women, respectively, were hospitalized for liveborn delivery. Because one delivery can result in multiple births, these numbers were smaller than the estimated totals for live births. In 1999, cocaine and cannabis were the most common drugs of abuse among women in this study. While maternal abuse of cocaine decreased over time, maternal abuse of cannabis and hallucinogens increased by 72.5% from 1999 to 2008 (Table 1). Besides cannabis, maternal abuse of opioids, amphetamines, sedatives, and other drugs also increased statistically significantly over the 10-year period (Table 1 and Figure 1B). The prevalence of maternal alcohol abuse remained relatively stable from 1999 to 2008 (Figure 1B), although the trend test result indicated a non-linear change over time (p-value of quadratic term = 0.04) (Table 1). Similar to what was observed in neonatal discharges, the proportion of alcohol-abusing mothers co-abusing drugs decreased from 61.3% in 1999 to 50.5% in 2008 (data not shown in table).
Substance-affected live births had longer hospital stays and higher costs than births without these specific diagnoses (Table 2). In 2008, live births diagnosed with drug withdrawal syndrome stayed in the hospital for an average of 16.3 days (95% confidence interval [CI]: 15.0–17.7 days) at a mean cost of $16,900 (95% CI: $14,600–$19,200), totaling $158 million. The majority of this medical expense (77.9%) was expected to be paid primarily by Medicaid. Mothers with at least one diagnosis of substance abuse at delivery did not have significantly longer hospital stays than those without it, but they had slightly higher average estimated medical costs (Table 2). About 60% or more of the medical expenses for the substance-abusing mothers were expected to be paid primarily by Medicaid, compared with 40% of such expenses for non-substance-abusing mothers. From 2001 to 2008, the mean costs of hospitalized live births and liveborn deliveries without substance-specific diagnosis increased annually by $74 (95% CI: $47–$101) and $55 (95% CI: $36–$75), respectively (Table 2). Over the same 8-year period, the mean cost of treating newborns affected by unspecified noxious substances increased annually by $517 (95% CI: $359–$676), which could be partially due to longer hospital stays (increasing annually by 0.37 day, 95% CI: 0.24–0.51 day). No other statistically significant trends were observed in the medical expenses for neonatal conditions.
Table 2.
Estimated cost, expected primary payer, and length of stay (LOS) for hospitalized live births and liveborn deliveries: United States, 2001–2008.
| Number |
Mean cost (2008 US$)a |
Paid by Medicaid (%) |
Mean LOS (days) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | 2001 | 2008 | 2001 | 2008 | Annual changeb | 2001 | 2008 | Annual changeb | 2001 | 2008 | Annual changeb |
| Neonatal Conditions c | |||||||||||
| Newborns affected by | |||||||||||
| Alcohol | 639 | 548 | $10,000 | $10,500 | $9 | 69.2 | 69.5 | −0.1 | 8.6 | 9.2 | 0.10 |
| Narcotics | 2,423 | 4,401 | $10,600 | $8,200 | −$285 | 64.7 | 76.0 | 1.0* | 9.9 | 7.9 | −0.23 |
| Cocaine | 7,095 | 6,302 | $7,700 | $7,900 | $56 | 69.7 | 76.8 | 0.8* | 7.7 | 8.1 | 0.03 |
| Hallucinogenic agents | 652 | 1,001 | ~ d | $6,300 | $447e | 63.1 | 74.0 | 0.4 | 3.8 | 6.4 | 0.36* |
| Unspecified noxious substance | 624 | 1,569 | $4,200 | $7,100 | $517* | 70.5 | 70.8 | 0.1 | 4.8 | 6.9 | 0.37* |
| Drug withdrawal syndrome | 3,688 | 9,368 | $18,100 | $16,900 | $259 | 64.3 | 77.9 | 1.1* | 16.1 | 16.3 | 0.03 |
| None of the above | 3,986,175 | 4,231,991 | $2,200 | $2,700 | $74* | 36.3 | 40.3 | 0.7* | 3.0 | 3.2 | 0.03* |
| Maternal substance abuse c | |||||||||||
| Alcohol | 3,687 | 3,885 | $4,000 | $4,400 | $70* | 61.7 | 59.8 | −0.4 | 3.0 | 2.8 | 0.04 |
| Opioids | 3,636 | 9,153 | $4,800 | $4,800 | −$20 | 67.9 | 75.7 | 0.9* | 3.0 | 3.0 | 0.03 |
| Cocaine | 9,284 | 9,902 | $4,100 | $4,300 | $37* | 69.1 | 73.8 | 0.2 | 2.8 | 2.8 | 0.01 |
| Cannabis and hallucinogens | 11,582 | 21,632 | $3,700 | $4,100 | $62* | 68.1 | 74.4 | 0.6 | 2.6 | 2.7 | 0.02* |
| Amphetamines | 3,620 | 4,657 | $3,700 | $4,800 | $99* | 67.1 | 69.1 | 0.4 | 2.4 | 2.6 | 0.02* |
| Sedatives | 396 | 859 | $3,700 | $4,100 | $21 | 73.3 | 71.9 | −0.5 | 2.6 | 2.6 | −0.01 |
| Other and unspecified drugs | 2,963 | 5,814 | $3,800 | $4,600 | $51 | 68.9 | 71.9 | 0.3 | 2.6 | 2.9 | 0.03* |
| None of the above | 3,795,093 | 4,107,117 | $3,400 | $3,800 | $55* | 36.6 | 39.9 | 0.7* | 2.5 | 2.6 | 0.02* |
p-value < 0.05.
All costs were adjusted to 2008 dollars using the overall Consumer Price Index.
Annual changes in mean cost, in percentage of hospitalizations paid by Medicaid, and in mean LOS, were estimated from weighted least squares linear regression models covering 2001 through 2008, in which year was an independent variable and the inverse of the square of standard errors of the estimates in each year was used as the weight.
A live birth and a liveborn delivery can be assigned to more than one group if more than one substance-specific diagnosis was coded.
~, the estimate is suppressed because it is imprecise and unreliable (i.e., its relative standard error, calculated as standard error/weighted estimate, is greater than 0.30).
Trend test in costs of newborns affected by hallucinogenic agents were based on annual estimates from 2003 to 2008 because the estimated mean costs in 2001 and 2002 were imprecise and unreliable.
Discussion
This descriptive study showed trends towards increases in the prevalence of neonatal drug withdrawal syndrome and of live births affected by drugs, specifically narcotics and hallucinogenic agents from 1999 to 2008. These increasing trends corresponded with those observed in maternal abuse of opioids, cannabis and hallucinogens, amphetamine, sedatives, and other/unspecified drugs diagnosed at time of delivery. The findings extend the historical increase in the prevalence of drug-affected newborns from 1979 to 1990 (14). These findings are consistent with increasing trends noted in previous reports on substance abuse among pregnant and reproductive-age women (21–23). They also reflect an overall increase in drug abuse problems in the U.S. general population based on numerous drug-related measures, such as illicit drug availability, prevalence of drug use/abuse, treatment services for abuse or dependence, and drug-related morbidity and mortality (24–33).
Cocaine was the only drug in this study whose prevalence in live births or parturient women declined over time. This was consistent with the declines in hospitalization rates for cocaine abuse among reproductive-age women and among pregnant women (21, 23) and with an overall decrease in cocaine-related measures in the U.S. general population (24–25, 34). These declines may reflect increasing public awareness of cocaine's harmful effects and successful nationwide efforts to curb cocaine use (24).
Alcohol has been documented as a substance widely used among pregnant women, second only to tobacco (25). In this study, however, fewer parturient women were diagnosed with alcohol abuse at delivery than with drug abuse. One reason for this may be that if pregnant women consume alcohol, most tend to drink only at low to moderate levels. For example, in the 2007–2008 NSDUH report, about 10.6% of pregnant women drank alcohol, but only 0.8% (95% CI: 0.3%–1.3%) were heavy alcohol users. This was lower than the 3.8% (95% CI: 2.6%–5.0%) of pregnant women using marijuana and hashish (35). Women who drink low to moderate levels of alcohol are unlikely to be diagnosed with alcohol dependence, alcohol intoxication, or excessive alcohol consumption on hospital records. Therefore, the NIS data may not capture women with moderate drinking behavior during pregnancy or at delivery.
Identifying an alcohol-exposed infant as alcohol-affected at birth can be a challenge because of the subtle nature of some facial dysmorphic features in newborns. This study's results reflect this difficulty, because the number of alcohol-affected live births identified from the neonatal discharge records was much lower than the number of parturient women diagnosed with alcohol abuse at delivery in the same year. It is possible, however, that some of the alcohol-exposed infants who might have been born asymptomatically healthy, may exhibit neurobehavioral or cognitive abnormality associated with fetal alcohol spectrum disorders later in life. Although systematic reviews have failed to confirm an association between low-level alcohol consumption during pregnancy and adverse birth outcomes (6, 36), no safe level of alcohol consumption in pregnancy has been established. All pregnant women are therefore encouraged to abstain from alcohol (Healthy People 2020 Objective).
One strength of this study is that it used the largest all-payer hospitalization database in the United States to estimate nationally representative prevalence and track national trends over time in live births affected by prenatal substance exposure. To validate that NIS data correctly represent national estimates, the analysis compared the total weighted number of live births and of liveborn deliveries identified from the NIS in 1999 and 2008 with the total number of registered live births reported in Births: Final Data for 1999 and Births: Final Data for 2008, published by the National Center for Health Statistics (37, 38). The comparisons showed that the 95% CI of the weighted estimates of hospitalized live births identified from the NIS in 1999 and 2008 covered the true number of registered live births in the same years (data not shown). This comparison found no differences in the distributions of maternal race (when assuming missing information on race in hospitalization data at random), maternal age, and geographic region between the NIS and the birth registries. Overall, the NIS provided reliable national estimates of live births.
Several limitations of this study should be noted. First, the NIS, which compiles cross-sectional hospital administrative data, is inevitably limited by the quality and variation of reporting practices across states and over time. However, no evidence can be obtained to evaluate the direction and extent of the reporting bias. The finding of different directions of changes in prevalence over time implies that no consistent bias across substance-specific diagnosis codes existed. Second, the NIS allowed maximum of 15 diagnosis codes for the data submitted before 2009, if the substance-related diagnosis was listed in the 16th fields or later, it could be omitted from the NIS data and would not be captured in the study. From 1999 to 2008, live births and liveborn deliveries that had more than 15 diagnosis codes increased from 0.02% to 0.27% and from <0.01% to 0.09%, respectively. There is a potential that we underestimated the prevalence, especially in the recent years. Third, the prevalence of parturient women diagnosed with substance abuse at liveborn delivery may underestimate maternal substance abuse during all stages of pregnancy. In general, substance use in the third trimester has been found to be lower than that in the first trimester (39, 40). Testing for alcohol and drugs in maternal blood and urine samples at childbirth can only detect recent use and cannot reflect a history of substance use during pregnancy. Fourth, underreporting is possible, because pregnant women may be reluctant to talk about their use of alcohol and other drugs. Healthcare providers may be reluctant to use certain substance-related diagnosis codes to avoid reimbursement difficulties, social stigma, or legal issues. Surveys of obstetrician-gynecologists (ob-gyns) in 1999 and 2007 showed that few ob-gyns asked all pregnant patients about alcohol use at initial and subsequent visits, and not many ob-gyns used a validated alcohol risk screening tool (41, 42). “Patient denial or resistance to treatment” and “time limitations” were the highest ranked barriers to alcohol screening and interventions (42). Despite dissemination efforts, most ob-gyns were not aware of the National Institute on Alcohol Abuse and Alcoholism Clinician's Guide and the American College of Obstetricians and Gynecologists (ACOG)'s Fetal Alcohol Spectrum Disorders Prevention Tool Kit (42). Fifth, screening practice varied by state, hospital, provider, women's race, and insurance type (43–46). Pregnant women may have unequal chances of being tested for substance use, which may affect the determination of whether an infant's health problems are caused by maternal substance abuse. For example, the fact that the healthcare expenses of more than 60% of substance-affected live births were paid by Medicaid may reflect a high substance exposure among infants of low-income mothers, common testing of poor mothers for substance abuse, or a combination. As of January 2007, new codes of the Healthcare Common Procedure Coding System are available through Medicaid for reimbursement of screening and brief intervention for alcohol and drug use disorders (46). Since code adoption takes time, its impact on our results should be limited.
The marked increase in drug-affected live births from 1999 to 2008 highlights the urgent need for behavioral intervention and early treatment for pregnant women with substance abuse problems. Some states have enacted legislation requiring health care providers to test for and/or report prenatal drug abuse when suspected, and some states have created or funded treatment programs or services for pregnant women (47, 48). Results from the Screening, Brief Intervention, and Referral to Treatment (SBIRT) service program have shown the feasibility of its implementation and the efficacy in reducing substance use and improving general health, mental health and social measures (49, 50). The ACOG's advisory committee has provided guidelines and rationales to urge healthcare providers to implement SBIRT protocols (51). Effective prevention efforts can mean not only fewer substance-affected babies but also lower related medical expenditures and lesser burdens on a mother and her family as well as on society as a whole.
Acknowledgements
This article is based on a study conducted for the Alcohol Epidemiologic Data System project funded by the National Institute on Alcohol Abuse and Alcoholism through contract No. HHSN267200800023C to CSR, Incorporated.
Footnotes
Disclosure statement: None of the authors have a conflict of interest.
Publisher's Disclaimer: The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the sponsoring agency or the Federal government.
References
- 1.Susbstance Abuse and Mental Health Services Administration (SAMSHA) Results from the 2008 National Survey on Drug Use and Health: National findings. SAMSHA, Office of Applied Studies; Rockville, MD: 2009. (NSDUH Series H-36). HHS Publication No. SMA 09-4434. [Google Scholar]
- 2.Warren KR, Thomas JD, editors. Fetal Alcohol Spectrum Disorders. Alcohol Research & Health. 2011;34(1) [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand J, Floyd RL, Weber MK, O'Connor M, Riley EP, Johnson KA, Cohen DE, National Task Force on FAS/FAE . Fetal Alcohol Syndrome: Guidelines for referral and diagnosis. Centers for Disease Control and Prevention; Atlanta, GA: 2004. [Google Scholar]
- 4.Addis A, Moretti ME, Ahmed Syed F, Einarson TR, Koren G. Fetal effects of cocaine: an updated meta-analysis. Reprod Toxicol. 2001;15(4):341–369. doi: 10.1016/s0890-6238(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 5.Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204:340.e1–e12. doi: 10.1016/j.ajog.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Savitz DA, Murnane P. Behavioral influences on preterm birth: a review. Epidemiology. 2010;21(3):291–299. doi: 10.1097/EDE.0b013e3181d3ca63. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Frassica JJ, Orav EJ. Cardiovascular abnormalities in infants prenatally exposed to cocaine. J Pediatr. 1991;118(1):44–51. doi: 10.1016/s0022-3476(05)81842-6. [DOI] [PubMed] [Google Scholar]
- 8.Ladhani NN, Shah PS, Murphy KE. Prenatal amphetamine exposure and birth outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:219.e1–e7. doi: 10.1016/j.ajog.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B. Neuroimaging of children following prenatal drug exposure. Semin Cell Dev Biol. 2009;20(4):441–454. doi: 10.1016/j.semcdb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roussotte F, Soderberg L, Sowell E. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychol Rev. 2010;20(4):376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Committee on Drugs Neonatal drug withdrawal. Pediatrics. 1998;101(6):1079–1088. [PubMed] [Google Scholar]
- 12.Robbins JM, Bird TM, Tilford JM, Reading JA, Cleves MA, Aitken ME, Druschel CM, Hobbs CA. Reduction in newborns with discharge coding of in utero alcohol effects in the United States, 1993 to 2002. Arch Pediatr Adolesc Med. 2006;160(12):1224–1231. doi: 10.1001/archpedi.160.12.1224. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics . Healthy People 2010 Final Review. Centers for Disease Control and Prevention (CDC), National Center for Health Statistics; Hyattsville, MD: 2011. cited November 29, 2011. Available from: http://www.cdc.gov/nchs/data/hpdata2010/hp2010_final_review.pdf. [Google Scholar]
- 14.Dicker M, Leighton EA. Trends in the US prevalence of drug-using parturient women and drug-affected newborns, 1979 through 1990. Am J Public Health. 1994;84(9):1433–1438. doi: 10.2105/ajph.84.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell M, Nassar N, Leonard H, Hagan R, Mathews R, Patterson Y, Stanley F. Increasing prevalence of neonatal withdrawal syndrome: population study of maternal factors and child protection involvement. Pediatrics. 2009;123(4):e614–e621. doi: 10.1542/peds.2008-2888. [DOI] [PubMed] [Google Scholar]
- 16.Healthcare Cost and Utilization Project (HCUP) Introduction to the HCUP Nationwide Inaptient Sample (NIS) U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; Rockville, MD: 2008. cited September 20, 2010. Available from: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2008.jsp. [Google Scholar]
- 17.Centers for Medicare and Medicaid Services, National Center for Health Statistics ICD-9-CM Official Guidelines for Coding and Reporting. Effective. 2010 Oct 1; cited December 1, 2010. Available from: http://www.cdc.gov/nchs/data/icd9/icdguide10.pdf.
- 18.Healthcare Cost and Utilization Project (HCUP) Cost-to-Charge Ratio Files. U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; Rockville, MD: 2011. cited April 17, 2012. Available from: http://www.hcupus.ahrq.gov/db/state/costtocharge.jsp. [Google Scholar]
- 19.US Department of Labor Bureau of Labor Statistics . Chained consumer price index for all urban consumers (C-CPI-U), US All Items. US Department of Labor; Washington, DC: 2012. Consumer Price Index. (Series ID: SUUR0000SA0). cited April 25, 2012. Available from: http://data.bls.gov/cgi-bin/surveymost?su. [Google Scholar]
- 20.Healthcare Cost and Utilization Project (HCUP) NIS Description of Data Elements. U.S. Department of Health and Human Services, Agency for Healthcare Research and Quality; Rockville, MD: 2011. cited March 29, 2011. Available from: http://www.hcupus.ahrq.gov/db/nation/nis/nisdde.jsp. [Google Scholar]
- 21.Cox S, Posner SF, Kourtis AP, Jamieson DJ. Hospitalizations with amphetamine abuse among pregnant women. Obstet Gynecol. 2008;111(2 Pt 1):341–347. doi: 10.1097/01.AOG.000300377.82722.ad. [DOI] [PubMed] [Google Scholar]
- 22.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113(6):1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 23.Cox S, Johnson CH, Meikle S, Jamieson DJ, Posner SF. Trends in rates of hospitalization with a diagnosis of substance abuse among reproductive-age women, 1998 to 2003. Womens Health Issues. 2007;17(2):75–83. doi: 10.1016/j.whi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.National Drug Intelligence Center . National Drug Threat Assessment 2011. U.S. Department of Justice, National Drug Intelligence Center; Washington, DC: 2011. cited September 20, 2011. Available from: http://www.justice.gov/ndic/pubs44/44849/44849p.pdf. [Google Scholar]
- 25.Substance Abuse and Mental Health Services Administration (SAMSHA) Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. SAMSHA, Office of Applied Studies; Rockville, MD: 2011. (NSDUH Series H-41). HHS Publication No. SMA 11-4658. [Google Scholar]
- 26.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Substance Abuse and Mental Health Services Administration (SAMSHA) Treatment Episode Data Set (TEDS): 1998–2008. National Admissions to Substance Abuse Treatment Services. SAMSHA, Office of Applied Studies; Rockville, MD: 2010. (DASIS Series: S-50). HHS Publication No. SMA 09-4471. [Google Scholar]
- 28.Centers for Disease Control and Prevention Emergency department visits involving nonmedical use of selected prescription drugs—United States, 2004–2008. MMWR. 2010;59(23):705–709. [PubMed] [Google Scholar]
- 29.Cox S, Kuo C, Jamieson DJ, Kourtis AP, McPheeters ML, Meikle SF, Posner SF. Poisoning hospitalisations among reproductive-aged women in the USA, 1998–2006. Inj Prev. 2011;17(5):332–337. doi: 10.1136/ip.2010.029793. [DOI] [PubMed] [Google Scholar]
- 30.Coben JH, Davis SM, Furbee PM, Sikora RD, Tillotson RD, Bossarte RM. Hospitalizations for poisoning by prescription opioids, sedatives, and tranquilizers. Am J Prev Med. 2010;38(5):517–524. doi: 10.1016/j.amepre.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Bohnert AS, Fudalej S, Ilgen MA. Increasing poisoning mortality rates in the United States, 1999–2006. Public Health Rep. 2010;125(4):542–547. doi: 10.1177/003335491012500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulozzi LJ, Annest JL. US data show sharply rising drug-induced death rates. Inj Prev. 2007;13(2):130–132. doi: 10.1136/ip.2006.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulozzi LJ, Centers for Disease Control and Prevention (CDC) Drug-induced deaths: United States, 2003–2007. MMWR Surveill Summ. 2011;60(Suppl.):60–61. [PubMed] [Google Scholar]
- 34.Harder VS, Chilcoat HD. Cocaine use and educational achievement: understanding a changing association over the past 2 decades. Am J Public Health. 2007;97(10):1790–1793. doi: 10.2105/AJPH.2006.091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Substance Abuse and Mental Health Services Administration (SAMSHA) Results from the 2008 National Survey on Drug Use and Health: Detailed tables. SAMSHA, Office of Applied Studies; Rockville, MD: 2009. cited August 2, 2011. Available from: http://oas.samhsa.gov/NSDUH/2K8NSDUH/tabs/INDEX.PDF. [Google Scholar]
- 36.Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114(3):243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 37.Ventura SJ, Martin JA, Curtin SC, Menacker F, Hamilton BE. Births: Final data for 1999. National Center for Health Statistics; Hyattsville, MD: 2001. [PubMed] [Google Scholar]
- 38.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews Osterman MJK. Births: Final data for 2008. National Center for Health Statistics; Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- 39.Havens JR, Simmons LA, Shannon LM, Hansen WF. Factors associated with substance use during pregnancy: results from a national sample. Drug Alcohol Depend. 2009;99(1–3):89–95. doi: 10.1016/j.drugalcdep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Ethen MK, Ramadhani TA, Scheuerle AE, Canfield MA, Wyszynski DF, Druschel CM, Romitti PA. Alcohol consumption by women before and during pregnancy. Matern Child Health J. 2009;13(2):274–285. doi: 10.1007/s10995-008-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diekman ST, Floyd RL, Découflé P, Schulkin J, Ebrahim SH, Sokol RJ. A survey of obstetrician-gynecologists on their patients' alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756–763. doi: 10.1016/s0029-7844(99)00616-x. [DOI] [PubMed] [Google Scholar]
- 42.Anderson BL, Dang EP, Floyd RL, Sokol R, Mahoney J, Schulkin J. Knowledge, opinions, and practice patterns of obstetrician-gynecologists regarding their patients' use of alcohol. J Addict Med. 2010;4(2):114–121. doi: 10.1097/ADM.0b013e3181b95015. [DOI] [PubMed] [Google Scholar]
- 43.Roberts SC, Nuru-Jeter A. Universal alcohol/drug screening in prenatal care: a strategy for reducing racial disparities? Questioning the assumptions. Matern Child Health J. 2011;15(8):1127–1134. doi: 10.1007/s10995-010-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellsworth MA, Stevens TP, D'Angio CT. Infant race affects application of clinical guidelines when screening for drugs of abuse in newborns. Pediatrics. 2010;125(6):e1379–85. doi: 10.1542/peds.2008-3525. [DOI] [PubMed] [Google Scholar]
- 45.Gavin NI, Adams EK, Hartmann KE, Benedict MB, Chireau M. Racial and ethnic disparities in the use of pregnancy-related health care among Medicaid pregnant women. Matern Child Health J. 2004;8(3):113–26. doi: 10.1023/b:maci.0000037645.63379.62. [DOI] [PubMed] [Google Scholar]
- 46.Fussell HE, Rieckmann TR, Quick MB. Medicaid reimbursement for screening and brief intervention for substance misuse. Psychiatr Serv. 2011;62(3):306–309. doi: 10.1176/ps.62.3.pss6203_0306. [DOI] [PubMed] [Google Scholar]
- 47.Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J. 2004;1(5):1–44. doi: 10.1186/1477-7517-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttmacher Institute State Policies in Brief – Substance Abuse during Pregnancy (as of November 1, 2011) cited November 23, 2011. Available from: http://www.guttmacher.org/statecenter/spibs/spib_SADP.pdf.
- 49.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 50.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1–3):280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ACOG Committee Opinion No. 422: at-risk drinking and illicit drug use: ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–60. doi: 10.1097/AOG.0b013e318192499b. [DOI] [PubMed] [Google Scholar]